The Effect of Diabetes Control Status on CT Findings in Pulmonary Tuberculosis: Emphasis on Bronchial Erosive Changes
Abstract
1. Introduction
2. Methods
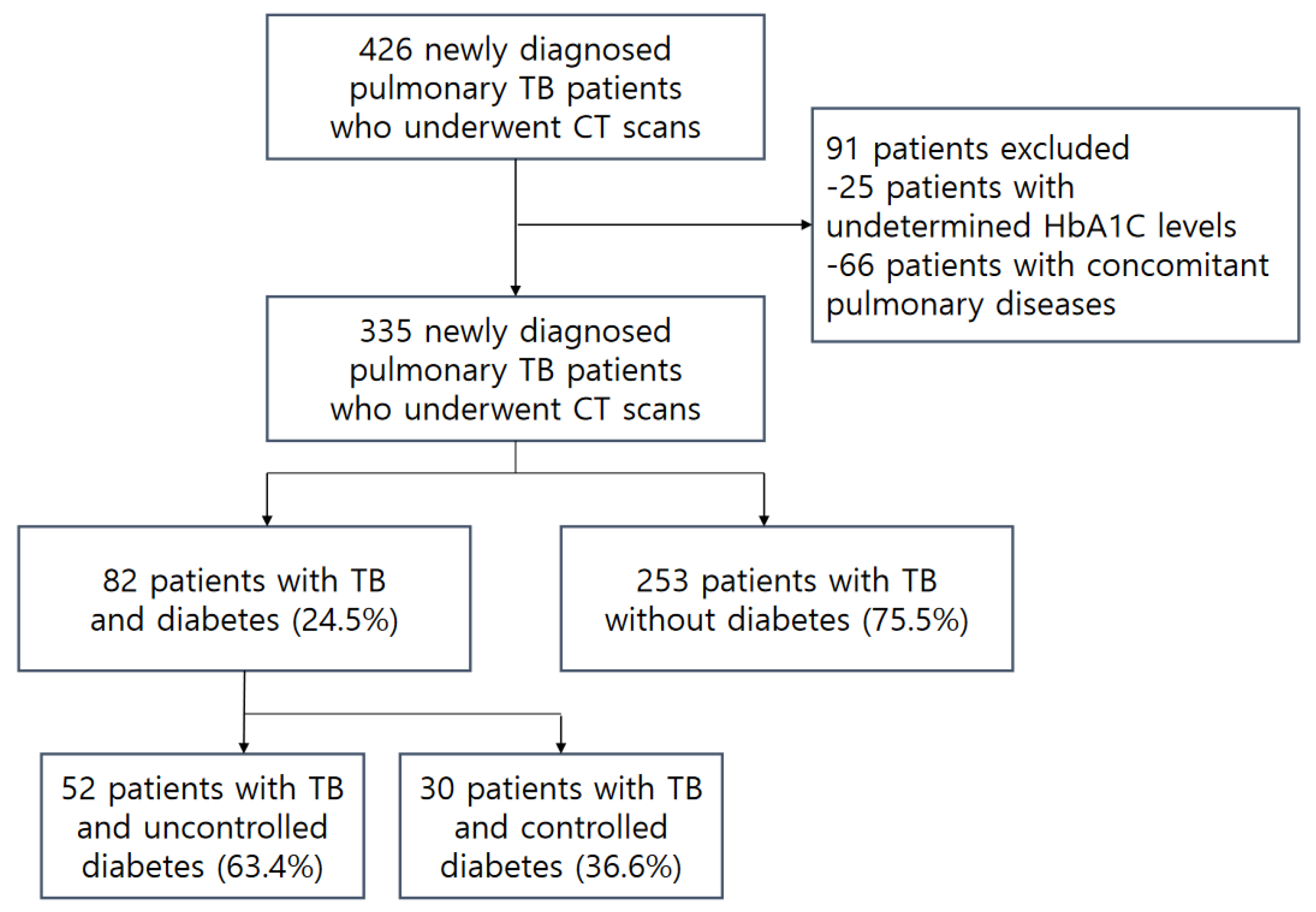
2.1. Study Design and Patients
2.2. Chest CT Protocol
2.3. Chest CT Interpretation
2.4. Statistical Analysis
2.5. Ethics Statement
3. Results
3.1. Patient Characteristics
3.2. Clinical Features and Microbiology Results
3.3. Chest CT Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ikezoe, J.; Takeuchi, N.; Johkoh, T.; Kohno, N.; Tomiyama, N.; Kozuka, T.; Noma, K.; Ueda, E. CT appearance of pulmonary tuberculosis in diabetic and immunocompromised patients: Comparison with patients who had no underlying disease. AJR Am. J. Roentgenol. 1992, 159, 1175–1179. [Google Scholar] [CrossRef]
- Umut, S.; Tosun, G.A.; Yildirim, N. Radiographic location of pulmonary tuberculosis in diabetic patients. Chest 1994, 106, 326. [Google Scholar] [CrossRef]
- Pérez-Guzman, C.; Torres-Cruz, A.; Villarreal-Velarde, H.; Salazar-Lezama, M.A.; Vargas, M.H. Atypical radiological images of pulmonary tuberculosis in 192 diabetic patients: A comparative study. Int. J. Tuberc. Lung Dis. 2001, 5, 455–461. [Google Scholar]
- Perez-Guzman, C.; Torres-Cruz, A.; Villarreal-Velarde, H.; Vargas, M.H. Progressive age-related changes in pulmonary tuberculosis images and the effect of diabetes. Am. J. Respir. Crit. Care Med. 2000, 162, 1738–1740. [Google Scholar] [CrossRef]
- Wang, C.S.; Yang, C.J.; Chen, H.C.; Chuang, S.H.; Chong, I.W.; Hwang, J.J.; Huang, M.S. Impact of type 2 diabetes on manifestations and treatment outcome of pulmonary tuberculosis. Epidemiol. Infect. 2009, 137, 203–210. [Google Scholar] [CrossRef]
- Wang, J.Y.; Lee, L.N.; Hsueh, P.R. Factors changing the manifestation of pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 2005, 9, 777–783. [Google Scholar]
- Chiang, C.Y.; Lee, J.J.; Chien, S.T.; Enarson, D.A.; Chang, Y.C.; Chen, Y.T.; Hu, T.Y.; Lin, C.B.; Suk, C.W.; Tao, J.M.; et al. Glycemic control and radiographic manifestations of tuberculosis in diabetic patients. PLoS ONE 2014, 9, e93397. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.K.; Wang, H.H.; Lai, Y.C.; Chang, S.C. The impact of glycemic status on radiological manifestations of pulmonary tuberculosis in diabetic patients. PLoS ONE 2017, 12, e0179750. [Google Scholar] [CrossRef]
- Morris, J.T.; Seaworth, B.J.; McAllister, C.K. Pulmonary tuberculosis in diabetics. Chest 1992, 102, 539–541. [Google Scholar] [CrossRef] [PubMed]
- Bacakoğlu, F.; Başoğlu, O.K.; Cok, G.; Sayiner, A.; Ateş, M. Pulmonary tuberculosis in patients with diabetes mellitus. Respiration 2001, 68, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Al-Tawfiq, J.A.; Saadeh, B.M. Radiographic manifestations of culture-positive pulmonary tuberculosis: Cavitary or non-cavitary? Int. J. Tuberc. Lung Dis. 2009, 13, 367–370. [Google Scholar] [PubMed]
- Weaver, R.A. Unusual radiographic presentation of pulmonary tuberculosis in diabetic patients. Am. Rev. Respir. Dis. 1974, 109, 162–163. [Google Scholar] [PubMed]
- Marais, R.M. Diabetes mellitus in black and coloured tuberculosis patients. S. Afr. Med. J. 1980, 57, 483–484. [Google Scholar] [PubMed]
- Kuaban, C.; Fotsin, J.G.; Koulla-Shiro, S.; Ekono, M.R.; Hagbe, P. Lower lung field tuberculosis in Yaounde, Cameroon. Cent. Afr. J. Med. 1996, 42, 62–65. [Google Scholar]
- Shaikh, M.A.; Singla, R.; Khan, N.B.; Sharif, N.S.; Saigh, M.O. Does diabetes alter the radiological presentation of pulmonary tuberculosis. Saudi Med. J. 2003, 24, 278–281. [Google Scholar]
- al-Wabel, A.H.; Teklu, B.; Mahfouz, A.A.; al-Ghamdi, A.S.; el-Amin, O.B.; Khan, A.S. Symptomatology and chest roentgenographic changes of pulmonary tuberculosis among diabetics. East Afr. Med. J. 1997, 74, 62–64. [Google Scholar]
- Park, S.W.; Shin, J.W.; Kim, J.Y.; Park, I.W.; Choi, B.W.; Choi, J.C.; Kim, Y.S. The effect of diabetic control status on the clinical features of pulmonary tuberculosis. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1305–1310. [Google Scholar] [CrossRef]
- Zhan, S.; Juan, X.; Ren, T.; Wang, Y.; Fu, L.; Deng, G.; Zhang, P. Extensive Radiological Manifestation in Patients with Diabetes and Pulmonary Tuberculosis: A Cross-Sectional Study. Ther. Clin. Risk Manag. 2022, 18, 595–602. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, K.S.; Yoon, J.H.; Chung, M.P.; Kim, H.; Kwon, O.J.; Rhee, C.H.; Han, Y.C. Tuberculosis of the trachea and main bronchi: CT findings in 17 patients. AJR Am. J. Roentgenol. 1997, 168, 1051–1056. [Google Scholar] [CrossRef]
- Im, J.G.; Itoh, H.; Shim, Y.S.; Lee, J.H.; Ahn, J.; Han, M.C.; Noma, S. Pulmonary tuberculosis: CT findings--early active disease and sequential change with antituberculous therapy. Radiology 1993, 186, 653–660. [Google Scholar] [CrossRef]
- Ko, J.M.; Kim, K.J.; Park, S.H.; Park, H.J. Bronchiectasis in active tuberculosis. Acta Radiol. 2013, 54, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.H.; Han, J.; Park, H.J.; Kim, T.S.; Jung, A.Y.; Sung, D.W.; Lee, J.H.; Kim, J.E.; Han, D.H. Aneurysmal appearance of medium-sized bronchi: A peripheral manifestation of endobronchial tuberculosis. AJR Am. J. Roentgenol. 2009, 193, W95–W99. [Google Scholar] [CrossRef]
- American Diabetes Association. (6) Glycemic targets. Diabetes Care 2015, 38 (Suppl. S1), S33–S40. [Google Scholar] [CrossRef]
- Yeh, J.J.; Chen, S.C.; Teng, W.B.; Chou, C.H.; Hsieh, S.P.; Lee, T.L.; Wu, M.T. Identifying the most infectious lesions in pulmonary tuberculosis by high-resolution multi-detector computed tomography. Eur. Radiol. 2010, 20, 2135–2145. [Google Scholar] [CrossRef] [PubMed]
- Perrin, F.M.; Woodward, N.; Phillips, P.P.; McHugh, T.D.; Nunn, A.J.; Lipman, M.C.; Gillespie, S.H. Radiological cavitation, sputum mycobacterial load and treatment response in pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 2010, 14, 1596–1602. [Google Scholar] [PubMed]
- Kim, T.S.; Koh, W.J.; Han, J.; Chung, M.J.; Lee, J.H.; Lee, K.S.; Kwon, O.J. Hypothesis on the evolution of cavitary lesions in nontuberculous mycobacterial pulmonary infection: Thin-section CT and histopathologic correlation. AJR Am. J. Roentgenol. 2005, 184, 1247–1252. [Google Scholar] [CrossRef]
- Ren, Y.; Ren, H.; Tian, Q.; Li, X.; Liu, Y. The relationship between computed tomography appearance of pulmonary tuberculosis and blood glucose levels in 763 diabetes mellitus patients with pulmonary tuberculosis: A comparative study. Endocrine 2022, 76, 584–592. [Google Scholar] [CrossRef]
- Yoon, Y.S.; Jung, J.W.; Jeon, E.J.; Seo, H.; Ryu, Y.J.; Yim, J.J.; Kim, Y.H.; Lee, B.H.; Park, Y.B.; Lee, B.J.; et al. The effect of diabetes control status on treatment response in pulmonary tuberculosis: A prospective study. Thorax 2017, 72, 263–270. [Google Scholar] [CrossRef]
- Martens, G.W.; Arikan, M.C.; Lee, J.; Ren, F.; Greiner, D.; Kornfeld, H. Tuberculosis susceptibility of diabetic mice. Am. J. Respir. Cell Mol. Biol. 2007, 37, 518–524. [Google Scholar] [CrossRef]
- Faurholt-Jepsen, D.; Aabye, M.G.; Jensen, A.V.; Range, N.; Praygod, G.; Jeremiah, K.; Changalucha, J.; Faurholt-Jepsen, M.; Jensen, L.; Jensen, S.M.; et al. Diabetes is associated with lower tuberculosis antigen-specific interferon gamma release in Tanzanian tuberculosis patients and non-tuberculosis controls. Scand. J. Infect. Dis. 2014, 46, 384–391. [Google Scholar] [CrossRef]



| Total | Non-DM | Controlled DM | Uncontrolled DM | p-Value (1) | p-Value (2) | p-Value (3) | p-Value (4) | |
|---|---|---|---|---|---|---|---|---|
| n = 335 | n = 253 | n = 30 | n = 52 | |||||
| Age, years median (IQR) | 59 (47, 73) | 57 (41, 72) | 71 (63, 81) ** | 60 (51.5, 68) ** | <0.001 | <0.001 | 0.127 | <0.001 ‡ |
| Sex, male | 224 (67) | 158 (63) | 21 (70) | 45 (87) | 0.003 | 0.417 | 0.001 | 0.069 |
| Co-morbidity | ||||||||
| CRF | 9 (3) | 5 (2) | - | 4 (8) | 0.086 † | >0.999 † | 0.049 † | 0.291 † |
| Malignancy | 31 (9) | 28 (11) | 2 (7) | 1 (2) | 0.089 † | 0.753 † | 0.039 † | 0.551 † |
| HIV infection | 1 (0) | 1 (0) | - | - | >0.999 † | >0.999 † | >0.999 † | - |
| CVD | 7 (2) | 5 (2) | 2 (7) | - | 0.112 † | 0.163 † | 0.593 † | 0.131 † |
| COPD, asthma | 11 (3) | 8 (3) | 1 (3) | 2 (4) | 0.875 † | >0.999 † | 0.681 † | >0.999 † |
| Chronic alcoholics, LC | 29 (9) | 23 (9) | 3 (10) | 3 (6) | 0.749 † | 0.746 † | 0.590 † | 0.664 † |
| Sputum | ||||||||
| Positive microbiology | 178/263 (68) | 129/198 (65) | 12/22 (55) | 37/43 (86) | 0.011 | 0.325 | 0.007 | 0.005 |
| Positive TB-PCR test | 117/226 (52) | 77/166 (46) | 8/20 (40) | 32/40 (80) | <0.001 | 0.588 | <0.001 | 0.002 |
| Positive AFB smear | 96/256 (38) | 65/191 (34) | 5/22 (23) | 26/43 (61) | 0.002 | 0.285 | 0.001 | 0.004 |
| AFB smear grade, mean ± SD | 0.9 ± 1.3 | 0.8 ± 1.3 | 0.5 ± 1.1 | 1.5 ± 1.4 | 0.002 | 0.238 | 0.002 | 0.005 |
| Positive culture | 154/239 (64) | 113/182 (62) | 6/19 (32) | 35/38 (92) | <0.001 | 0.01 | <0.001 | <0.001 |
| Bronchial washing fluid | ||||||||
| Positive microbiology | 204/221 (92) | 142/158 (90) | 24/25 (96) | 38/38 (100) | 0.076 † | 0.475 † | 0.045 † | 0.397 † |
| Positive TB-PCR test | 169/219 (77) | 116/156 (74) | 19/25 (76) | 34/38 (90) | 0.136 | 0.861 | 0.046 | 0.176 † |
| Positive AFB smear | 76/218 (35) | 51/155 (33) | 5/25 (20) | 20/38 (53) | 0.019 | 0.196 | 0.024 | 0.001 |
| AFB smear grade, mean ± SD | 0.7 ± 1.1 | 0.7 ± 1.1 | 0.3 ± 0.7 | 1.2 ± 1.4 | 0.008 | 0.144 | 0.014 | 0.005 |
| Positive culture | 191/220 (87) | 136/157 (87) | 19/25 (76) | 36/38 (95) | 0.098 | 0.220 † | 0.261 † | 0.0498 † |
| Total | Non-DM | Controlled DM | Uncontrolled DM | p-Value (1) | p-Value (2) | p-Value (3) | p-Value (4) | |
|---|---|---|---|---|---|---|---|---|
| n = 335 | n = 253 | n = 30 | n = 52 | |||||
| Micronodule | ||||||||
| Centrilobular | 168 (50) | 125 (49) | 14 (47) | 29 (56) | 0.651 | 0.777 | 0.403 | 0.427 |
| Peribronchovascular | 261 (78) | 193 (76) | 26 (87) | 42 (81) | 0.373 | 0.199 | 0.484 | 0.494 |
| Septal | 171 (51) | 123 (49) | 17 (57) | 31 (60) | 0.286 | 0.404 | 0.149 | 0.794 |
| Subpleural | 113 (34) | 94 (37) | 6 (20) | 13 (25) | 0.06 | 0.063 | 0.094 | 0.605 |
| Miliary | 15 (5) | 8 (3) | 3 (10) | 4 (8) | 0.066 † | 0.099 † | 0.129 † | 0.703 † |
| Tree-in-bud | 134 (40) | 98 (39) | 12 (40) | 24 (46) | 0.610 † | 0.893 | 0.32 | 0.589 |
| Consolidation/macronodule | 294 (89) | 221 (87) | 25 (83) | 48 (92) | 0.452 | 0.566 † | 0.313 | 0.276 † |
| Cavitation | 157 (47) | 109 (43) | 7 (23) | 41 (79) | <0.001 | 0.038 | <0.001 | <0.001 |
| Ground glass opacity | 52 (16) | 35 (14) | 5 (17) | 12 (23) | 0.241 | 0.590 † | 0.093 | 0.49 |
| Bronchovascular bundle thickening | 249 (74) | 184 (73) | 21 (70) | 44 (85) | 0.172 | 0.752 | 0.072 | 0.116 |
| Interlobular septal thickening | 235 (70) | 176 (70) | 20 (67) | 39 (75) | 0.671 | 0.745 | 0.434 | 0.419 |
| Reverse halo sign | 9 (3) | 6 (2) | 1 (3) | 2 (4) | 0.587 † | 0.548 † | 0.628 † | >0.999 † |
| Galaxy/cluster sign | 24 (7) | 19 (8) | 2 (7) | 3 (6) | >0.999 † | >0.999 † | >0.999 † | >0.999 † |
| Endobronchial involvement | 64 (19) | 51 (20) | 6 (20) | 7 (14) | 0.53 | 0.984 | 0.262 | 0.534 † |
| Pleurisy | 108 (32) | 88 (35) | 6 (20) | 14 (27) | 0.176 | 0.104 | 0.274 | 0.482 |
| Lymphadenopathy | 110 (33) | 86 (34) | 11 (37) | 13 (25) | 0.406 | 0.77 | 0.207 | 0.263 |
| Extrathoracic involvement | 19 (6) | 16 (6) | 1 (3) | 2 (4) | 0.847 † | >0.999 † | 0.748 † | >0.999 † |
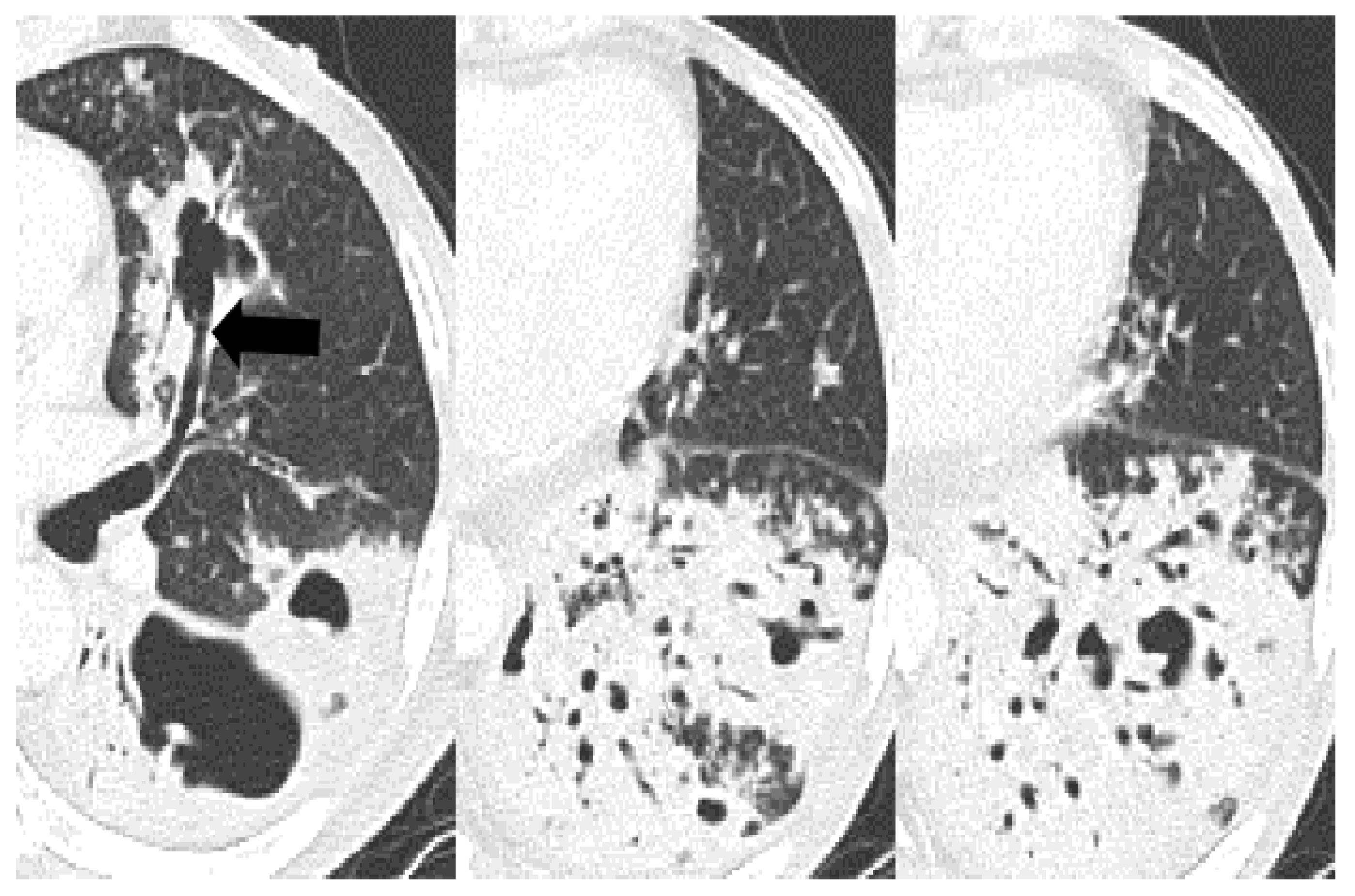
| Bronchial erosion | 157 (47) | 110 (44) | 9 (30) | 38 (73) | <0.001 | 0.157 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, M.K.; Lee, S.Y.; Ko, J.M.; Im, S.-A. The Effect of Diabetes Control Status on CT Findings in Pulmonary Tuberculosis: Emphasis on Bronchial Erosive Changes. J. Clin. Med. 2023, 12, 4725. https://doi.org/10.3390/jcm12144725
Jung MK, Lee SY, Ko JM, Im S-A. The Effect of Diabetes Control Status on CT Findings in Pulmonary Tuberculosis: Emphasis on Bronchial Erosive Changes. Journal of Clinical Medicine. 2023; 12(14):4725. https://doi.org/10.3390/jcm12144725
Chicago/Turabian StyleJung, Min Kyung, Sang Young Lee, Jeong Min Ko, and Soo-Ah Im. 2023. "The Effect of Diabetes Control Status on CT Findings in Pulmonary Tuberculosis: Emphasis on Bronchial Erosive Changes" Journal of Clinical Medicine 12, no. 14: 4725. https://doi.org/10.3390/jcm12144725
APA StyleJung, M. K., Lee, S. Y., Ko, J. M., & Im, S.-A. (2023). The Effect of Diabetes Control Status on CT Findings in Pulmonary Tuberculosis: Emphasis on Bronchial Erosive Changes. Journal of Clinical Medicine, 12(14), 4725. https://doi.org/10.3390/jcm12144725





