Hematological Indices Are Useful in Predicting Complications of Liver Cirrhosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Evaluation of Esophageal Varices
2.3. Statistical Analysis
3. Results
3.1. Demographic Characteristics
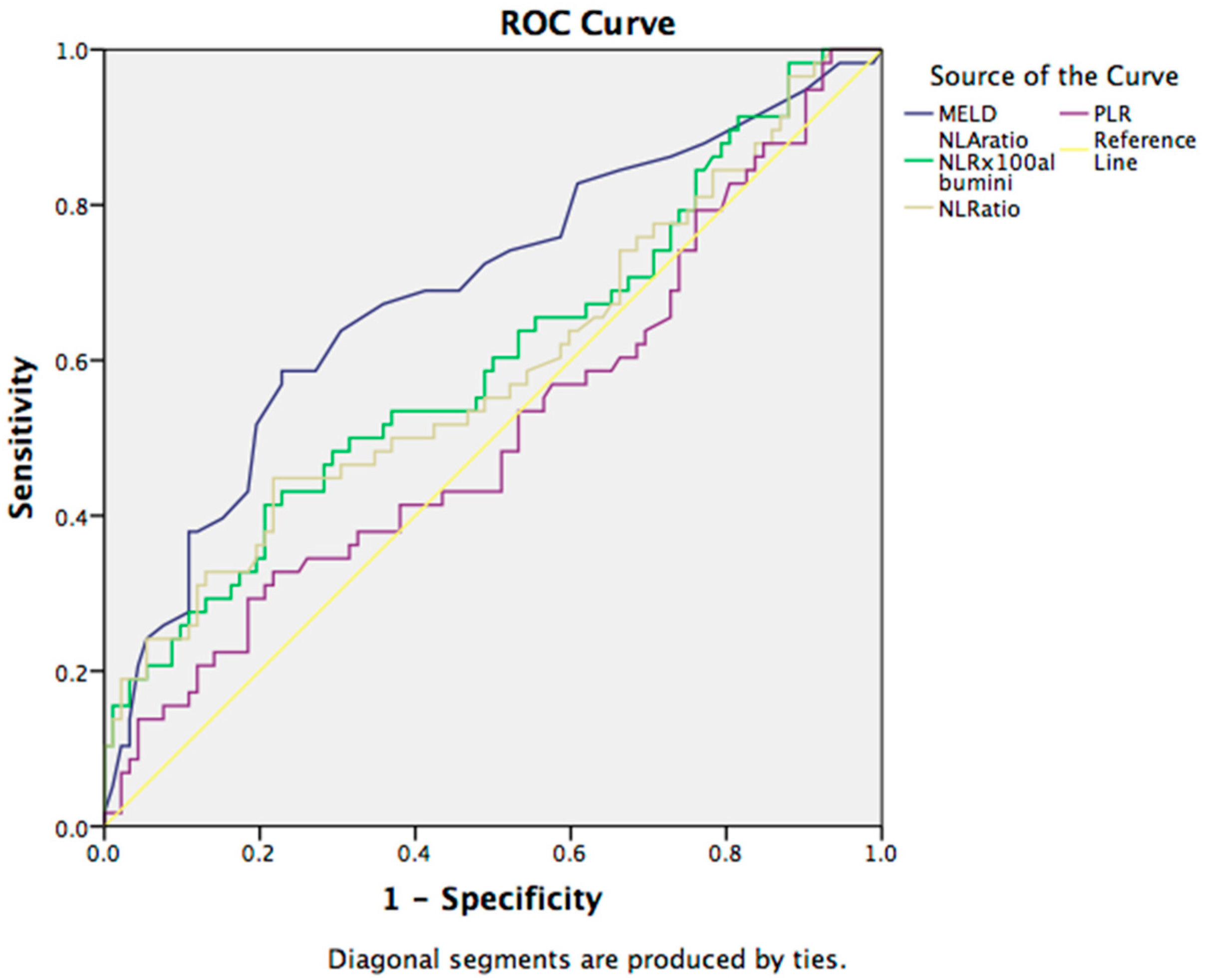
3.2. Prognostic Markers for 30-Day Mortality in Cirrhotic Patients
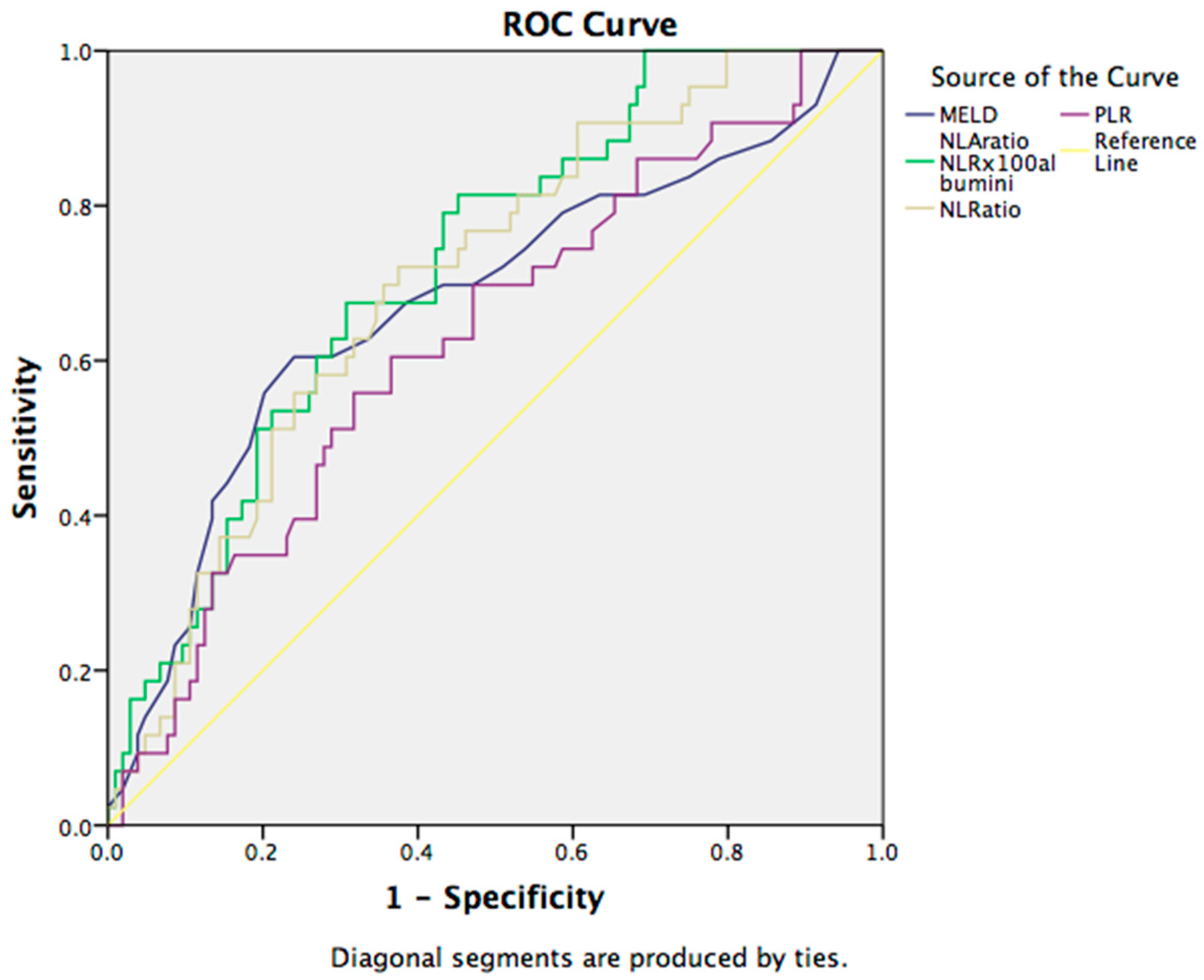
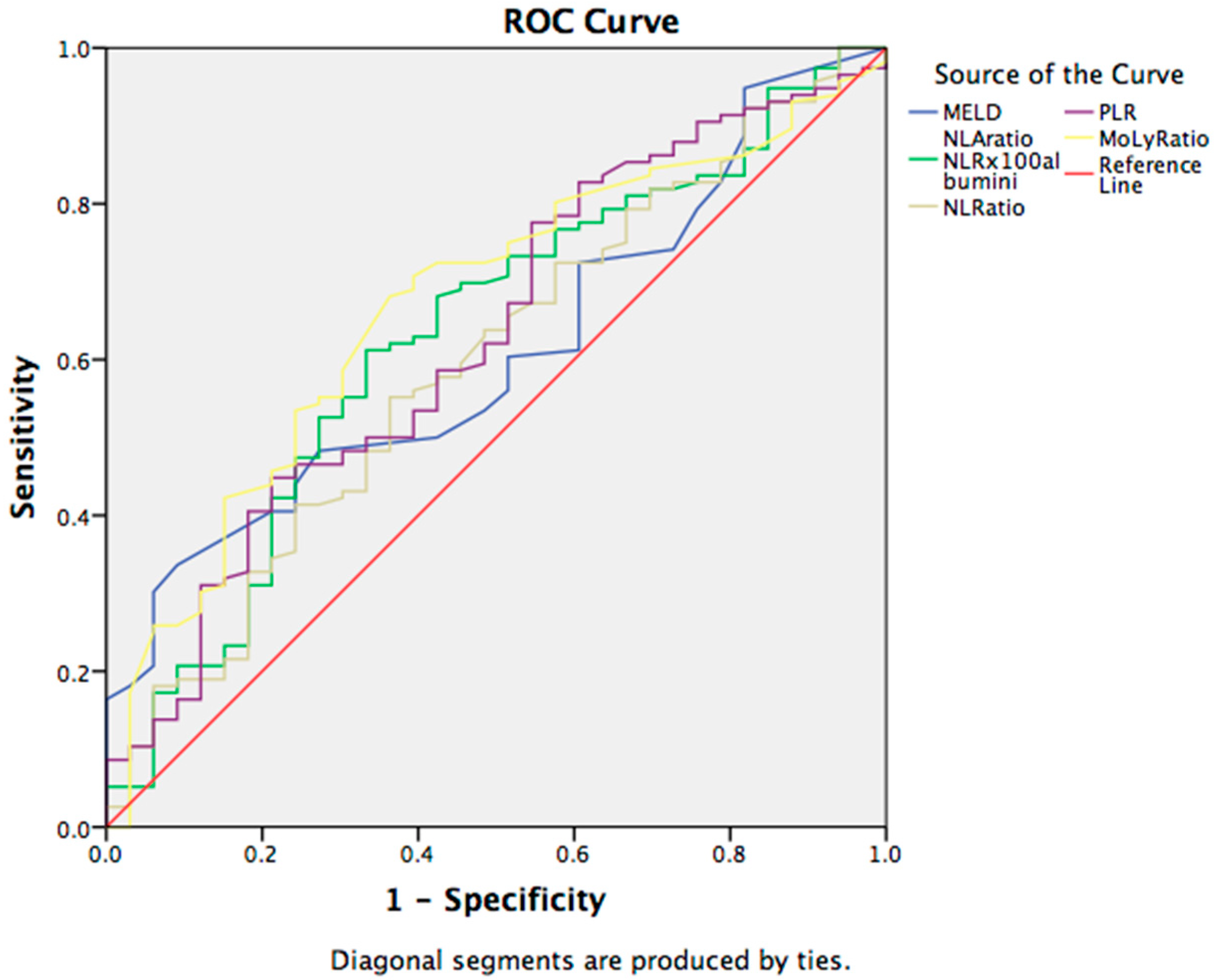
3.3. Prognostic Markers for the Complications in Cirrhotic Patients
4. Discussion
Limitation of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Asrani, S.K.; Kamath, P.S. Natural history of cirrhosis. Curr. Gastroenterol. Rep. 2013, 15, 308. [Google Scholar] [CrossRef] [PubMed]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Zahorec, R. Ratio of neutrophil to lymphocyte counts—Rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl. Lek. Listy 2001, 102, 5–14. [Google Scholar] [PubMed]
- Aktas, G.; Alcelik, A.; Tekce, B.K.; Tekelioglu, V.; Sit, M.; Savli, H. Red cell distribution width and mean platelet volume in patients with irritable bowel syndrome. Gastroenterol. Rev. 2014, 9, 160–163. [Google Scholar] [CrossRef]
- Paliogiannis, P.; Fois, A.G.; Sotgia, S.; Mangoni, A.A.; Zinellu, E.; Pirina, P.; Carru, C.; Zinellu, A. The neutrophil-to-lymphocyte ratio as a marker of chronic obstructive pulmonary disease and its exacerbations: A systematic review and meta-analysis. Eur. J. Clin. Investig. 2018, 48, e12984. [Google Scholar] [CrossRef]
- Duman, T.T.; Aktas, G.; Atak, B.M.; Kocak, M.Z.; Erkus, E.; Savli, H. Neutrophil to lymphocyte ratio as an indicative of diabetic control level in type 2 diabetes mellitus. Afr. Health Sci. 2019, 19, 1602–1606. [Google Scholar] [CrossRef]
- Guthrie, G.J.K.; Charles, K.A.; Roxburgh, C.S.D.; Horgan, P.G.; McMillan, D.C.; Clarke, S.J. The systemic inflammation-based neutrophil–lymphocyte ratio: Experience in patients with cancer. Crit. Rev. Oncol. Hematol. 2013, 88, 218–230. [Google Scholar] [CrossRef]
- Fu, H.; Qin, B.; Hu, Z.; Ma, N.; Yang, M.; Wei, T.; Tang, Q.; Huang, Y.; Huang, F.; Liang, Y.; et al. Neutrophil- and Platelet-to-Lymphocyte Ratios are Correlated with Disease Activity in Rheumatoid Arthritis. Clin. Lab. 2015, 61, 269–273. [Google Scholar] [CrossRef]
- Kokcu, A.; Kurtoglu, E.; Celik, H.; Tosun, M.; Malatyalıoglu, E.; Ozdemir, A.Z. May the platelet to lymphocyte ratio be a prognostic factor for epithelial ovarian cancer? Asian Pac. J. Cancer Prev. 2014, 15, 9781–9784. [Google Scholar] [CrossRef]
- Atak, B.M.; Duman, T.T.; Aktas, G.; Kocak, M.Z.; Savli, H. Platelet Distribution Width is Associated with Type 2 Diabetes Mellitus and Diabetic Nephropathy and Neuropathy. Natl. J. Health Sci. 2018, 3, 95–98. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, D.-Y.; Xu, X.-Z.; Liu, Y.-Y.; Yin, T.-T.; Li, D. Platelet/Lymphocyte, Lymphocyte/Monocyte, and Neutrophil/Lymphocyte Ratios as Biomarkers in Patients with Rheumatoid Arthritis and Rheumatoid Arthritis-Associated Interstitial Lung Disease. Experiment 2019, 25, 6474–6481. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Liu, J.; Wang, J.; Xie, T.; Zhang, Q.; Feng, S.; Deng, H.; Zhong, B. Platelet-to-lymphocyte ratio (PLR) and neutrophil-to-lymphocyte ratio (NLR) are associated with chronic hepatitis B virus (HBV) infection. Int. Immunopharmacol. 2017, 51, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Wei, G.; Chang, Q.; Peng, R.; Shi, G.; Zheng, P.; He, F.; Wang, W.; Ming, L. The platelet-to lymphocyte ratio, superior to the neutrophil-to-lymphocyte ratio, correlates with hepatitis C virus infection. Int. J. Infect. Dis. 2016, 45, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Biyik, M.; Ucar, R.; Solak, Y.; Gungor, G.; Polat, I.; Gaipov, A.; Cakir, O.O.; Ataseven, H.; Demir, A.; Turk, S.; et al. Blood neutrophil-to-lymphocyte ratio independently predicts survival in patients with liver cirrhosis. Eur. J. Gastroenterol. Hepatol. 2013, 25, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Kara, M.; Dogru, T.; Genc, H.; Sertoglu, E.; Celebi, G.; Gurel, H.; Kayadibi, H.; Cicek, A.F.; Ercin, C.N.; Sonmez, A. Neutrophil-to-lymphocyte ratio is not a predictor of liver histology in patients with nonalcoholic fatty liver disease. Eur. J. Gastroenterol. Hepatol. 2015, 27, 1144–1148. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, Y.; He, Y.; Wei, Q.; Xie, Q.; Zhang, L.; Xia, Y.; Zhou, X.; Zhang, L.; Feng, X.; et al. The role of neutrophil to lymphocyte ratio for the assessment of liver fibrosis and cirrhosis: A systematic review. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Graubard, B.I.; Rabkin, C.S.; Engels, E.A. Neutrophil-to-lymphocyte ratio and mortality in the United States general population. Sci. Rep. 2021, 11, 464. [Google Scholar] [CrossRef]
- Mortaz, E.; Alipoor, S.D.; Adcock, I.M.; Mumby, S.; Koenderman, L. Update on Neutrophil Function in Severe Inflammation. Front. Immunol. 2018, 9, 2171. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Yang, F.; Xu, Y.; Feng, C.; Zhao, Y. The regulatory roles of neutrophils in adaptive immunity. Cell Commun. Signal. 2019, 17, 147. [Google Scholar] [CrossRef]
- Lowsby, R.; Gomes, C.; Jarman, I.; Lisboa, P.; Nee, P.A.; Vardhan, M.; Eckersley, T.; Saleh, R.; Mills, H. Neutrophil to lymphocyte count ratio as an early indicator of blood stream infection in the emergency department. Emerg. Med. J. 2015, 32, 531–534. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, R.; Yu, X.; Yang, R.; Xu, H.; Mao, Z.; Wang, Y. The neutrophil-lymphocyte count ratio as a diagnostic marker for bacteraemia: A systematic review and meta-analysis. Am. J. Emerg. Med. 2019, 37, 1482–1489. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.; Huang, Q.; Yang, F.; Tian, W.; Li, C.; Ding, L.; Fang, H.-C.; Zhao, Y. Serum biomarkers to differentiate Gram-negative, Gram-positive and fungal infection in febrile patients. J. Med. Microbiol. 2021, 70, 001360. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Hou, M.; Ding, Z.; Liu, X.; Shao, Y.; Li, X. Prognostic Value of Neutrophil-to-Lymphocyte Ratio in Stroke: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 686–983. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-J.; Park, S.-D.; Kwon, S.W.; Woo, S.-I.; Lee, M.-D.; Shin, S.-H.; Kim, D.-H.; Kwan, J.; Park, K.-S. Relation between Neutrophil-to-Lymphocyte Ratio and Index of Microcirculatory Resistance in Patients with ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Am. J. Cardiol. 2016, 118, 1323–1328. [Google Scholar] [CrossRef]
- Adamstein, N.H.; MacFadyen, J.G.; Rose, L.M.; Glynn, R.J.; Dey, A.K.; Libby, P.; Tabas, I.A.; Mehta, N.N.; Ridker, P.M. The neutrophil–lympho cyte ratio and incident atherosclerotic events: Analyses from five contemporary randomized trials. Eur. Heart J. 2021, 42, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M. Neutrophil-to-lymphocyte ratio in trauma patients. J. Trauma Inj. Infect. Crit. Care 2017, 82, 225–226. [Google Scholar] [CrossRef]
- Lee, P.Y.; Oen, K.Q.X.; Lim, G.R.S.; Hartono, J.L.; Muthiah, M.; Huang, D.Q.; Teo, F.S.W.; Li, A.Y.; Mak, A.; Chandran, N.S.; et al. Neutrophil-to-Lymphocyte Ratio Predicts Development of Immune-Related Adverse Events and Outcomes from Immune Checkpoint Blockade: A Case-Control Study. Cancers 2021, 13, 1308. [Google Scholar] [CrossRef]
- Josse, J.M.; Cleghorn, M.C.; Ramji, K.M.; Jiang, H.; Elnahas, A.; Jackson, T.D.; Okrainec, A.; Quereshy, F.A. The neutrophil/lymphocyte ratio predicts major perioperative complications in patients undergoing colorectal surgery. Color. Dis. 2016, 18, O236–O242. [Google Scholar] [CrossRef]
- LaRosa, D.F.; Orange, J. 1. Lymphocytes. J. Allergy Clin. Immunol. 2008, 121, S364–S369. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Liu, L.; Prithweeraj, M.; Xu, H.; Wu, R.; Wen, X.; Niu, J. Neutrophil-to-Lymphocyte Ratio and Albumin: New Serum Biomarkers to Predict the Prognosis of Male Alcoholic Cirrhosis Patients. BioMed Res. Int. 2020, 2020, 7268459. [Google Scholar] [CrossRef]
- Kamath, P.S.; Kim, W.R. The model for end-stage liver disease (MELD). Hepatology 2007, 45, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Pugh, R.N.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Roayaie, S.; Jibara, G.; Berhane, S.; Tabrizian, P.; Park, J.W.; Yang, J.; Yan, L.; Han, G.; Izzo, F.; Chen, M.; et al. 851 PALBI-An Objective Score Based on Platelets, Albumin Bilirubin Stratifies HCC Patients Undergoing Resection & Ablation Better than Child’s Classification. Hepatology 2015, 62, 624A–690A. [Google Scholar]
- Lemoine, M.; Shimakawa, Y.; Nayagam, S.; Khalil, M.; Suso, P.; Lloyd, J.; Goldin, R.; Njai, H.-F.; Ndow, G.; Taal, M.; et al. The gamma-glutamyl transpeptidase to platelet ratio (GPR) predicts significant liver fibrosis and cirrhosis in patients with chronic HBV infection in West Africa. Gut 2015, 65, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Tsao, G.; Abraldes, J.G.; Berzigotti, A.; Bosch, J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 2017, 65, 310–335. [Google Scholar] [CrossRef]
- Qamar, A.A.; Grace, N.D. Abnormal hematological indices in cirrhosis. Can. J. Gastroenterol. 2009, 23, 441–445. [Google Scholar] [CrossRef]
- Bajaj, J.S.; O’Leary, J.G.; Reddy, K.R.; Wong, F.; Biggins, S.W.; Patton, H.; Fallon, M.B.; Garcia-Tsao, G.; Maliakkal, B.; Malik, R.; et al. Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology 2014, 60, 250–256. [Google Scholar] [CrossRef]
- Fan, Z.; EnQiang, C.; Yao, D.L.; LiBo, Y.; Hong, L.; Lang, B.; Ping, F.; Hong, T. Neutrophil–lymphocyte ratio predicts short term mortality in patients with hepatitis B virus-related acute-on-chronic liver failure treated with an artificial liver support system. PLoS ONE 2017, 12, e0175332. [Google Scholar] [CrossRef]
- Lu, W.; Zhang, Y.; Zhu, H.; Zhang, T.; Zhang, L.; Gao, N.; Chang, D.; Yin, J.; Zhou, X.; Li, M.; et al. Evaluation and comparison of the diagnostic performance of routine blood tests in predicting liver fibrosis in chronic hepatitis B infection. Br. J. Biomed. Sci. 2019, 76, 137–142. [Google Scholar] [CrossRef]
- Alsebaey, A.; Elhelbawy, M.; Waked, I. Platelets-to-lymphocyte ratio is a good predictor of liver fibrosis and insulin resistance in hepatitis C virus-related liver disease. Eur. J. Gastroenterol. Hepatol. 2018, 30, 207–211. [Google Scholar] [CrossRef]
- Hanberg, J.S.; Freiberg, M.S.; Goetz, M.B.; Rodriguez-Barradas, M.C.; Gibert, C.; Oursler, K.A.; Justice, A.C.; Tate, J.P. VACS Project Team Neutrophil-to-Lymphocyte and Platelet-to-Lymphocyte Ratios as Prognostic Inflammatory Biomarkers in Human Immunodeficiency Virus (HIV), Hepatitis C Virus (HCV), and HIV/HCV Coinfection. Open Forum Infect. Dis. 2019, 6, ofz347. [Google Scholar]
- Wang, D.; Bai, N.; Hu, X.; Ouyang, X.W.; Yao, L.; Tao, Y.; Wang, Z. Preoperative inflammatory markers of NLR and PLR as indicators of poor prognosis in resectable HCC. PeerJ 2019, 7, e7132. [Google Scholar] [CrossRef] [PubMed]
- Ismael, M.N.; Forde, J.; Milla, E.; Khan, W.; Cabrera, R. Utility of Inflammatory Markers in Predicting Hepatocellular Carcinoma Survival after Liver Transplantation. BioMed Res. Int. 2019, 2019, 7284040. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, G.; Garcia-Tsao, G.; Pagliaro, L. Natural history and prognostic indicators of survival in cirrhosis: A systematic review of 118 studies. J. Hepatol. 2006, 44, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Banas, C.; Sargeant, C.; Luketic, V.A.; Sterling, R.K.; Stravitz, R.T.; Shiffman, M.L.; Heuman, D.; Coterrell, A.; Fisher, R.A.; et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology 2006, 43, 682–689. [Google Scholar] [CrossRef]
- Michalak, A.; Cichoż-Lach, H.; Guz, M.; Kozicka, J.; Cybulski, M.; Jeleniewicz, W.; Stepulak, A. Towards an evaluation of alcoholic liver cirrhosis and nonalcoholic fatty liver disease patients with hematological scales. World J. Gastroenterol. 2020, 26, 7538–7549. [Google Scholar] [CrossRef]
- Pomacu, M.M.; Trașcă, M.D.; Pădureanu, V.; Bugă, A.M.; Andrei, A.M.; Stănciulescu, E.C.; Baniță, I.M.; Rădulescu, D.; Pisoschi, C.G. Interrelation of inflammation and oxidative stress in liver cirrhosis. Exp. Ther. Med. 2021, 21, 602. [Google Scholar] [CrossRef]
- Cananzi, F.C.M.; Minerva, E.M.; Samà, L.; Ruspi, L.; Sicoli, F.; Conti, L.; Fumagalli Romario, U.; Quagliuolo, V.L. Preoperative monocyte-to-lymphocyte ratio predicts recurrence in gastrointestinal stromal tumors. J. Surg. Oncol. 2019, 119, 12–20. [Google Scholar] [CrossRef]
- Kadiyoran, C.; Zengin, O.; Cizmecioglu, H.A.; Tufan, A.; Kucuksahin, O.; Cure, M.C.; Cure, E.; Kucuk, A.; Ozturk, M.A. Monocyte to Lymphocyte Ratio, Neutrophil to Lymphocyte Ratio, and Red Cell Distribution Width are the Associates with Gouty Arthritis. Acta Med. 2019, 62, 99–104. [Google Scholar] [CrossRef]
- Aktas, G.; Duman, T.T.; Kurtkulagi, D.O.; Atak Tel, B.M.; Bilgin, S.; Kahveci, G.; Oku, A.; Kosekli, M.A. Liver steatosis is associated both with platelet distribution width, neutrophil lymphocyte and monocyte lymphocyte ratios. Prim. Health Care 2020, 10, 346. [Google Scholar]
- Vineeth, V.K.; Kellarai, A.; Prakash, P.S. Utility of Neutrophil to Lymphocyte Ratio as a Predictor of Complications in Patients with Liver Cirrhosis. J. Evol. Med. Dent. Sci. 2020, 9, 2197–2201. [Google Scholar] [CrossRef]
- Chalasani, N.; Gorski, J.C.; Asghar, M.S.; Asghar, A.; Foresman, B.; Hall, S.D.; Crabb, D.W. Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology 2003, 37, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Pessayre, D.; Fromenty, B.; Mansouri, A. Mitochondrial injury in steatohepatitis. Eur. J. Gastroenterol. Hepatol. 2004, 16, 1095–1105. [Google Scholar] [CrossRef]
- Crespo, J.; Fern, P.; Hern, M.; Mayorga, M.; Pons-Romero, F. Gene expression of tumor necrosis factor α and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology 2001, 34, 1158–1163. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology 2002, 123, 1705–1725. [Google Scholar] [CrossRef] [PubMed]
- Bertolani, C.; Marra, F. The role of adipokines in liver fibrosis. Pathophysiology 2008, 15, 91–101. [Google Scholar] [CrossRef]
- Lucchesi, A.N.; De Freitas, N.T.; Cassettari, L.L.; Marques, S.F.G.; Spadella, C.T. Diabetes mellitus triggers oxidative stress in the liver of alloxan-treated rats: A mechanism for diabetic chronic liver disease. Acta Cir. Bras. 2013, 28, 502–508. [Google Scholar] [CrossRef]
- Kikuchi, M.; Horie, Y.; Ebinuma, H.; Taniki, N.; Nakamoto, N.; Kanai, T. Alcoholic liver cirrhosis and signifcant riskfactorsfor the development of alcohol-related hepatocellular carcinoma–Japan, 2012. Nihon Arukoru Yakubutsu Igakkai Zasshi 2015, 50, 222–234. [Google Scholar]
- Volzke, H. Multicausality in fatty liver disease: Is there a rationale to distinguish between alcoholic and non-alcoholic origin? World J. Gastroenterol. 2012, 18, 3492–3501. [Google Scholar] [CrossRef]
| MELD score = 9.57 × ln(Cr) + 3.78 × ln(TBIL) + 11.2 × ln(INR) + 6.43 |
| ALBI = ((log10 bilirubin × 0.66) + (albumin × (−0.085))) |
| NLR = Neu/Ly |
| PLR = Plt/Ly |
| MoLR = Mo/Ly |
| GPR = GGT/Plt |
| NLA = NLR/albumin |
| Variables | Total Patients (n = 150) |
|---|---|
| Sex (m/f) | 116/34 |
| Age (years) | 58.83 ± 11.79 |
| Etiology | |
| Alcohol | 109 |
| Hepatitis B | 3 |
| Hepatitis C | 17 |
| Autoimmune disease | 18 |
| Wilson disease | 3 |
| Toxic | 0 |
| Cryptogenic DM | 20 39 |
| Laboratory test | |
| Hg (g/L) a | 96.17 ± 27.24 |
| WBC (109/L) a Lymphocytes Neutrophiles Eosinophiles Monocytes | 8.98 ± 5.28 1.24 6.81 0.12 0.7 |
| PLT (109/L) a | 115.58 ± 63.83 |
| TBIL (mmol/L) b | 103.25 |
| Alb (g/L) b | 29.81 |
| AST (U/L) b | 104.08 |
| ALT (U/L) b | 44.29 |
| ALP (U/L)b | 134.27 |
| GGT (U/L) b | 171.42 |
| BUN (mmol/L) b | 15.39 |
| Cr (µmol/L) b | 141.90 |
| INRb | 1.65 |
| NH4 (µmol/L) b | 72.34 |
| CRP (mg/L) b | 32.56 |
| Pct (ng/L) b | 1.76 |
| Na (mmol/L) b | 135.70 |
| LDH (U/L) b | 554.04 |
| Cholesterol (mmol/L) | 2.97 |
| Triglycerides (mmol/L) Esophageal varices Mild Moderate Large | 1.13 117 (78.5%) 43 (28.7%) 43 (28.7%) 31 (20.7%) |
| MELD score b | 19.83 |
| ALBI score b | −1.40 |
| NLR b | 7.08 |
| NLA b | 25.71 |
| PLR b | 111.75 |
| MoLR b | 0.67 |
| LMR b | 2.13 |
| MPR b | 0.13 |
| GPR b | 2.16 |
| Variables | Survived pts | Non-Survived pts | p Value |
|---|---|---|---|
| Sex (m/f) | 75/25 | 36/7 | 0.924 |
| Variceal bleeding | 103 | 42 | 0.333 |
| MELD | 17.99 | 23.79 | 0.001 |
| NLA | 19.98 | 37.75 | 0.000 |
| NLR | 5.95 | 9.52 | 0.000 |
| PLR | 103.84 | 127.91 | 0.014 |
| MoLR | 0.642 | 0.758 | 0.192 |
| LMR | 2.21 | 1.97 | 0.171 |
| MPR | 0.130 | 0.121 | 0.213 |
| GPR | 2.08 | 2.46 | 0.984 |
| ALBI | −1.51 | −1.15 | 0.003 |
| Variables | Hepatic Encephalopathy | Ascites | Outcome | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes | No | p Value | Yes | No | p Value | Live | Dead | p Value | |
| MELD | 23.55 | 17.48 | 0.000 | 20.72 | 17.03 | 0.056 | 17.99 | 23.79 | 0.001 |
| NLA | 35.08 | 19.79 | 0.014 | 28.02 | 18.09 | 0.011 | 19.98 | 37.75 | 0.000 |
| NLR | 9.24 | 5.72 | 0.040 | 7.52 | 5.67 | 0.022 | 5.95 | 9.52 | 0.000 |
| PLR | 121.67 | 105.49 | 0.065 | 118.32 | 89.55 | 0.092 | 103.84 | 127.91 | 0.014 |
| MoLR | 0.79 | 0.60 | 0.760 | 0.73 | 0.52 | 0.025 | 0.642 | 0.758 | 0.192 |
| LMR | 2.02 | 2.20 | 0.173 | 1.94 | 2.71 | 0.003 | 2.21 | 1.97 | 0.171 |
| MPR | 0.13 | 0.13 | 0.175 | 0.12 | 0.14 | 0.000 | 0.130 | 0.121 | 0.213 |
| GPR | 2.56 | 1.90 | 0.931 | 2.08 | 2.46 | 0.026 | 2.08 | 2.46 | 0.984 |
| ALBI | −1.25 | −1.50 | 0.063 | −1.32 | −1.68 | 0.909 | −1.51 | −1.15 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glisic, T.; Popovic, D.D.; Lolic, I.; Toplicanin, A.; Jankovic, K.; Dragasevic, S.; Aleksic, M.; Stjepanovic, M.; Oluic, B.; Matovic Zaric, V.; et al. Hematological Indices Are Useful in Predicting Complications of Liver Cirrhosis. J. Clin. Med. 2023, 12, 4820. https://doi.org/10.3390/jcm12144820
Glisic T, Popovic DD, Lolic I, Toplicanin A, Jankovic K, Dragasevic S, Aleksic M, Stjepanovic M, Oluic B, Matovic Zaric V, et al. Hematological Indices Are Useful in Predicting Complications of Liver Cirrhosis. Journal of Clinical Medicine. 2023; 12(14):4820. https://doi.org/10.3390/jcm12144820
Chicago/Turabian StyleGlisic, Tijana, Dusan D. Popovic, Iva Lolic, Aleksandar Toplicanin, Katarina Jankovic, Sanja Dragasevic, Marko Aleksic, Mihailo Stjepanovic, Branislav Oluic, Vera Matovic Zaric, and et al. 2023. "Hematological Indices Are Useful in Predicting Complications of Liver Cirrhosis" Journal of Clinical Medicine 12, no. 14: 4820. https://doi.org/10.3390/jcm12144820
APA StyleGlisic, T., Popovic, D. D., Lolic, I., Toplicanin, A., Jankovic, K., Dragasevic, S., Aleksic, M., Stjepanovic, M., Oluic, B., Matovic Zaric, V., Radisavljevic, M. M., & Stojkovic Lalosevic, M. (2023). Hematological Indices Are Useful in Predicting Complications of Liver Cirrhosis. Journal of Clinical Medicine, 12(14), 4820. https://doi.org/10.3390/jcm12144820





