Acute Effects of Blood Flow Restriction Training on Movement Velocity and Neuromuscular Signal during the Back Squat Exercise
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
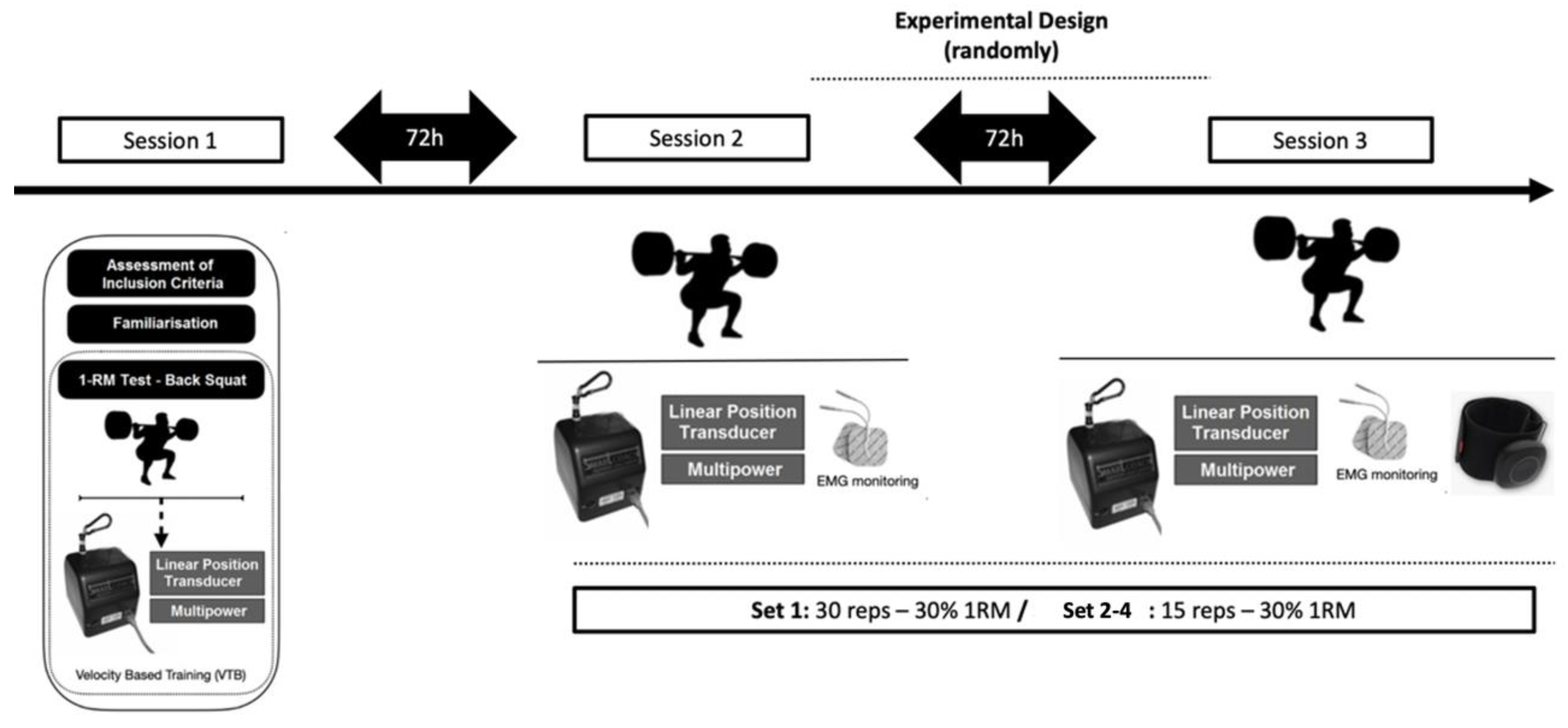
2.2. Trial Design
2.3. Procedures
2.3.1. Back Squat One-Repetition Maximum Test
2.3.2. Back Squat Exercise Protocol
2.3.3. BFRT Protocol
2.3.4. Surface EMG Protocol
2.3.5. Statistical Analysis
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferraz, R.B.; Gualano, B.; Rodrigues, R.; Kurimori, C.O.; Fuller, R.; Lima, F.R.; De Sá-Pinto, A.L.; Roschel, H. Benefits of Resistance Training with Blood Flow Restriction in Knee Osteoarthritis. Med. Sci. Sports Exerc. 2018, 50, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, S.S.; Næss-Schmidt, E.T.; Hansen, G.M.; Nielsen, J.F.; Stubbs, P.W. Neuromuscular effects of dorsiflexor training with and without blood flow restriction. Heliyon 2019, 5, e02341. [Google Scholar] [CrossRef] [PubMed]
- Bowman, E.N.; Elshaar, R.; Milligan, H.; Jue, G.; Mohr, K.; Brown, P.; Watanabe, D.M.; Limpisvasti, O. Proximal, Distal, and Contralateral Effects of Blood Flow Restriction Training on the Lower Extremities: A Randomized Controlled Trial. Sports Health 2019, 11, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Labata-Lezaun, N.; Llurda-Almuzara, L.; González-Rueda, V.; López-de-Celis, C.; Cedeño-Bermúdez, S.; Bañuelos-Pago, J.; Perez-Bellmunt, A. Effectiveness of Blood Flow Restriction Training on Muscle Strength and Physical Performance in Older Adults: A Systematic Review and Meta-analysis. Arch. Phys. Med. Rehabil. 2022, 103, 1848–1857. [Google Scholar] [CrossRef]
- Maga, M.; Wachsmann-Maga, A.; Batko, K.; Włodarczyk, A.; Kłapacz, P.; Krężel, J.; Szopa, N.; Sliwka, A. Impact of Blood-Flow-Restricted Training on Arterial Functions and Angiogenesis—A Systematic Review with Meta-Analysis. Biomedicines 2023, 11, 1601. [Google Scholar] [CrossRef]
- Pant, G.; Bhutia, U.D. Effect of restricted blood flow on muscle hypotrophy & O2 saturation level on weight training. Int. J. Phys. Educ. Sport. Health 2017, 4, 316–317. [Google Scholar]
- Flocco, P.; Galeoto, G. Effect of blood flow restriction training on physiological outcomes in healthy athletes: A systematic review and meta-analysis. Muscles Ligaments Tendons J. 2021, 11, 101–117. [Google Scholar] [CrossRef]
- Centner, C.; Jerger, S.; Lauber, B.; Seynnes, O.; Friedrich, T.; Lolli, D.; Gollhofer, A.; König, D. Low-Load Blood Flow Restriction and High-Load Resistance Training Induce Comparable Changes in Patellar Tendon Properties. Med. Sci. Sports Exerc. 2021, 54, 582–589. [Google Scholar] [CrossRef]
- Schwiete, C.; Franz, A.; Roth, C.; Behringer, M. Effects of Resting vs. Continuous Blood-Flow Restriction-Training on Strength, Fatigue Resistance, Muscle Thickness, and Perceived Discomfort. Front Physiol. 2021, 12, 663665. [Google Scholar] [CrossRef]
- Wortman, R.J.; Brown, S.M.; Savage-Elliott, I.; Finley, Z.J.; Mulcahey, M.K. Blood Flow Restriction Training for Athletes: A Systematic Review. Am. J. Sports Med. 2021, 49, 1938–1944. [Google Scholar] [CrossRef]
- Yasuda, T.; Loenneke, J.P.; Thiebaud, R.S.; Abe, T. Effects of Blood Flow Restricted Low-Intensity Concentric or Eccentric Training on Muscle Size and Strength. PLoS ONE 2012, 7, e52843. [Google Scholar] [CrossRef] [PubMed]
- Chulvi-Medrano, I.; Picón-Martínez, M.; Cortell-Tormo, J.M.; Tortosa-Martínez, J.; Alonso-Aubin, D.A.; Alakhdar, Y. Different time course of recovery in achilles tendon thickness after low-load resistance training with and without blood flow restriction. J. Sport Rehabil. 2021, 30, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, M.; Cramer, J.T.; Abe, T.; Sato, Y.; Bemben, M.G. Neuromuscular fatigue following low-intensity dynamic exercise with externally applied vascular restriction. J. Electromyogr. Kinesiol. 2010, 20, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Centner, C.; Ritzmann, R.; Schur, S.; Gollhofer, A.; König, D. Blood flow restriction increases myoelectric activity and metabolic accumulation during whole-body vibration. Eur. J. Appl. Physiol. 2019, 119, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Queiros, V.S.; De França, I.M.; De Trybulski, R. Myoelectric Activity and Fatigue in Low-Load Resistance Exercise with Different Pressure of Blood Flow Restriction: A Systematic Review and Meta-Analysis. Front Physiol. 2021, 12, 786752. [Google Scholar] [CrossRef]
- González-Badillo, J.J.; Sánchez-Medina, L.; Ribas-Serna, J.; Rodríguez-Rosell, D. Toward a New Paradigm in Resistance Training by Means of Velocity Monitoring: A Critical and Challenging Narrative. Sport Med—Open 2022, 8, 118. [Google Scholar] [CrossRef]
- García-Ramos, A.; Suzovic, D.; Pérez-Castilla, A. The load-velocity profiles of three upper-body pushing exercises in men and women. Sport Biomech. 2019, 20, 693–705. [Google Scholar] [CrossRef]
- Sánchez-Medina, L.; Pallarés, J.; Pérez, C.; Morán-Navarro, R.; González-Badillo, J. Estimation of Relative Load From Bar Velocity in the Full Back Squat Exercise. Sport Med. Int. Open 2017, 1, E80–E88. [Google Scholar] [CrossRef]
- Sánchez-Medina, L.; González-Badillo, J.J. Velocity loss as an indicator of neuromuscular fatigue during resistance training. Med. Sci. Sports Exerc. 2011, 43, 1725–1734. [Google Scholar] [CrossRef]
- Rodríguez-Rosell, D.; Yáñez-García, J.M.; Torres-Torrelo, J.; Mora-Custodio, R.; Marques, M.C.; González-Badillo, J.J. Effort index as a novel variable for monitoring the level of effort during resistance exercises. J. Strength Cond. Res. 2018, 32, 2139–2153. [Google Scholar] [CrossRef]
- Wilk, M.; Gepfert, M.; Krzysztofik, M.; Stastny, P.; Zajac, A.; Bogdanis, G.C. Acute Effects of Continuous and Intermittent Blood Flow Restriction on Movement Velocity During Bench Press Exercise Against Different Loads. Front Physiol. 2020, 11, 1–9. [Google Scholar] [CrossRef]
- Cancela, J.; Ayán, C.; Vila, H.; Gutiérrez, J.; Gutiérrez-Santiago, A. Validez de Constructo del Cuestionario Internacional de Actividad Física en Universitarios Españoles. Rev. Iberoam. Diagnóstico Evaluación Avaliação Psicológica 2019, 52, 5–14. [Google Scholar] [CrossRef]
- Shrestha, B.; Dunn, L. The Declaration of Helsinki on Medical Research involving Human Subjects: A Review of Seventh Revision. J. Nepal. Health Res. Counc. 2020, 17, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Medina, L.; Perez, C.E.; Gonzalez-Badillo, J.J. Importance of the propulsive phase in strength assessment. Int. J. Sports Med. 2010, 31, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J.; Pope, Z.K.; Benik, F.M.; Hester, G.M.; Sellers, J.; Nooner, J.L.; Schnaiter, J.A.; Bond-Williams, K.E.; Carter, A.S.; Ross, C.L.; et al. Longer interset rest periods enhance muscle strength and hypertrophy in resistance-trained men. J. Strength Cond. Res. 2016, 30, 1805–1812. [Google Scholar] [CrossRef]
- Garcia-Sillero, M.; Chulvi-Medrano, I.; Maroto-Izquierdo, S.; Bonilla, D.A.; Vargas-Molina, S.; Benítez-Porres, J. Effects of Preceding Transcranial Direct Current Stimulation on Movement Velocity and EMG Signal during the Back Squat Exercise. J. Clin. Med. 2022, 11, 5220. [Google Scholar] [CrossRef]
- García-Sillero, M.; Jurado-Castro, J.M.; Benítez-Porres, J.; Vargas-Molina, S. Acute effects of a percussive massage treatment on movement velocity during resistance training. Int. J. Environ. Res. Public Health 2021, 18, 7726. [Google Scholar] [CrossRef]
- Pope, Z.K.; Willardson, J.M.; Schoenfeld, B.J. Exercise and blood flow restriction. J. Strength Cond. Res. 2013, 27, 2914–2926. [Google Scholar] [CrossRef]
- Stegeman, D.; Hermens, H. Standards for Surface Electromyography: The European Project Surface EMG for Non-Invasive Assessment of Muscles (SENIAM). Enschede: Roessingh Res. Dev. 2007, 10, 8–12. [Google Scholar]
- Hermens, H.J.; Merletti, R.; Rix, H.; Freriks, B. The State of the Art on Signal Processing Methods for Surface ElectroMyoGraphy. In Seniam; Springer: Singapore, 1998; pp. 7–9. [Google Scholar]
- Gobbo, M.; Maffiuletti, N.A.; Orizio, C.; Minetto, M.A. Muscle motor point identification is essential for optimizing neuromuscular electrical stimulation use. J. Neuroeng. Rehabil. 2014, 11, 1–6. [Google Scholar] [CrossRef]
- Napoli, N.J.; Mixco, A.R.; Bohorquez, J.E.; Signorile, J.F. An EMG comparative analysis of quadriceps during isoinertial strength training using nonlinear scaled wavelets. Hum. Mov. Sci. 2015, 40, 134–153. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Ty Hopkins, J. Quadriceps activation normative values and the affect of subcutaneous tissue thickness. J. Electromyogr. Kinesiol. 2011, 21, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.; Kuenze, C.; Saliba, S.; Hart, J.M. Accessory muscle activation during the superimposed burst technique. J. Electromyogr. Kinesiol. 2012, 22, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Molina-Molina, A.; Ruiz-Malagón, E.J.; Carrillo-Pérez, F.; Roche-Seruendo, L.E.; Damas, M.; Banos, O.; García-Pinillos, F. Validation of mDurance, A Wearable Surface Electromyography System for Muscle Activity Assessment. Front. Physiol. 2020, 11, 11. [Google Scholar] [CrossRef]
- Jakobsen, M.D.; Sundstrup, E.; Andersen, C.H.; Aagaard, P.; Andersen, L.L. Muscle activity during leg strengthening exercise using free weights and elastic resistance: Effects of ballistic vs controlled contractions. Hum. Mov. Sci. 2013, 32, 65–78. [Google Scholar] [CrossRef]
- Gepfert, M.; Krzysztofik, M.; Kostrzewa, M.; Jarosz, J.; Trybulski, R.; Zajac, A.; Wilk, M. The acute impact of external compression on back squat performance in competitive athletes. Int. J. Environ. Res. Public Health 2020, 17, 4674. [Google Scholar] [CrossRef]
- Wilk, M.; Trybulski, R.; Krzysztofik, M.; Wojdala, G.; Campos, Y.; Zajac, A.; Lulińska, E.; Stastny, P. Acute Effects of Different Blood Flow Restriction Protocols on Bar Velocity During the Squat Exercise. Front. Physiol. 2021, 12, 1–8. [Google Scholar] [CrossRef]
- Serrano-Ramon, J.; Cortell-Tormo, J.; Bautista, I.; García Jaén, M.; Chulvi-Medrano, I. Acute effects of different external compression with blood flow restriction on force-velocity profile during squat and bench press exercises. Biol. Sport 2023, 40, 209–216. [Google Scholar] [CrossRef]
- Farup, J.; de Paoli, F.; Bjerg, K.; Riis, S.; Ringgard, S.; Vissing, K. Blood flow restricted and traditional resistance training performed to fatigue produce equal muscle hypertrophy. Scand. J. Med. Sci. Sport 2015, 25, 754–763. [Google Scholar] [CrossRef]
- Teixeira, E.L.; Painelli V de, S.; Schoenfeld, B.J.; Silva-Batista, C.; Longo, A.R.; Aihara, A.Y.; Cardoso, F.N.; Peres, B.D.A.; Tricoli, V. Perceptual and Neuromuscular Responses Adapt Similarly Between High-Load Resistance Training and Low-Load Resistance Training with Blood Flow Restriction. J. Strength Cond. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Laurentino, G.; Ugrinowitsch, C.; Aihara, A.Y.; Fernandes, A.R.; Parcell, A.C.; Ricard, M.; Tricoli, V. Effects of strength training and vascular occlusion. Int. J. Sports Med. 2008, 29, 664–667. [Google Scholar] [CrossRef]
- Yasuda, T.; Ogasawara, R.; Sakamaki, M.; Ozaki, H.; Sato, Y.; Abe, T. Combined effects of low-intensity blood flow restriction training and high-intensity resistance training on muscle strength and size. Eur. J. Appl. Physiol. 2011, 111, 2525–2533. [Google Scholar] [CrossRef]
- Chang, H.; Yao, M.; Chen, B.; Qi, Y.; Zhang, J. Effects of Blood Flow Restriction Combined with Low-Intensity Resistance Training on Lower-Limb Muscle Strength and Mass in Post-Middle-Aged Adults: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 15691. [Google Scholar] [CrossRef]
- Gołaś, A.; Maszczyk, A.; Petr, M.; Statsny, P.; Wilk, M.; Wróbel, G. Changes in Bar Velocity and Muscular Activity during the Bench Press in Relation to the Load Lifted. Cent. Eur. J. Sport Sci. Med. 2015, 11, 95–101. [Google Scholar] [CrossRef]
- Gabriel, D.A.; Kamen, G.; Frost, G. Neural adaptations to resistive exercise: Mechanisms and recommendations for training practices. Sport Med. 2006, 36, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.R.; Burgomaster, K.A.; Schofield, L.M.; Gibala, M.J.; Sale, D.G.; Phillips, S.M. Neuromuscular adaptations in human muscle following low intensity resistance training with vascular occlusion. Eur. J. Appl. Physiol. 2004, 92, 399–406. [Google Scholar] [CrossRef]
- Neto, G.R.; Santos, H.H.; Sousa, J.B.C.; Júnior, A.T.A.; Araújo, J.P.; Aniceto, R.R.; Sousa, M.S.C. Effects of high-intensity blood flow restriction exercise on muscle fatigue. J. Hum. Kinet. 2014, 41, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Takarada, Y.; Takazawa, H.; Sato, Y.; Takebayashi, S.; Tanaka, Y.; Ishii, N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J. Appl. Physiol. 2000, 88, 2097–2106. [Google Scholar] [CrossRef]
- Gandevia, S.C. Spinal and supraspinal factors in human muscle fatigue. Physiol. Rev. 2001, 81, 1725–1789. [Google Scholar] [CrossRef]
- Sharifi, S.; Monazzami, A.; Nikousefat, Z.; Heyrani, A.; Yari, K. The acute and chronic effects of resistance training with blood flow restriction on hormonal responses in untrained young men: A comparison of frequency. Cell Mol. Biol. 2020, 66, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Incognito, A.V.; Burr, J.F.; Millar, P.J. The Effects of Ischemic Preconditioning on Human Exercise Performance. Sport Med. 2016, 46, 531–544. [Google Scholar] [CrossRef] [PubMed]
- de Souza, H.L.R.; Arriel, R.A.; Hohl, R.; da Mota, G.R.; Marocolo, M. Is Ischemic Preconditioning Intervention Occlusion-Dependent to Enhance Resistance Exercise Performance? J. Strength Cond. Res. 2021, 35, 2706–2712. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.G.; Birk, G.K.; Timothy Cable, N.; Atkinson, G.; Green, D.J.; Jones, H.; Thijssen, D.H.J. Remote ischemic preconditioning prevents reduction in brachial artery flow-mediated dilation after strenuous exercise. Am. J. Physiol. Hear. Circ. Physiol. 2012, 303, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Andreas, M.; Schmid, A.I.; Keilani, M.; Doberer, D.; Bartko, J.; Crevenna, R.; Moser, E.; Wolzt, M. Effect of ischemic preconditioning in skeletal muscle measured by functional magnetic resonance imaging and spectroscopy: A randomized crossover trial. J. Cardiovasc. Magn. Reson. 2011, 13, 32. [Google Scholar] [CrossRef] [PubMed]
| BFRT Condition (n = 21) | Non-BFRT Condition (n = 21) | |||||||
|---|---|---|---|---|---|---|---|---|
| Set 1 | Set 2 | Set 3 | Set 4 | Set 1 | Set 2 | Set 3 | Set 4 | |
| Movement velocity | ||||||||
| MPV (m·s−1) | 0.85 ± 0.11 (0.70–1.05) | 0.85 ± 0.10 (0.69–1.06) | 0.85 ± 0.11 (0.67–1.07) | 0.85 ± 0.11 (0.63–1.05) | 0.85 ± 0.11 (0.65–1.02) | 0.85 ± 0.11 (0.62–1.07) | 0.84 ± 0.12 (0.61–1.05) | 0.86 ± 0.12 (0.63–1.11) |
| Vmax (m·s−1) | 1.39 ± 0.17 (1.11–1.71) | 1.37 ± 0.16 (1.10–1.67) | 1.36 ± 0.17 (1.10–1.69) | 1.38 ± 0.18 (1.04–1.71) | 1.41 ± 0.17 (1.13–1.66) | 1.39 ± 0.18 (1.03–1.75) | 1.39 ± 0.20 (1.01–1.73) | 1.38 ± 0.20 (1.08–1.85) |
| EI | 20.7 ± 14.6 *,^ (6.6–58.7) | 12.1 ± 7.4 (3.9–32.1) | 13.1 ± 6.0 (3.4–26.1) | 12.5 ± 6.0 (3.9–23.9) | 17.3 ± 9.2 (6.6–48.4) | 11.2 ± 3.9 (4.2–20.5) | 10.7 ± 3.9 (6.2–20.6) | 11.9 ± 6.3 (6.0–30.1) |
| Muscle activity | ||||||||
| RF EMGmax (μV) | 82.1 ± 19.8 * (29.0–102.0) | 72.8 ± 16.6 (29.4–100.0) | 73.8 ± 17.2 (26.3–97.8) | 74.8 ± 18.3 (26.5–104.0) | 77.0 ± 18.8 (25.4–102.0) | 72.0 ± 17.0 (24.7–99.4) | 75.9 ± 19.7 (24.8–100.9) | 73.7 ± 18.0 (24.0–100.0) |
| RF EMGrms (%) | 34.9 ± 9.1 * (15.1–46.1) | 29.2 ± 6.9 (16.6–46.4) | 29.7 ± 6.8 (13.8–40.0) | 30.1 ± 6.4 (17.2–40.6) | 34.4 ± 11.9 (0.3–49.2) | 30.4 ± 8.0 (10.5–48.3) | 30.0 ± 8.6 (11.4–48.6) | 30.4 ± 8.3 (10.1–48.3) |
| VL EMGmax(μV) | 83.3 ± 18.0 (44.9–110.4) | 79.9 ± 17.0 (43.9–100.0) | 79.7 ± 16.6 (43.9–99.3) | 78.8 ± 16.3 (41.7–100.2) | 84.7 ± 15.0 (46.8–103.0) | 78.9 ± 13.4 (44.2–95.9) | 80.5 ± 11.3 (50.3–101.1) | 78.6 ± 12.2 (52.5–100.1) |
| VL EMGrms (%) | 39.7 ± 8.3 (22.8–56.2) | 35.5 ± 7.6 (23.2–51.0) | 36.1 ± 7.4 (22.9–48.3) | 36.5 ± 8.2 (21.0–52.6) | 39.9 ± 11.9 (0.5–53.0) | 36.3 ± 6.9 (18.7–50.7) | 35.8 ± 6.8 (18.6–50.1) | 36.1 ± 7.2 (20.7–50.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Sillero, M.; Maroto-Izquierdo, S.; Galván-García, M.; Benitez-Porres, J.; Vargas-Molina, S.; Jurado-Castro, J.M. Acute Effects of Blood Flow Restriction Training on Movement Velocity and Neuromuscular Signal during the Back Squat Exercise. J. Clin. Med. 2023, 12, 4824. https://doi.org/10.3390/jcm12144824
García-Sillero M, Maroto-Izquierdo S, Galván-García M, Benitez-Porres J, Vargas-Molina S, Jurado-Castro JM. Acute Effects of Blood Flow Restriction Training on Movement Velocity and Neuromuscular Signal during the Back Squat Exercise. Journal of Clinical Medicine. 2023; 12(14):4824. https://doi.org/10.3390/jcm12144824
Chicago/Turabian StyleGarcía-Sillero, Manuel, Sergio Maroto-Izquierdo, María Galván-García, Javier Benitez-Porres, Salvador Vargas-Molina, and Jose Manuel Jurado-Castro. 2023. "Acute Effects of Blood Flow Restriction Training on Movement Velocity and Neuromuscular Signal during the Back Squat Exercise" Journal of Clinical Medicine 12, no. 14: 4824. https://doi.org/10.3390/jcm12144824
APA StyleGarcía-Sillero, M., Maroto-Izquierdo, S., Galván-García, M., Benitez-Porres, J., Vargas-Molina, S., & Jurado-Castro, J. M. (2023). Acute Effects of Blood Flow Restriction Training on Movement Velocity and Neuromuscular Signal during the Back Squat Exercise. Journal of Clinical Medicine, 12(14), 4824. https://doi.org/10.3390/jcm12144824










