Peri-Procedural Management of Direct-Acting Oral Anticoagulants (DOACs) in Transcatheter Miniaturized Leadless Pacemaker Implantation
Abstract
1. Introduction
- To assess the effects of a standardized DOAC management approach, consisting in withholding one dose prior to the procedure and reinitiation 6–24 h, on peri-procedural adverse events;
- To identify clinical characteristics associated with this DOAC management strategy.
2. Materials and Methods
2.1. Definition of Peri-Procedural Anticoagulation Regimens and General Patient Management
- -
- Group 1A included patients treated with the standardized approach, which consisted of DOAC interruption of 1 dose prior to the procedure, irrespective of the half-life of the drug. The therapy was then reinitiated 6–24 h after the procedure;
- -
- Group 1B gathered all other peri-procedural DOAC regimens, including:
- Interrupted and delayed reinitiation: DOAC interruption before the procedure of at least 2 consecutive doses for either dabigatran/apixaban or for rivaroxaban/edoxaban. DOAC anticoagulation therapy was then reinitiated >24 h after the procedure;
- Uninterrupted: no peri-procedural DOAC interruption was performed;
- Interrupted with “Bridging”: in patients treated with DOACs, the oral anticoagulant was interrupted before the procedure for at least 2 consecutive doses when treated with dabigatran/apixaban or 1 dose for rivaroxaban/edoxaban, and either fractionated or unfractionated heparin was administered peri-procedurally.
- -
- Interrupted with “Bridging”: in patients under chronic VKA, this approach consisted in suspending VKA at least 72 h before, performing TPS positioning with INR < 1.5, and either fractionated or unfractionated heparin was administered peri-procedurally;
- -
- Interrupted without “Bridging”: in patients under chronic VKA, this approach consisted in suspending VKA at least 72 h before, performing TPS positioning with INR < 1.5, and reinitiating VKA after the procedure without resorting to “bridging” with either fractionated or unfractionated heparin;
- -
- Uninterrupted: TPS positioning was performed without discontinuing VKA with INR level < 3 [12].
2.2. Leadless Pacemaker Implantation
2.3. Study Endpoints and Classification of Adverse Events
2.4. Statistics
3. Results
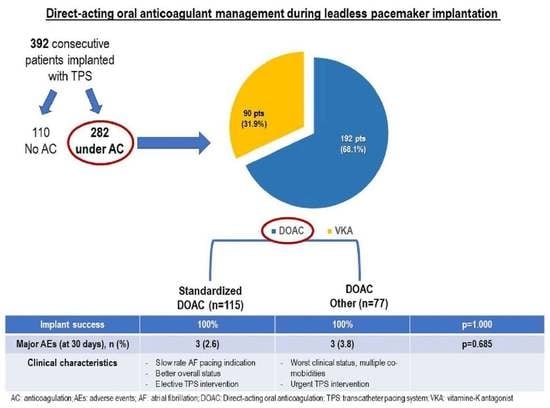
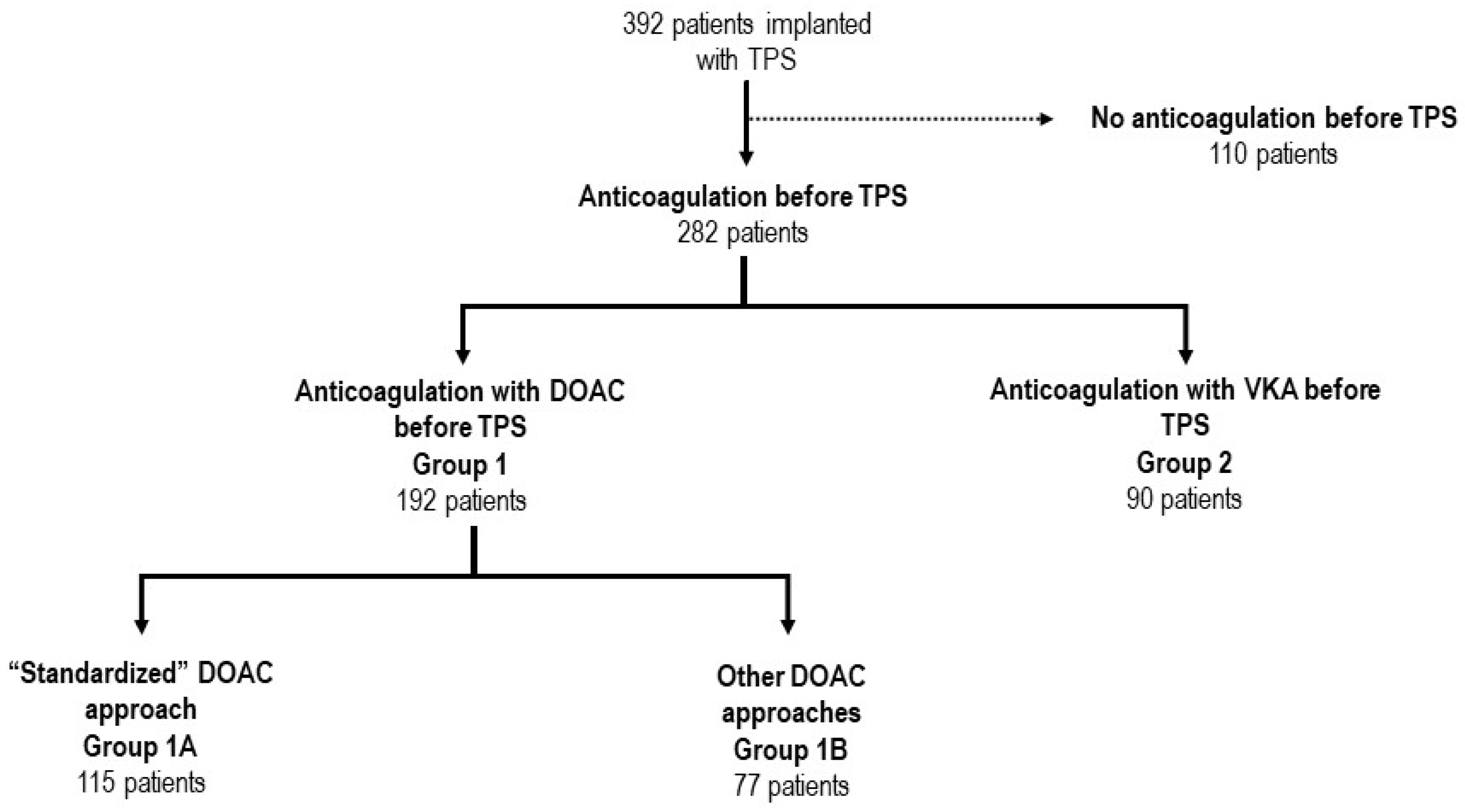
3.1. Patient Characteristics and Management of Anticoagulation during TPS
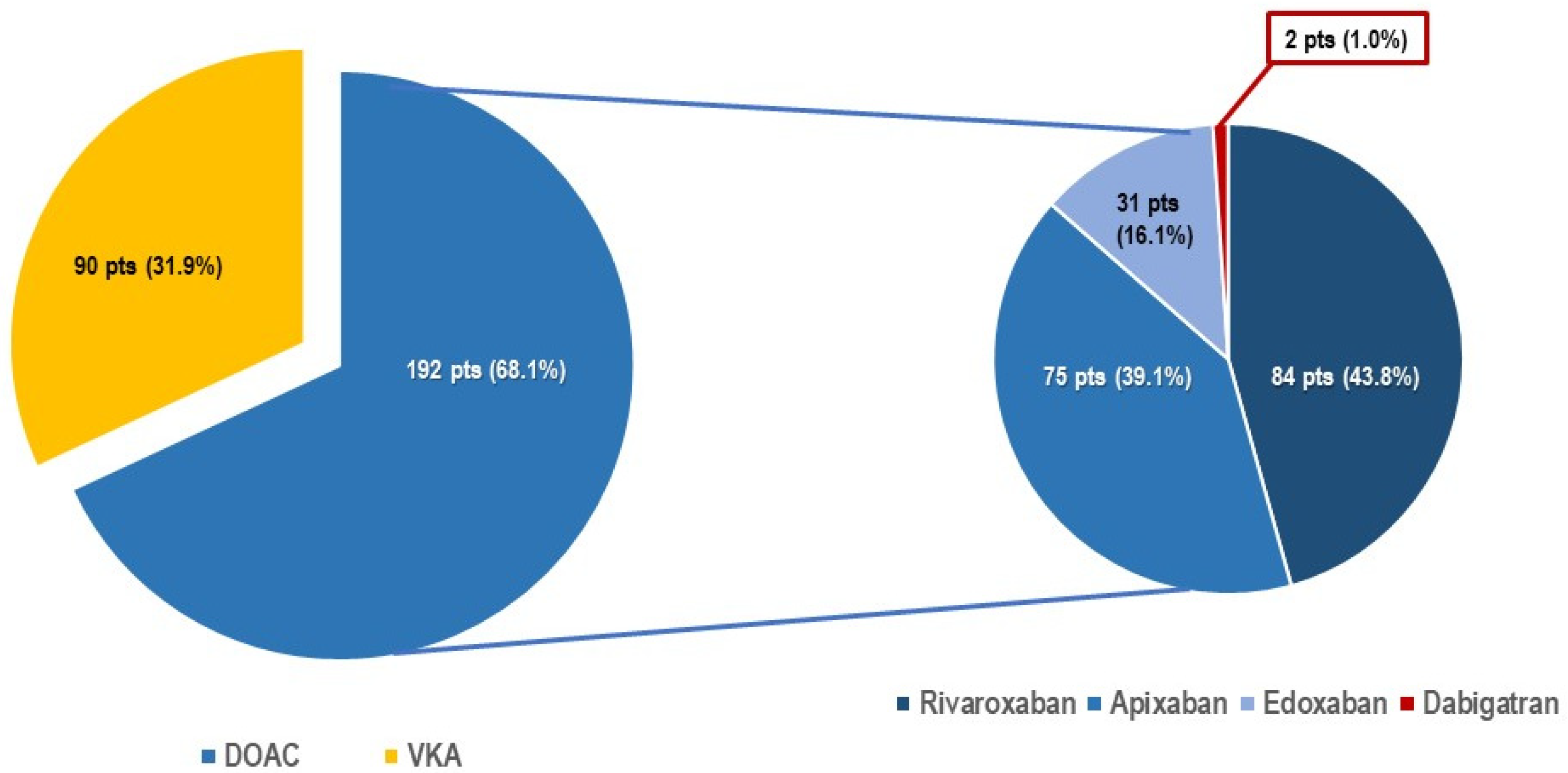
3.2. Differing Peri-Procedural DOAC Regimens during TPS Procedure
3.3. Differing Peri-Procedural DOAC Regimens and Clinical Outcomes
4. Discussion
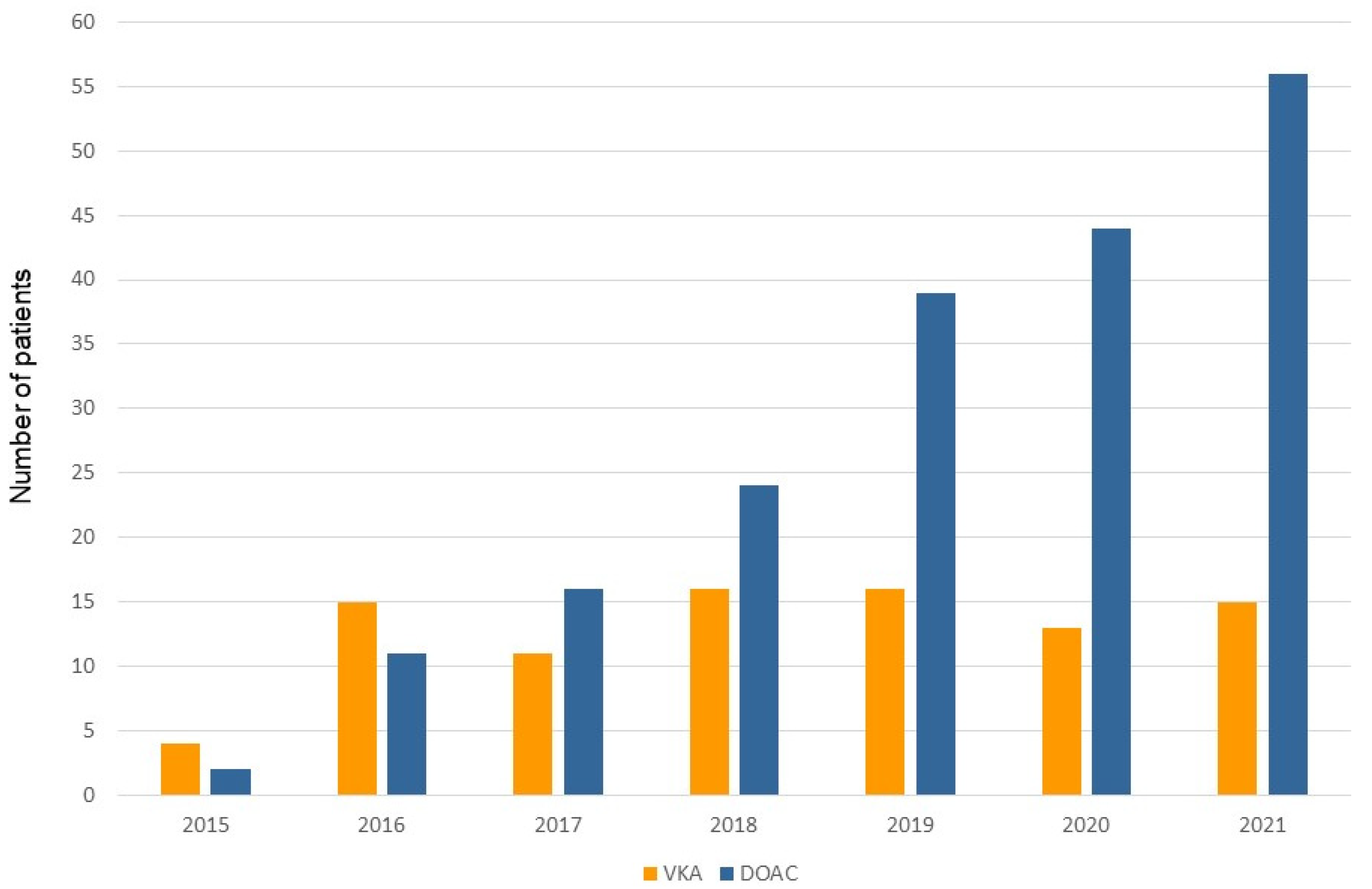
4.1. The Growing Importance of DOAC during TPS Procedure
4.2. Peri-Procedural Management of DOAC during TPS Procedure: Which Is the Best Approach?
4.3. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Regoli, F.D. Reducing CIED-Related Morbidity: “LESS Is More”. J. Clin. Med. 2022, 11, 4782. [Google Scholar] [CrossRef]
- El-Chami, M.F.; Al-Samadi, F.; Clementy, N.; Garweg, C.; Martinez-Sande, J.L.; Piccini, J.P.; Iacopino, S.; Lloyd, M.; Prat, X.V.; Jacobsen, M.D.; et al. Updated performance of the Micra transcatheter pacemaker in the real-world setting: A comparison to the investigational study and a transvenous historical control. Heart Rhythm 2018, 15, 1800–1807. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Exner, D.V.; Cantillon, D.J.; Doshi, R.; Bunch, T.J.; Tomassoni, G.F.; Friedman, P.A.; Estes, N.M.; Ip, J.; Niazi, I.; et al. Percutaneous Implantation of an Entirely Intracardiac Leadless Pacemaker. N. Engl. J. Med. 2015, 373, 1125–1135. [Google Scholar] [CrossRef]
- Boveda, S.; Higuera, L.; Longacre, C.; Wolff, C.; Wherry, K.; Stromberg, K.; El-Chami, M.F. Two-year outcomes of leadless vs. transvenous single-chamber ventricular pacemaker in high-risk subgroups. Europace 2023, 25, 1041–1050. [Google Scholar] [CrossRef]
- Piccini, J.P.; Cunnane, R.; Steffel, J.; El-Chami, M.F.; Reynolds, D.; Roberts, P.R.; Soejima, K.; Steinwender, C.; Garweg, C.; Chinitz, L.; et al. Development and validation of a risk score for predicting pericardial effusion in patients undergoing leadless pacemaker implantation: Experience with the Micra transcatheter pacemaker. Europace 2022, 24, 1119–1126. [Google Scholar] [CrossRef]
- Valiton, V.; Graf, D.; Pruvot, E.; Carroz, P.; Fromer, M.; Bisch, L.; Tran, V.N.; Cook, S.; Scharf, C.; Burri, H. Leadless pacing using the transcatheter pacing system (Micra TPS) in the real world: Initial Swiss experience from the Romandie region. Europace 2019, 21, 275–280. [Google Scholar] [CrossRef]
- Kiani, S.; Black, G.B.; Rao, B.; Thakkar, N.; Massad, C.; Patel, A.; Merchant, F.M.; Hoskins, M.H.; De Lurgio, D.B.; Patel, A.M.; et al. Outcomes of Micra leadless pacemaker implantation with uninterrupted anticoagulation. J. Cardiovasc. Electrophysiol. 2019, 30, 1313–1318. [Google Scholar] [CrossRef]
- Antonio, R.S.; Chipa-Ccasani, F.; Apolo, J.; Linhart, M.; Trotta, O.; Pujol-López, M.; Niebla, M.; Alarcón, F.; Trucco, E.; Arbelo, E.; et al. Management of anticoagulation in patients undergoing leadless pacemaker implantation. Heart Rhythm 2019, 16, 1849–1854. [Google Scholar] [CrossRef]
- El-Chami, M.F.; Garweg, C.; Iacopino, S.; Al-Samadi, F.; Martinez-Sande, J.L.; Tondo, C.; Johansen, J.B.; Prat, X.V.; Piccini, J.P.; Cha, Y.M.; et al. Leadless pacemaker implant, anticoagulation status, and outcomes: Results from the Micra Transcatheter Pacing System Post-Approval Registry. Heart Rhythm 2022, 19, 228–234. [Google Scholar] [CrossRef]
- Sticherling, C.; Marin, F.; Birnie, D.; Boriani, G.; Calkins, H.; Dan, G.-A.; Gulizia, M.; Halvorsen, S.; Hindricks, G.; Kuck, K.-H.; et al. Antithrombotic management in patients undergoing electrophysiological procedures: A European Heart Rhythm Association (EHRA) position document endorsed by the ESC Working Group Thrombosis, Heart Rhythm Society (HRS), and Asia Pacific Heart Rhythm Society (APHRS). Europace 2015, 17, 1197–1214. [Google Scholar] [CrossRef]
- Steffel, J.; Verhamme, P.; Potpara, T.S.; Albaladejo, P.; Antz, M.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur. Heart J. 2018, 39, 1330–1393. [Google Scholar] [CrossRef]
- Birnie, D.H.; Healey, J.S.; Wells, G.A.; Verma, A.; Tang, A.S.; Krahn, A.D.; Simpson, C.S.; Ayala-Paredes, F.; Coutu, B.; Leiria, T.L.; et al. Pacemaker or Defibrillator Surgery without Interruption of Anticoagulation. N. Engl. J. Med. 2013, 368, 2084–2093. [Google Scholar] [CrossRef]
- Regoli, F.; Araco, M.; Moccetti, T.; Caputo, M.L.; Conte, G.; Auricchio, A.; Moccetti, M. Potential Clinical Utility and Feasibility of Combined Left Atrial Appendage Closure and Positioning of Miniaturized Pacemaker Through a Single Right Femoral Vein Access. Am. J. Cardiol. 2017, 120, 236–242. [Google Scholar] [CrossRef]
- Ritter, P.; Duray, G.Z.; Steinwender, C.; Soejima, K.; Omar, R.; Mont, L.; Boersma, L.V.; Knops, R.E.; Chinitz, L.; Zhang, S.; et al. Early performance of a miniaturized leadless cardiac pacemaker: The Micra Transcatheter Pacing Study. Eur. Heart J. 2015, 36, 2510–2519. [Google Scholar] [CrossRef]
- Regoli, F.; Roberto, M.; Grazioli-Gauthier, L.; Cioffi, G.; Pasotti, E.; Caputo, M.L.; Conte, G.; Breitenstein, A.; Moccetti, T. Feasibility and clinical efficacy of double suture-mediated closure device technique for hemostasis during positioning of miniaturized wireless pacemaker. J. Interv. Card. Electrophysiol. 2022, 64, 129–135. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.E.S.C.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.E.; Dan, G.A.; Dilaveris, P.E.; et al. ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar]
- Fox, K.A.; Piccini, J.P.; Wojdyla, D.; Becker, R.C.; Halperin, J.L.; Nessel, C.C.; Paolini, J.F.; Hankey, G.J.; Mahaffey, K.W.; Patel, M.R.; et al. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur. Heart J. 2011, 32, 2387–2394. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Birnie, D.H.; Healey, J.S.; Wells, G.A.; Ayala-Paredes, F.; Coutu, B.; Sumner, G.L.; Becker, G.; Verma, A.; Philippon, F.; Kalfon, E.; et al. Continued vs. interrupted direct oral anticoagulants at the time of device surgery, in patients with moderate to high risk of arterial thrombo-embolic events (BRUISE CONTROL-2). Eur. Heart J. 2018, 39, 3973–3979. [Google Scholar] [CrossRef]
- Kim, K.; Won, S.; Kim, J.; Lee, E.; Kim, K.; Park, S. Meta-analysis of complication as a risk factor for early ambulation after percutaneous coronary intervention. Eur. J. Cardiovasc. Nurs. 2013, 12, 429–436. [Google Scholar] [CrossRef]
- Busca, E.; Airoldi, C.; Bertoncini, F.; Buratti, G.; Casarotto, R.; Gaboardi, S.; Faggiano, F.; Barisone, M.; White, I.R.; Allara, E.; et al. Bed rest duration and complications after transfemoral cardiac catheterization: A network meta-analysis. Eur. J. Cardiovasc. Nurs. 2022; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | All (n = 392) | DOAC (Group 1, n = 192) | VKA (Group 2, n = 90) | p Value |
|---|---|---|---|---|
| Demographic, clinical | ||||
| Age, years | 81.4 ± 7.3 | 81.2 ± 7.2 | 81.7 ± 7.4 | 0.928 |
| Male | 260 (66.3) | 118 (60.9) | 63 (70.0) | 0.184 |
| Structural heart disease | 279 (71.2) | 112 (58.3) | 88 (97.7) | <0.001 |
| Ischemic | 145 (37.0) | 58 (30.2) | 39 (43.3) | |
| Valvular | 111 (28.3) | 41 (21.3) | 43 (47.8) | |
| Other | 23 (6.0) | 13 (6.8) | 6 (6.7) | |
| Hypertension | 334 (85.2) | 173 (90.2) | 84 (93.6) | 0.501 |
| Diabetes mellitus | 77 (19.6) | 31 (16.0) | 31 (21.9) | 0.001 |
| Renal impairment (≥1.5 mg/dL) | 208 (53.1) | 82 (42.9) | 49 (54.6) | 0.074 |
| Dialysis | 34 (8.7) | 0 | 7 (7.8) | <0.001 |
| Chronic obstructive lung disease | 60 (15.3) | 29 (15.1) | 9 (10.0) | 0.268 |
| Peripheral artery disease | 57 (14.5) | 21 (10.7) | 10 (11.1) | 1.000 |
| Previous stroke | 53 (13.5) | 25 (13.2) | 13 (14.4) | 1.000 |
| Tumoral disease | 60 (15.3) | 29 (15.2) | 13 (14.1) | 1.000 |
| Other comorbidities | 8 (2.0) | 6 (3.1) | 1 (1.1) | 0.437 |
| Left ventricular ejection fraction (%) | 55.5 ± 9.6 | 55.7 ± 8.9 | 55.1 ± 9.7 | 0.609 |
| Planned hospitalization for implantation | 156 (39.8) | 83 (43.5) | 30 (33.3) | 0.153 |
| Pacemaker indication | ||||
| Slow rate atrial fibrillation | 158 (40.3) | 84 (43.5) | 42 (46.9) | 0.700 |
| Brady-tachycardia atrial fibrillation | 71 (18.1) | 42 (21.7) | 17 (18.8) | 0.639 |
| AV block and permanent atrial fibrillation | 49 (12.5) | 27 (14.1) | 11 (12.5) | 0.713 |
| AV block and underlying sinus rhythm | 49 (12.5) | 10 (5.4) | 7 (7.8) | 0.426 |
| Sick sinus syndrome | 20 (5.1) | 17 (8.7) | 0 | 0.002 |
| Other | 44 (11.2) | 12 (6.5) | 13 (14.1) | 0.041 |
Oral anticoagulation therapy Vitamin K antagonist Directing-acting anticoagulant No oral anticoagulation therapy Atrial appendage occlusion Pre-existing Combined strategy | 282 (71.9) 90 (22.9) 192 (49.0) 110 (28.1) 12 (7.2) 4 (2.4) 4 (4.8) * | 192 / 192 / 2 (1.1) | 90 90 / 10 (10.9) | <0.001 |
| Complication | Standardized DOAC (Group 1A, n = 115) | Other DOAC Regimens (Group 1B, n = 77) | p Value |
|---|---|---|---|
| Demographic, clinical | |||
| Age, years | 80.5 ± 7.2 | 81.4 ± 6.6 | 0.381 |
| Male | 72 (62.6) | 51 (66.2) | 0.647 |
| Structural heart disease | 49 (42.6) | 56 (72.7) | <0.001 |
| Ischemic | 36 (31.3) | 23 (29.9) | |
| Valvular | 10 (8.9) | 21 (27.2) | |
| Other | 2 (1.7) | 12 (15.6) | |
| Hypertension | 98 (85.2) | 74 (96.6) | 0.016 |
| Diabetes mellitus | 20 (17.4) | 13 (16.9) | 1.000 |
| Renal impairment | 38 (33.0) | 51 (66.2) | <0.001 |
| Dialysis | 0 | 0 | 1.000 |
| COPD | 28 (24.3) | 7 (9.1) | 0.008 |
| Peripheral artery disease | 5 (4.3) | 16 (20.8) | <0.001 |
| Previous stroke | 13 (11.3) | 11 (14.3) | 0.657 |
| Tumoral disease | 13 (11.3) | 13 (16.0) | 0.288 |
| Other comorbidities | 2 (1.7) | 2 (2.6) | 1.000 |
| Left ventricular ejection fraction (%) | 55.1 ± 9.3 | 55.1 ± 9.3 | 0.819 |
| Planned hospitalization for implantation | 67 (57.4) | 18 (23.4) | <0.001 |
| Pacemaker indication | |||
| Slow rate AF | 64 (55.7) | 25 (32.5) | 0.002 |
| Atrial brady-tachi syndrome | 28 (24.3) | 15 (19.5) | 0.482 |
| Atrioventricular block and AF | 8 (7.0) | 16 (20.8) | 0.007 |
| Atrioventricular block and sinus rhythm | 2 (1.7) | 7 (9.1) | 0.031 |
| Sick sinus syndrome | 5 (4.3) | 10 (13.0) | 0.051 |
| Other | 8 (7.0) | 5 (6.5) | 1.000 |
| Anticoagulation therapy | |||
| Dabigatran | 2 (1.7) | 0 | 0.517 |
| Rivaroxaban | 63 (54.7) | 21 (27.2) | <0.001 |
| Apixaban | 36 (31.3) | 39 (50.6) | 0.010 |
| Edoxaban | 14 (12.2) | 17 (22.1) | 0.075 |
| Atrial appendage occlusion | 0 | 1 (1.2) | 1.000 |
| Standardized DOAC (Gp 1A, n= 115) | Other DOAC Regimens (Gp 1B, n = 77) | p Value | |
|---|---|---|---|
| Procedure duration, min | 45.1 ± 14.0 | 56.2 ± 27.6 | <0.001 |
| Fluoroscopy time, min | 7.0 ± 6.1 | 9.2 ± 7.5 | 0.027 |
| Implant success rate | 115 (100) | 77 (100) | 1.000 |
| DOAC management | |||
| DOAC stopped (hours) | 21.4 ± 5.2 | 27.0 ± 27.1 | 0.032 |
| DOAC reinitiation (hours) | 14.8 ± 9.3 | 35.7 ± 33.4 | <0.001 |
| Complications | |||
| Major | |||
| Intraprocedural bleeding | 3 (2.6) | 3 (3.8) | 0.685 |
| Pericardial effusion | 1 | 2 | |
| Major femoral access bleeding | 2 | 1 | |
| Minor | |||
| Puncture site hematoma (<6 cm) | 6 (5.2) | 3 (3.9) | 0.743 |
Length of hospital stay, days (IQR) | 3.0 (2.0:3.8) | 4.0 (3.0:12.5) | <0.019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regoli, F.D.; Saguner, A.M.; Auricchio, A.; Demarchi, A.; Pasotti, E.; Conte, G.; Caputo, M.L.; Özkartal, T.; Breitenstein, A. Peri-Procedural Management of Direct-Acting Oral Anticoagulants (DOACs) in Transcatheter Miniaturized Leadless Pacemaker Implantation. J. Clin. Med. 2023, 12, 4814. https://doi.org/10.3390/jcm12144814
Regoli FD, Saguner AM, Auricchio A, Demarchi A, Pasotti E, Conte G, Caputo ML, Özkartal T, Breitenstein A. Peri-Procedural Management of Direct-Acting Oral Anticoagulants (DOACs) in Transcatheter Miniaturized Leadless Pacemaker Implantation. Journal of Clinical Medicine. 2023; 12(14):4814. https://doi.org/10.3390/jcm12144814
Chicago/Turabian StyleRegoli, François Diederik, Ardan M. Saguner, Angelo Auricchio, Andrea Demarchi, Elena Pasotti, Giulio Conte, Maria Luce Caputo, Tardu Özkartal, and Alexander Breitenstein. 2023. "Peri-Procedural Management of Direct-Acting Oral Anticoagulants (DOACs) in Transcatheter Miniaturized Leadless Pacemaker Implantation" Journal of Clinical Medicine 12, no. 14: 4814. https://doi.org/10.3390/jcm12144814
APA StyleRegoli, F. D., Saguner, A. M., Auricchio, A., Demarchi, A., Pasotti, E., Conte, G., Caputo, M. L., Özkartal, T., & Breitenstein, A. (2023). Peri-Procedural Management of Direct-Acting Oral Anticoagulants (DOACs) in Transcatheter Miniaturized Leadless Pacemaker Implantation. Journal of Clinical Medicine, 12(14), 4814. https://doi.org/10.3390/jcm12144814