A Worldwide Analysis of Adipose-Derived Stem Cells and Stromal Vascular Fraction in Orthopedics: Current Evidence and Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Exclusion and Inclusion Criteria and Study Selection
2.3. Quality Assessment
2.4. Data Extraction, Collected Variables, Grouping, and Analysis
2.5. Statistical Analysis
3. Results
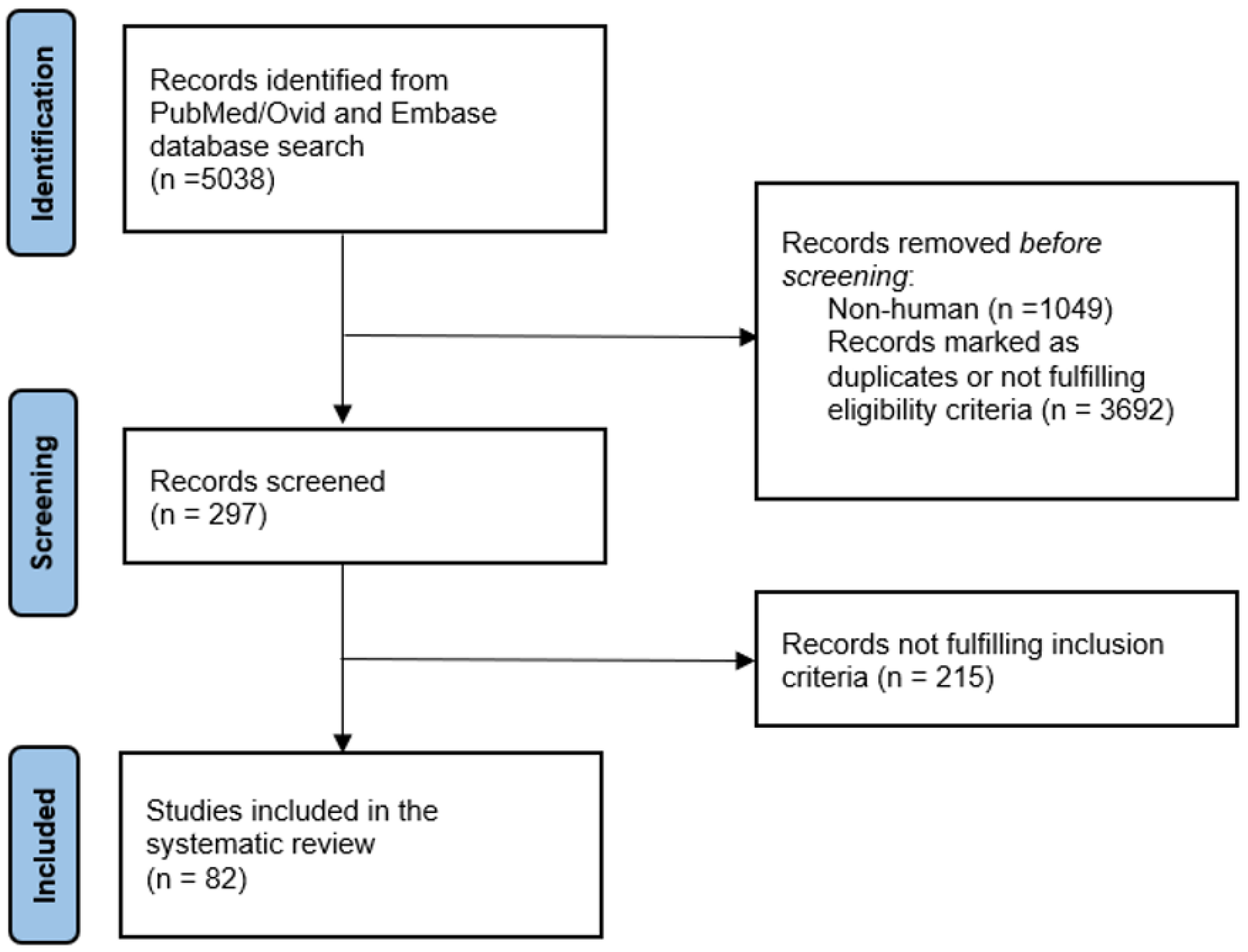
3.1. Review Process and Included Studies
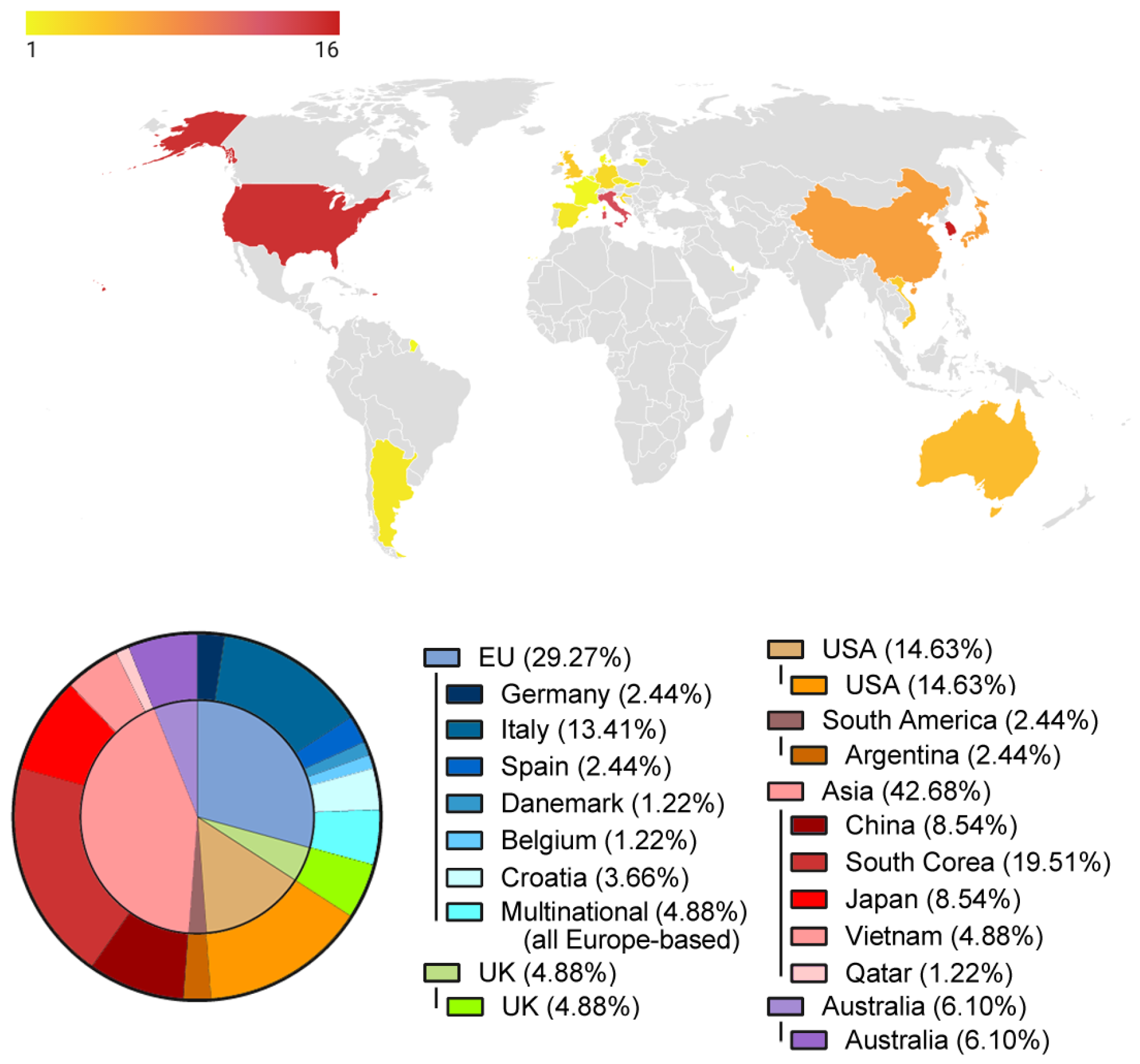
3.2. Worldwide Distribution
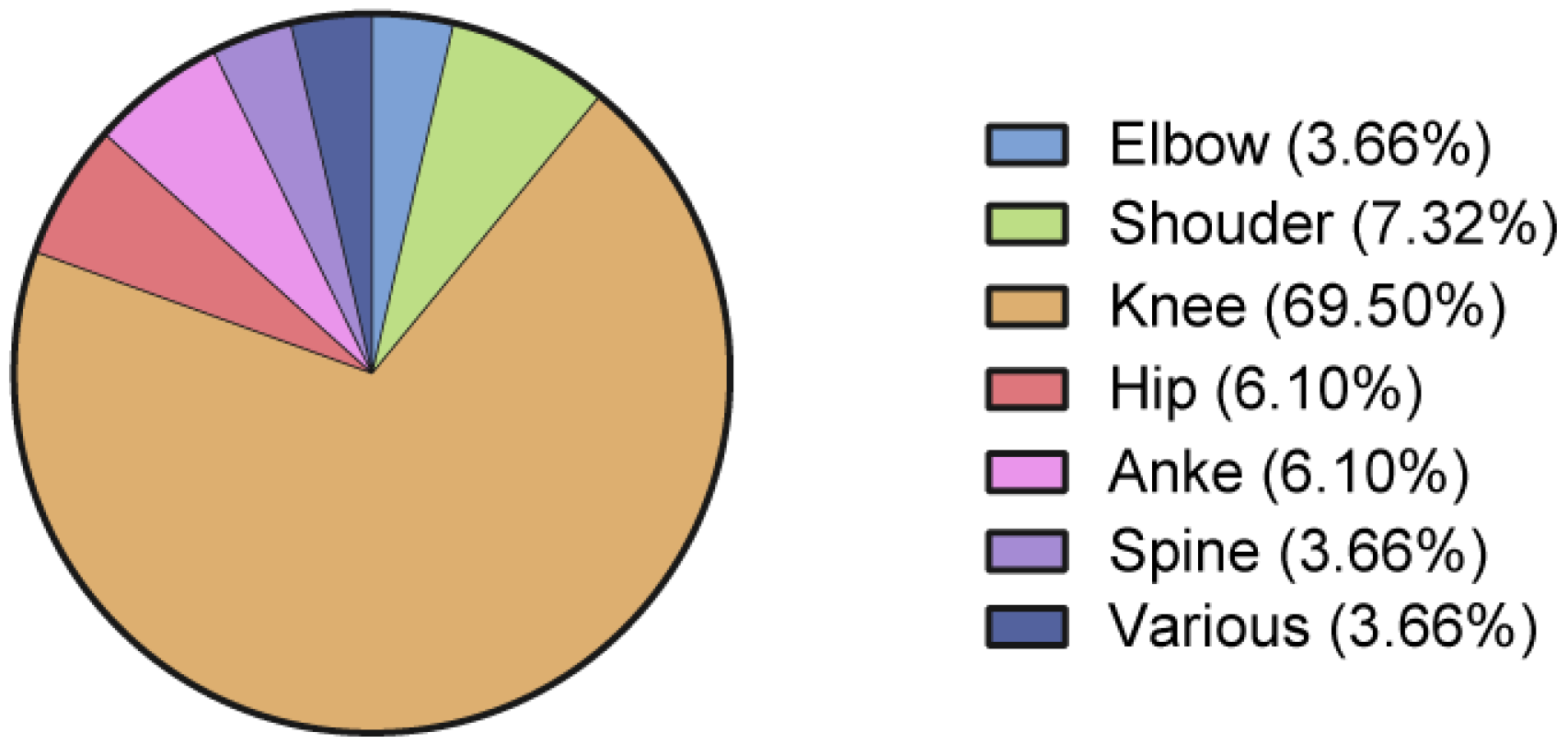
3.3. Anatomical Area and Treated Pathologies
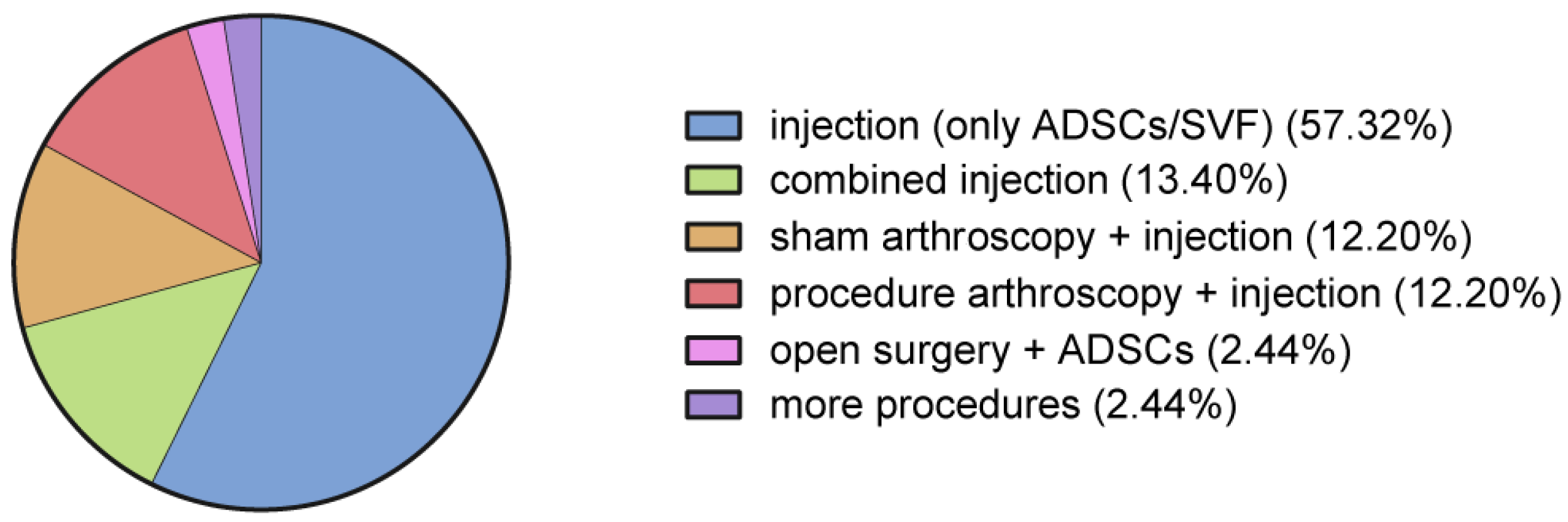
3.4. Procedures
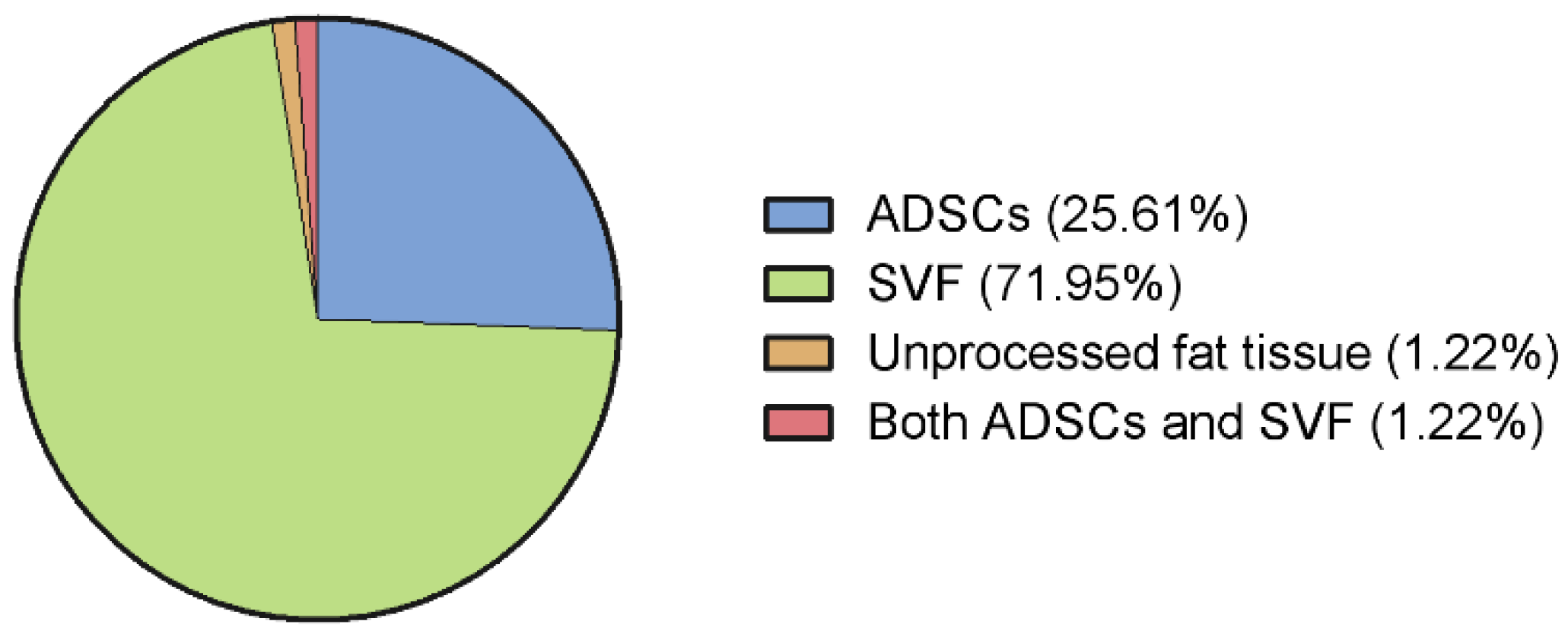
3.5. Adipose Tissue Derivate Types
3.6. Adipose Tissue Processing
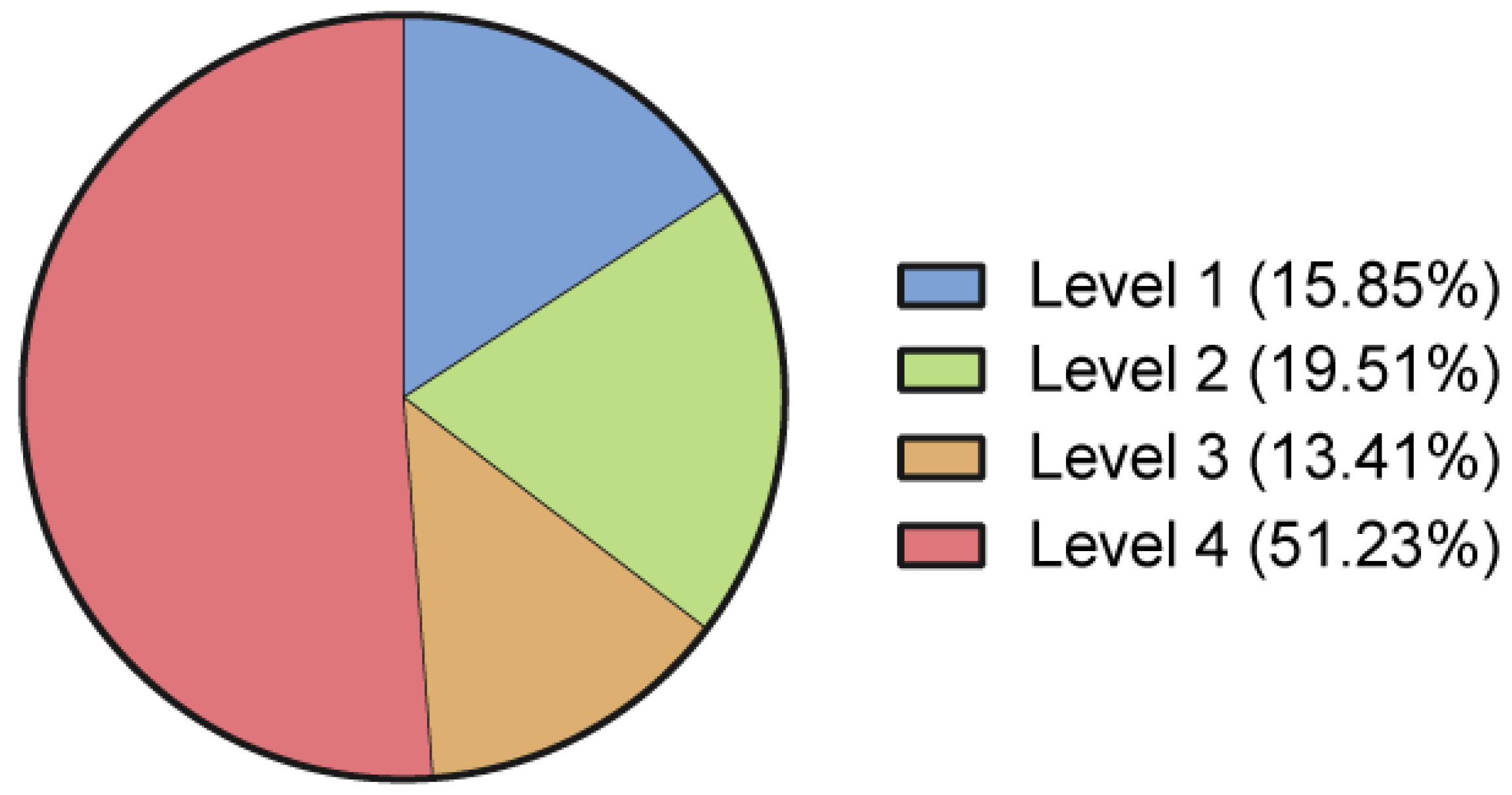
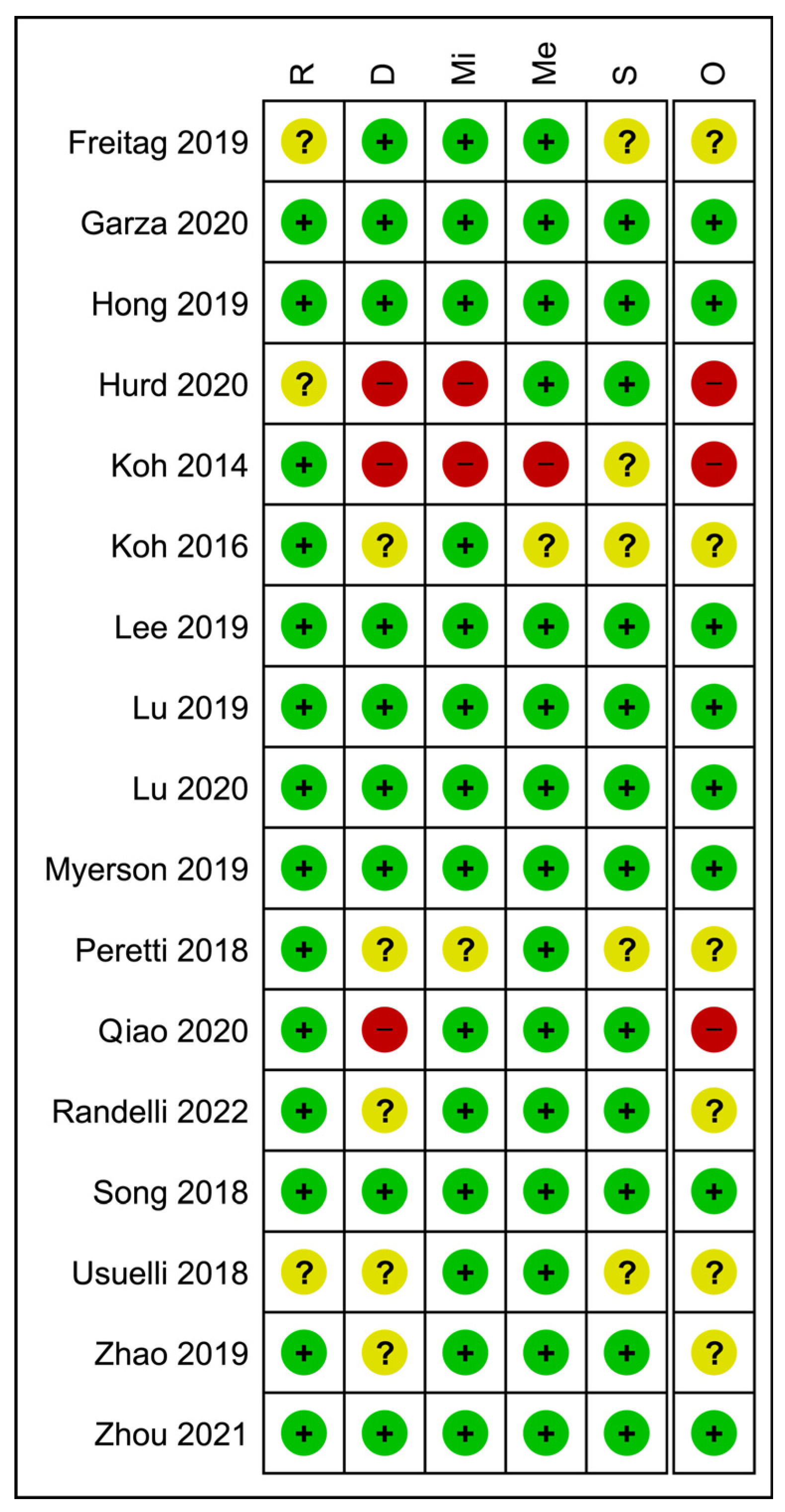
3.7. Quality of Evidence Appraisal
4. Discussion
4.1. Historical Remarks on MSC Sources for Orthopedic Applications
4.2. Adipose Tissue and Its Derivates for Regenerative Medicine Applications
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perucca Orfei, C.; Boffa, A.; Sourugeon, Y.; Laver, L.; Magalon, J.; Sánchez, M.; Tischer, T.; Filardo, G.; de Girolamo, L. Cell-Based Therapies Have Disease-Modifying Effects on Osteoarthritis in Animal Models. A Systematic Review by the ESSKA Orthobiologic Initiative. Part 1: Adipose Tissue-Derived Cell-Based Injectable Therapies. Knee Surg. Sport. Traumatol. Arthrosc. 2022, 31, 641–655. [Google Scholar] [CrossRef]
- Prakoso, A.T.; Basri, H.; Adanta, D.; Yani, I.; Ammarullah, M.I.; Akbar, I.; Ghazali, F.A.; Syahrom, A.; Kamarul, T. The Effect of Tortuosity on Permeability of Porous Scaffold. Biomedicines 2023, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Randelli, P.; Randelli, F.; Ragone, V.; Menon, A.; D’Ambrosi, R.; Cucchi, D.; Cabitza, P.; Banfi, G. Regenerative Medicine in Rotator Cuff Injuries. Biomed Res. Int. 2014, 2014, 129515. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-I.; Kim, M.-S.; Kim, J.-H. Intra-Articular Injection of Autologous Adipose-Derived Stem Cells or Stromal Vascular Fractions: Are They Effective for Patients with Knee Osteoarthritis? A Systematic Review with Meta-Analysis of Randomized Controlled Trials. Am. J. Sports Med. 2023, 51, 837–848. [Google Scholar] [CrossRef]
- Satija, N.K.; Singh, V.K.; Verma, Y.K.; Gupta, P.; Sharma, S.; Afrin, F.; Sharma, M.; Sharma, P.; Tripathi, R.P.; Gurudutta, G.U. Mesenchymal Stem Cell-Based Therapy: A New Paradigm in Regenerative Medicine. J. Cell. Mol. Med. 2009, 13, 4385–4402. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Andia, I.; Zumstein, M.A.; Zhang, C.-Q.; Pinto, N.R.; Bielecki, T. Classification of Platelet Concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for Topical and Infiltrative Use in Orthopedic and Sports Medicine: Current Consensus, Clinical Implications and Perspectives. Muscles Ligaments Tendons J. 2014, 4, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Angeline, M.E.; Rodeo, S.A. Biologics in the Management of Rotator Cuff Surgery. Clin. Sports Med. 2012, 31, 645–663. [Google Scholar] [CrossRef]
- Schmitt, A.; van Griensven, M.; Imhoff, A.B.; Buchmann, S. Application of Stem Cells in Orthopedics. Stem Cells Int. 2012, 2012, 394962. [Google Scholar] [CrossRef]
- Gnecchi, M.; Zhang, Z.; Ni, A.; Dzau, V.J. Paracrine Mechanisms in Adult Stem Cell Signaling and Therapy. Circ. Res. 2008, 103, 1204–1219. [Google Scholar] [CrossRef]
- Ménard, C.; Tarte, K. Immunoregulatory Properties of Clinical Grade Mesenchymal Stromal Cells: Evidence, Uncertainties, and Clinical Application. Stem Cell Res. Ther. 2013, 4, 64. [Google Scholar] [CrossRef]
- Shi, M.; Liu, Z.-W.; Wang, F.-S. Immunomodulatory Properties and Therapeutic Application of Mesenchymal Stem Cells. Clin. Exp. Immunol. 2011, 164, 1–8. [Google Scholar] [CrossRef]
- Soleymaninejadian, E.; Pramanik, K.; Samadian, E. Immunomodulatory Properties of Mesenchymal Stem Cells: Cytokines and Factors. Am. J. Reprod. Immunol. 2012, 67, 1–8. [Google Scholar] [CrossRef]
- Krasnodembskaya, A.; Song, Y.; Fang, X.; Gupta, N.; Serikov, V.; Lee, J.-W.; Matthay, M.A. Antibacterial Effect of Human Mesenchymal Stem Cells Is Mediated in Part from Secretion of the Antimicrobial Peptide LL-37. Stem Cells 2010, 28, 2229–2238. [Google Scholar] [CrossRef]
- Mei, S.H.J.; Haitsma, J.J.; Dos Santos, C.C.; Deng, Y.; Lai, P.F.H.; Slutsky, A.S.; Liles, W.C.; Stewart, D.J. Mesenchymal Stem Cells Reduce Inflammation While Enhancing Bacterial Clearance and Improving Survival in Sepsis. Am. J. Respir. Crit. Care Med. 2010, 182, 1047–1057. [Google Scholar] [CrossRef]
- Randelli, P.; Cucchi, D.; Cabitza, F.; Compagnoni, R.; Menon, A. Tendon-Derived Stem Cells for Rotator Cuff Repair. Oper. Tech. Orthop. 2016, 26, 147–154. [Google Scholar] [CrossRef]
- Potapov, I.; García-Prat, L.; Ravichandran, S.; Muñoz-Cánoves, P.; Del Sol, A. Computational Modelling of Stem Cell-Niche Interactions Facilitates Discovery of Strategies to Enhance Tissue Regeneration and Counteract Ageing. FEBS J. 2022, 289, 1486–1491. [Google Scholar] [CrossRef] [PubMed]
- Nedunchezian, S.; Banerjee, P.; Lee, C.-Y.; Lee, S.-S.; Lin, C.-W.; Wu, C.-W.; Wu, S.-C.; Chang, J.-K.; Wang, C.-K. Generating Adipose Stem Cell-Laden Hyaluronic Acid-Based Scaffolds Using 3D Bioprinting via the Double Crosslinked Strategy for Chondrogenesis. Mater. Sci. Eng. C 2021, 124, 112072. [Google Scholar] [CrossRef] [PubMed]
- Torres-Torrillas, M.; Rubio, M.; Damia, E.; Cuervo, B.; del Romero, A.; Peláez, P.; Chicharro, D.; Miguel, L.; Sopena, J.J. Adipose-Derived Mesenchymal Stem Cells: A Promising Tool in the Treatment of Musculoskeletal Diseases. Int. J. Mol. Sci. 2019, 20, 3105. [Google Scholar] [CrossRef] [PubMed]
- Lana, J.F.S.D.; Lana, A.V.S.D.; da Fonseca, L.F.; Coelho, M.A.; Marques, G.G.; Mosaner, T.; Ribeiro, L.L.; Azzini, G.O.M.; Santos, G.S.; Fonseca, E.; et al. Stromal Vascular Fraction for Knee Osteoarthritis—An Update. J. Stem Cells Regen. Med. 2022, 18, 11–20. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269, W64. [Google Scholar] [CrossRef]
- Marx, R.G.; Wilson, S.M.; Swiontkowski, M.F. Updating the Assignment of Levels of Evidence. J. Bone Jt. Surg. 2015, 97, 1–2. [Google Scholar] [CrossRef]
- Ramponi, L.; Yasui, Y.; Murawski, C.D.; Ferkel, R.D.; DiGiovanni, C.W.; Kerkhoffs, G.M.M.J.; Calder, J.D.F.; Takao, M.; Vannini, F.; Choi, W.J.; et al. Lesion Size Is a Predictor of Clinical Outcomes After Bone Marrow Stimulation for Osteochondral Lesions of the Talus: A Systematic Review. Am. J. Sports Med. 2017, 45, 1698–1705. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Wahab, T.A.; Betancourt, J.P.; Hassan, F.; Al Thani, S.; Choueiri, H.; Jain, N.B.; Malanga, G.A.; Murrell, W.D.; Prasad, A.; Verborgt, O. Initial Treatment of Complete Rotator Cuff Tear and Transition to Surgical Treatment: Systematic Review of the Evidence. Muscle Ligaments Tendons J. 2019, 06, 35. [Google Scholar] [CrossRef]
- Patrick, C.M.; Fernandez, I.; Gonzalez, G.A.; Nesti, L.J.; Dunn, J.C. Analysis of the Quality of Prospective Randomized Controlled Trials for Treatment of Boxer’s Fractures. HAND 2021, 18, 294–299. [Google Scholar] [CrossRef]
- Jakobsen, R.B.; Engebretsen, L.; Slauterbeck, J.R. An Analysis of the Quality of Cartilage Repair Studies. J. Bone Joint Surg. Am. 2005, 87, 2232–2239. [Google Scholar] [CrossRef]
- Coleman, B.D.; Khan, K.M.; Maffulli, N.; Cook, J.L.; Wark, J.D. Studies of Surgical Outcome after Patellar Tendinopathy: Clinical Significance of Methodological Deficiencies and Guidelines for Future Studies. Victorian Institute of Sport Tendon Study Group. Scand. J. Med. Sci. Sports 2000, 10, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- Michalek, J.; Moster, R.; Lukac, L.; Proefrock, K.; Petrasovic, M.; Rybar, J.; Chaloupka, A.; Darinskas, A.; Michalek, J.; Kristek, J.; et al. Stromal Vascular Fraction Cells of Adipose and Connective Tissue in People with Osteoarthritis: A Case Control Prospective Multi-Centric Non-Randomized Study. Glob. Surg. 2017, 3, 163. [Google Scholar] [CrossRef]
- Russo, A.; Coco, V.; Zaffagnini, S. The Effect of Autologous Adipose Derived Mesenchymal Stem Cell Therapy on Juvenile Osteochondritis Dissecans of the Patella: A Case Study. J. Surg. Case Rep. 2020, 2020, rjaa274. [Google Scholar] [CrossRef] [PubMed]
- Khoury, M.A.; Chamari, K.; Tabben, M.; Alkhelaifi, K.; Ricardo, T.; Damián, C.; D’hooghe, P. Expanded Adipose Derived Mesenchymal Stromal Cells Are Effective in Treating Chronic Insertional Patellar Tendinopathy: Clinical and MRI Evaluations of a Pilot Study. J. Exp. Orthop. 2021, 8, 49. [Google Scholar] [CrossRef]
- Alentorn-Geli, E.; Seijas, R.; Martínez-De la Torre, A.; Cuscó, X.; Steinbacher, G.; Álvarez-Díaz, P.; Barastegui, D.; Navarro, J.; Serra-Renom, J.M.; Nishishinya, B.; et al. Effects of Autologous Adipose-Derived Regenerative Stem Cells Administered at the Time of Anterior Cruciate Ligament Reconstruction on Knee Function and Graft Healing. J. Orthop. Surg. 2019, 27, 2309499019867580. [Google Scholar] [CrossRef] [PubMed]
- Heidari, N.; Slevin, M.; Zeinolabediny, Y.; Meloni, D.; Olgiati, S.; Wilson, A.; Noorani, A.; Azamfirei, L. Comparison of the Effect of MFAT and MFAT + PRP on Treatment of Hip Osteoarthritis: An Observational, Intention-to-Treat Study at One Year. J. Clin. Med. 2022, 11, 1056. [Google Scholar] [CrossRef] [PubMed]
- Pak, J.; Lee, J.H.; Park, K.S.; Lee, S. Efficacy of Autologous Adipose Tissue-Derived Stem Cells with Extracellular Matrix and Hyaluronic Acid on Human Hip Osteoarthritis. Biomed. Res. 2017, 28, 1654–1658. [Google Scholar]
- Ivone, A.; Fioruzzi, A.; Jannelli, E.; Castelli, A.; Ghiara, M.; Ferranti Calderoni, E.; Fontana, A. Micro-Fragmented Adipose Tissue Transplantation (MATT) for the Treatment of Acetabular Delamination. A Two Years Follow up Comparison Study with Microfractures. Acta Biomed. 2019, 90, 69–75. [Google Scholar] [CrossRef]
- Pak, J. Autologous Adipose Tissue-Derived Stem Cells Induce Persistent Bone-like Tissue in Osteonecrotic Femoral Heads. Pain Physician 2012, 15, 75–85. [Google Scholar] [CrossRef]
- Pak, J.; Lee, J.H.; Jeon, J.H.; Lee, S.H. Complete Resolution of Avascular Necrosis of the Human Femoral Head Treated with Adipose Tissue-Derived Stem Cells and Platelet-Rich Plasma. J. Int. Med. Res. 2014, 42, 1353–1362. [Google Scholar] [CrossRef]
- Niazi, N.; Islam, A.; Aljawadi, A.; Akbar, Z.; Pillai, A. Autologous Micro Fragmented Adipose Cell Therapy for End-Stage Ankle Osteoarthritis—Case Report and Review of Literature. SN Compr. Clin. Med. 2021, 3, 909–913. [Google Scholar] [CrossRef]
- Myerson, C.L.; Myerson, M.S.; Coetzee, J.C.; Stone McGaver, R.; Giveans, M.R. Subtalar Arthrodesis with Use of Adipose-Derived Cellular Bone Matrix Compared with Autologous Bone Graft: A Multicenter, Randomized Controlled Trial. J. Bone Joint Surg. Am. 2019, 101, 1904–1911. [Google Scholar] [CrossRef]
- Usuelli, F.G.; Grassi, M.; Maccario, C.; Vigano’, M.; Lanfranchi, L.; Alfieri Montrasio, U.; de Girolamo, L. Intratendinous Adipose-Derived Stromal Vascular Fraction (SVF) Injection Provides a Safe, Efficacious Treatment for Achilles Tendinopathy: Results of a Randomized Controlled Clinical Trial at a 6-Month Follow-Up. Knee Surg. Sport. Traumatol. Arthrosc. 2018, 26, 2000–2010. [Google Scholar] [CrossRef] [PubMed]
- Lo Presti, M.; Costa, G.G.; Agrò, G.; Vasco, C.; Zunarelli, P.; Zaffagnini, S. Adipose-Derived Stromal Vascular Fraction Injection in a Competitive High-Level Athlete Affected by Insertional Achilles Tendinopathy. J. Foot Ankle Surg. 2021, 60, 626–629. [Google Scholar] [CrossRef]
- Freitag, J.; Wickham, J.; Shah, K.; Tenen, A. Effect of Autologous Adipose-Derived Mesenchymal Stem Cell Therapy in the Treatment of an Osteochondral Lesion of the Ankle. BMJ Case Rep. 2020, 13, 234595. [Google Scholar] [CrossRef] [PubMed]
- Randelli, P.S.; Cucchi, D.; Fossati, C.; Boerci, L.; Nocerino, E.; Ambrogi, F.; Menon, A. Arthroscopic Rotator Cuff Repair Augmentation with Autologous Microfragmented Lipoaspirate Tissue Is Safe and Effectively Improves Short-Term Clinical and Functional Results: A Prospective Randomized Controlled Trial With 24-Month Follow-Up. Am. J. Sports Med. 2022, 50, 1344–1357. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Sung, C.H.; Chung, S.H.; Kwak, S.J.; Koh, Y.G. Does an Injection of Adipose-Derived Mesenchymal Stem Cells Loaded in Fibrin Glue Influence Rotator Cuff Repair Outcomes? A Clinical and Magnetic Resonance Imaging Study. Am. J. Sports Med. 2017, 45, 2010–2018. [Google Scholar] [CrossRef] [PubMed]
- Cherian, C.; Malanga, G.A.; Hogaboom, N.; Pollack, M.A.; Dyson-Hudson, T.A. Autologous, Micro-Fragmented Adipose Tissue as a Treatment for Chronic Shoulder Pain in a Wheelchair Using Individual with Spinal Cord Injury: A Case Report. Spinal Cord Ser. Cases 2019, 5, 46. [Google Scholar] [CrossRef]
- Hurd, J.L.; Facile, T.R.; Weiss, J.; Hayes, M.M.; Hayes, M.M.; Furia, J.P.; Maffulli, N.; Winnier, G.E.; Alt, C.; Schmitz, C.; et al. Safety and Efficacy of Treating Symptomatic, Partial-Thickness Rotator Cuff Tears with Fresh, Uncultured, Unmodified, Autologous Adipose-Derived Regenerative Cells (UA-ADRCs) Isolated at the Point of Care: A Prospective, Randomized, Controlled First-in-Hu. J. Orthop. Surg. Res. 2020, 15, 122. [Google Scholar] [CrossRef]
- Alt, E.; Rothoerl, R.; Hoppert, M.; Frank, H.-G.; Wuerfel, T.; Alt, C.; Schmitz, C. First Immunohistochemical Evidence of Human Tendon Repair Following Stem Cell Injection: A Case Report and Review of Literature. World J. Stem Cells 2021, 13, 944–970. [Google Scholar] [CrossRef] [PubMed]
- Hogaboom, N.; Malanga, G.; Cherian, C.; Dyson-Hudson, T. A Pilot Study to Evaluate Micro-Fragmented Adipose Tissue Injection under Ultrasound Guidance for the Treatment of Refractory Rotator Cuff Disease in Wheelchair Users with Spinal Cord Injury. J. Spinal Cord Med. 2021, 44, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Kim, W.; Lim, C.; Chung, S.G. Treatment of Lateral Epicondylosis by Using Allogeneic Adipose-Derived Mesenchymal Stem Cells: A Pilot Study. Stem Cells 2015, 33, 2995–3005. [Google Scholar] [CrossRef]
- Freitag, J.; Shah, K.; Wickham, J.; Tenen, A. Effect of Autologous Adipose-Derived Mesenchymal Stem Cell Therapy in Combination with Autologous Platelet-Rich Plasma in the Treatment of Elbow Tendinopathy. BMJ Case Rep. 2020, 13, 234592. [Google Scholar] [CrossRef]
- Khoury, M.; Tabben, M.; Rolón, A.U.; Levi, L.; Chamari, K.; D’Hooghe, P. Promising Improvement of Chronic Lateral Elbow Tendinopathy by Using Adipose Derived Mesenchymal Stromal Cells: A Pilot Study. J. Exp. Orthop. 2021, 8, 6. [Google Scholar] [CrossRef]
- Comella, K.; Silbert, R.; Parlo, M. Effects of the Intradiscal Implantation of Stromal Vascular Fraction plus Platelet Rich Plasma in Patients with Degenerative Disc Disease. J. Transl. Med. 2017, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Ha, D.-H.; Lee, E.-J.; Park, J.H.; Shim, J.H.; Ahn, T.-K.; Kim, K.-T.; Ropper, A.E.; Sohn, S.; Kim, C.-H.; et al. Safety and Tolerability of Intradiscal Implantation of Combined Autologous Adipose-Derived Mesenchymal Stem Cells and Hyaluronic Acid in Patients with Chronic Discogenic Low Back Pain: 1-Year Follow-up of a Phase I Study. Stem Cell Res. Ther. 2017, 8, 262. [Google Scholar] [CrossRef]
- Bright, B.; Bright, R.; Bright, P.; Limaye, A. Ankylosing Spondylitis, Chronic Fatigue and Depression Improved after Stromal Vascular Fraction Treatment for Osteoarthritis: A Case Report. J. Med. Case Rep. 2018, 12, 238. [Google Scholar] [CrossRef]
- Pak, J. Regeneration of Human Bones in Hip Osteonecrosis and Human Cartilage in Knee Osteoarthritis with Autologous Adipose-Tissue-Derived Stem Cells: A Case Series. J. Med. Case Rep. 2011, 5, 296. [Google Scholar] [CrossRef]
- Michalek, J.; Vrablikova, A.; Darinskas, A.; Lukac, L.; Prucha, J.; Skopalik, J.; Travnik, J.; Cibulka, M.; Dudasova, Z. Stromal Vascular Fraction Cell Therapy for Osteoarthritis in Elderly: Multicenter Case-Control Study. J. Clin. Orthop. Trauma 2019, 10, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.-G.; Kwon, O.-R.; Kim, Y.-S.; Choi, Y.-J. Comparative Outcomes of Open-Wedge High Tibial Osteotomy with Platelet-Rich Plasma Alone or in Combination with Mesenchymal Stem Cell Treatment: A Prospective Study. Arthroscopy 2014, 30, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.-G.; Choi, Y.-J. Infrapatellar Fat Pad-Derived Mesenchymal Stem Cell Therapy for Knee Osteoarthritis. Knee 2012, 19, 902–907. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, H.; Xiang, D.; Shao, J.; Fu, Q.; Han, Y.; Zhu, J.; Chen, Y.; Qian, Q. The Clinical Efficacy of Arthroscopic Therapy with Knee Infrapatellar Fat Pad Cell Concentrates in Treating Knee Cartilage Lesion: A Prospective, Randomized, and Controlled Study. J. Orthop. Surg. Res. 2021, 16, 87. [Google Scholar] [CrossRef]
- Zhao, X.; Ruan, J.; Tang, H.; Li, J.; Shi, Y.; Li, M.; Li, S.; Xu, C.; Lu, Q.; Dai, C. Multi-Compositional MRI Evaluation of Repair Cartilage in Knee Osteoarthritis with Treatment of Allogeneic Human Adipose-Derived Mesenchymal Progenitor Cells. Stem Cell Res. Ther. 2019, 10, 308. [Google Scholar] [CrossRef]
- Lu, L.; Dai, C.; Du, H.; Li, S.; Ye, P.; Zhang, L.; Wang, X.; Song, Y.; Togashi, R.; Vangsness, C.T.; et al. Intra-Articular Injections of Allogeneic Human Adipose-Derived Mesenchymal Progenitor Cells in Patients with Symptomatic Bilateral Knee Osteoarthritis: A Phase I Pilot Study. Regen. Med. 2020, 15, 1625–1636. [Google Scholar] [CrossRef]
- Hong, Z.; Chen, J.; Zhang, S.; Zhao, C.; Bi, M.; Chen, X.; Bi, Q. Intra-Articular Injection of Autologous Adipose-Derived Stromal Vascular Fractions for Knee Osteoarthritis: A Double-Blind Randomized Self-Controlled Trial. Int. Orthop. 2019, 43, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Peretti, G.M.; Ulivi, M.; De Girolamo, L.; Meroni, V.; Lombardo, M.D.; Mangiavini, L. Evaluation of the Use of Autologous Micro-Fragmented Adipose Tissue in the Treatment of Knee Osteoarthritis: Preliminary Results of a Randomized Controlled Trial. J. Biol. Regul. Homeost. Agents 2018, 32, 193–199. [Google Scholar]
- Freitag, J.; Bates, D.; Wickham, J.; Shah, K.; Huguenin, L.; Tenen, A.; Paterson, K.; Boyd, R. Adipose-Derived Mesenchymal Stem Cell Therapy in the Treatment of Knee Osteoarthritis: A Randomized Controlled Trial. Regen. Med. 2019, 14, 213–230. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-S.; Kim, H.J.; Kim, K.-I.; Kim, G.B.; Jin, W. Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl. Med. 2019, 8, 504–511. [Google Scholar] [CrossRef]
- Lu, L.; Dai, C.; Zhang, Z.; Du, H.; Li, S.; Ye, P.; Fu, Q.; Zhang, L.; Wu, X.; Dong, Y.; et al. Treatment of Knee Osteoarthritis with Intra-Articular Injection of Autologous Adipose-Derived Mesenchymal Progenitor Cells: A Prospective, Randomized, Double-Blind, Active-Controlled, Phase IIb Clinical Trial. Stem Cell Res. Ther. 2019, 10, 143. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Z.; Tang, J.; Yue, B.; Wang, J.; Zhang, J.; Xuan, L.; Dai, C.; Li, S.; Li, M.; Xu, C.; et al. Human Adipose-Derived Mesenchymal Progenitor Cells plus Microfracture and Hyaluronic Acid for Cartilage Repair: A Phase IIa Trial. Regen. Med. 2020, 15, 1193–1214. [Google Scholar] [CrossRef]
- Garza, J.R.; Campbell, R.E.; Tjoumakaris, F.P.; Freedman, K.B.; Miller, L.S.; Santa Maria, D.; Tucker, B.S. Clinical Efficacy of Intra-Articular Mesenchymal Stromal Cells for the Treatment of Knee Osteoarthritis: A Double-Blinded Prospective Randomized Controlled Clinical Trial. Am. J. Sports Med. 2020, 48, 588–598. [Google Scholar] [CrossRef]
- Song, Y.; Du, H.; Dai, C.; Zhang, L.; Li, S.; Hunter, D.J.; Lu, L.; Bao, C. Human Adipose-Derived Mesenchymal Stem Cells for Osteoarthritis: A Pilot Study with Long-Term Follow-up and Repeated Injections. Regen. Med. 2018, 13, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.-G.; Kwon, O.-R.; Kim, Y.-S.; Choi, Y.-J.; Tak, D.-H. Adipose-Derived Mesenchymal Stem Cells with Microfracture Versus Microfracture Alone: 2-Year Follow-up of a Prospective Randomized Trial. Arthroscopy 2016, 32, 97–109. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Krampera, M.; Galipeau, J.; Shi, Y.; Tarte, K.; Sensebe, L. Immunological Characterization of Multipotent Mesenchymal Stromal Cells-The International Society for Cellular Therapy (ISCT) Working Proposal. Cytotherapy 2013, 15, 1054–1061. [Google Scholar] [CrossRef]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A. Clarification of the Nomenclature for MSC: The International Society for Cellular Therapy Position Statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. What’s in a Name? Tissue Eng. Part A 2010, 16, 2415–2417. [Google Scholar] [CrossRef] [PubMed]
- Salmikangas, P.; Schuessler-Lenz, M.; Ruiz, S.; Celis, P.; Reischl, I.; Menezes-Ferreira, M.; Flory, E.; Renner, M.; Ferry, N. Marketing Regulatory Oversight of Advanced Therapy Medicinal Products (ATMPs) in Europe: The EMA/CAT Perspective. In Regulatory Aspects of Gene Therapy and Cell Therapy Products; Springer: Berlin/Heidelberg, Germany, 2015; pp. 103–130. [Google Scholar]
- Guess, A.J.; Daneault, B.; Wang, R.; Bradbury, H.; La Perle, K.M.D.; Fitch, J.; Hedrick, S.L.; Hamelberg, E.; Astbury, C.; White, P.; et al. Safety Profile of Good Manufacturing Practice Manufactured Interferon γ-Primed Mesenchymal Stem/Stromal Cells for Clinical Trials. Stem Cells Transl. Med. 2017, 6, 1868–1879. [Google Scholar] [CrossRef] [PubMed]
- Shin, D., II; Kim, M.; Park, D.Y.; Min, B.-H.; Yun, H.-W.; Chung, J.Y.; Min, K.J. Motorized Shaver Harvest Results in Similar Cell Yield and Characteristics Compared with Rongeur Biopsy During Arthroscopic Synovium-Derived Mesenchymal Stem Cell Harvest. Arthroscopy 2021, 37, 2873–2882. [Google Scholar] [CrossRef]
- Fu, W.; Li, Q.; Tang, X.; Chen, G.; Zhang, C.; Li, J. Mesenchymal Stem Cells Reside in Anterior Cruciate Ligament Remnants in Situ. Int. Orthop. 2016, 40, 1523–1530. [Google Scholar] [CrossRef]
- Randelli, P.; Conforti, E.; Piccoli, M.; Ragone, V.; Creo, P.; Cirillo, F.; Masuzzo, P.; Tringali, C.; Cabitza, P.; Tettamanti, G.; et al. Isolation and Characterization of 2 New Human Rotator Cuff and Long Head of Biceps Tendon Cells Possessing Stem Cell-Like Self-Renewal and Multipotential Differentiation Capacity. Am. J. Sports Med. 2013, 41, 1653–1664. [Google Scholar] [CrossRef]
- Tsai, C.C.; Huang, T.F.; Ma, H.L.; Chiang, E.R.; Hung, S.C. Isolation of Mesenchymal Stem Cells from Shoulder Rotator Cuff: A Potential Source for Muscle and Tendon Repair. Cell Transpl. 2013, 22, 413–422. [Google Scholar] [CrossRef]
- Utsunomiya, H.; Uchida, S.; Sekiya, I.; Sakai, A.; Moridera, K.; Nakamura, T. Isolation and Characterization of Human Mesenchymal Stem Cells Derived from Shoulder Tissues Involved in Rotator Cuff Tears. Am. J. Sports Med. 2013, 41, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Moravcikova, E.; Meyer, E.M.; Corselli, M.; Donnenberg, V.S.; Donnenberg, A.D. Proteomic Profiling of Native Unpassaged and Culture-Expanded Mesenchymal Stromal Cells (MSC). Cytometry A 2018, 93, 894–904. [Google Scholar] [CrossRef]
- Tevlin, R.; DesJardins-Park, H.; Huber, J.; DiIorio, S.E.; Longaker, M.T.; Wan, D.C. Musculoskeletal Tissue Engineering: Adipose Derived Stromal Cell Implementation for the Treatment of Osteoarthritis. Biomaterials 2022, 286, 121544. [Google Scholar] [CrossRef]
- Ossendorff, R.; Walter, S.; Schildberg, F.; Khoury, M.; Salzmann, G. Controversies in Regenerative Medicine: Should Knee Joint Osteoarthritis Be Treated with Mesenchymal Stromal Cells? Eur. Cells Mater. 2022, 43, 89–111. [Google Scholar] [CrossRef]
- Aronowitz, J.A.; Lockhart, R.A.; Hakakian, C.S. Mechanical versus Enzymatic Isolation of Stromal Vascular Fraction Cells from Adipose Tissue. Springerplus 2015, 4, 713. [Google Scholar] [CrossRef]
- Walter, S.; Randau, T.; Hilgers, C.; Haddouti, E.-M.; Masson, W.; Gravius, S.; Burger, C.; Wirtz, D.; Schildberg, F. Molecular and Functional Phenotypes of Human Bone Marrow-Derived Mesenchymal Stromal Cells Depend on Harvesting Techniques. Int. J. Mol. Sci. 2020, 21, 4382. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, V.; Clausen, P.; Matuska, A.M. Micro-Fragmented Adipose Tissue Cellular Composition Varies by Processing Device and Analytical Method. Sci. Rep. 2022, 12, 16107. [Google Scholar] [CrossRef]
- Bora, P.; Majumdar, A.S. Adipose Tissue-Derived Stromal Vascular Fraction in Regenerative Medicine: A Brief Review on Biology and Translation. Stem Cell Res. Ther. 2017, 8, 145. [Google Scholar] [CrossRef]
- Jo, C.H.; Lee, Y.G.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.C.; Kim, J.E.; Shim, H.; Shin, J.S.; Shin, I.S.; et al. Intra-Articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritis of the Knee: A Proof-of-Concept Clinical Trial. Stem Cells 2014, 32, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Pers, Y.-M.; Rackwitz, L.; Ferreira, R.; Pullig, O.; Delfour, C.; Barry, F.; Sensebe, L.; Casteilla, L.; Fleury, S.; Bourin, P.; et al. Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial. Stem Cells Transl. Med. 2016, 5, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.D.; Tran, T.D.-X.; Nguyen, H.T.-N.; Vu, H.T.; Le, P.T.-B.; Phan, N.L.-C.; Vu, N.B.; Phan, N.K.; Van Pham, P. Comparative Clinical Observation of Arthroscopic Microfracture in the Presence and Absence of a Stromal Vascular Fraction Injection for Osteoarthritis. Stem Cells Transl. Med. 2017, 6, 187–195. [Google Scholar] [CrossRef]
- Tran, T.D.X.; Wu, C.-M.; Dubey, N.K.; Deng, Y.-H.; Su, C.-W.; Pham, T.T.; Thi Le, P.B.; Sestili, P.; Deng, W.-P. Time- and Kellgren–Lawrence Grade-Dependent Changes in Intra-Articularly Transplanted Stromal Vascular Fraction in Osteoarthritic Patients. Cells 2019, 8, 308. [Google Scholar] [CrossRef] [PubMed]
- Tsubosaka, M.; Matsumoto, T.; Sobajima, S.; Matsushita, T.; Iwaguro, H.; Kuroda, R. Comparison of Clinical and Imaging Outcomes of Different Doses of Adipose-Derived Stromal Vascular Fraction Cell Treatment for Knee Osteoarthritis. Cell Transplant. 2021, 30, 9636897211067454. [Google Scholar] [CrossRef] [PubMed]
- Labarre, K.W.; Zimmermann, G. Infiltration of the Hoffa’s Fat Pad with Stromal Vascular Fraction in Patients with Osteoarthritis of the Knee -Results after One Year of Follow-Up. Bone Rep. 2022, 16, 101168. [Google Scholar] [CrossRef] [PubMed]
- Screpis, D.; Natali, S.; Farinelli, L.; Piovan, G.; Iacono, V.; de Girolamo, L.; Viganò, M.; Zorzi, C. Autologous Microfragmented Adipose Tissue for the Treatment of Knee Osteoarthritis: Real-World Data at Two Years Follow-Up. J. Clin. Med. 2022, 11, 1268. [Google Scholar] [CrossRef] [PubMed]
- Hudetz, D.; Borić, I.; Rod, E.; Jeleč, Ž.; Kunovac, B.; Polašek, O.; Vrdoljak, T.; Plečko, M.; Skelin, A.; Polančec, D.; et al. Early Results of Intra-Articular Micro-Fragmented Lipoaspirate Treatment in Patients with Late Stages Knee Osteoarthritis: A Prospective Study. Croat. Med. J. 2019, 60, 227–236. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ossendorff, R.; Menon, A.; Schildberg, F.A.; Randelli, P.S.; Scheidt, S.; Burger, C.; Wirtz, D.C.; Cucchi, D. A Worldwide Analysis of Adipose-Derived Stem Cells and Stromal Vascular Fraction in Orthopedics: Current Evidence and Applications. J. Clin. Med. 2023, 12, 4719. https://doi.org/10.3390/jcm12144719
Ossendorff R, Menon A, Schildberg FA, Randelli PS, Scheidt S, Burger C, Wirtz DC, Cucchi D. A Worldwide Analysis of Adipose-Derived Stem Cells and Stromal Vascular Fraction in Orthopedics: Current Evidence and Applications. Journal of Clinical Medicine. 2023; 12(14):4719. https://doi.org/10.3390/jcm12144719
Chicago/Turabian StyleOssendorff, Robert, Alessandra Menon, Frank A. Schildberg, Pietro S. Randelli, Sebastian Scheidt, Christof Burger, Dieter C. Wirtz, and Davide Cucchi. 2023. "A Worldwide Analysis of Adipose-Derived Stem Cells and Stromal Vascular Fraction in Orthopedics: Current Evidence and Applications" Journal of Clinical Medicine 12, no. 14: 4719. https://doi.org/10.3390/jcm12144719
APA StyleOssendorff, R., Menon, A., Schildberg, F. A., Randelli, P. S., Scheidt, S., Burger, C., Wirtz, D. C., & Cucchi, D. (2023). A Worldwide Analysis of Adipose-Derived Stem Cells and Stromal Vascular Fraction in Orthopedics: Current Evidence and Applications. Journal of Clinical Medicine, 12(14), 4719. https://doi.org/10.3390/jcm12144719









