Patient Perspectives on Digital Interventions to Manage Heart Failure Medications: The VITAL-HF Pilot
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Study Design
2.3. Intervention and Study Procedures
2.4. Outcomes
2.5. Statistical Analyses
3. Results
Participant Experiences
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.M.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Beaton, A.Z.; Boehme, A.K.; Buxton, A.E.; et al. Heart Disease and Stroke Statistics—2023 Update: A Report From the American Heart Association. Circulation 2023, 147, e93–e621. [Google Scholar]
- Dixit, N.M.; Parikh, N.U.; Ziaeian, B.; Jackson, N.; Fonarow, G.C. Cost-Effectiveness of Comprehensive Quadruple Therapy for Heart Failure with Reduced Ejection Fraction. JACC Heart Fail 2023, 11, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.J.; Butler, J.; Fonarow, G.C. Simultaneous or Rapid Sequence Initiation of Quadruple Medical Therapy for Heart Failure—Optimizing Therapy with the Need for Speed. JAMA Cardiol. 2021, 6, 743–744. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar]
- DeVore, A.D.; Granger, B.B.; Fonarow, G.C.; Al-Khalidi, H.R.; Albert, N.M.; Lewis, E.F.; Butler, J.; Piña, I.L.; Allen, L.A.; Yancy, C.W.; et al. Effect of a Hospital and Postdischarge Quality Improvement Intervention on Clinical Outcomes and Quality of Care for Patients with Heart Failure with Reduced Ejection Fraction: The CONNECT-HF Randomized Clinical Trial. JAMA 2021, 326, 314–323. [Google Scholar] [CrossRef]
- Greene, S.J.; Butler, J.; Albert, N.M.; DeVore, A.D.; Sharma, P.P.; Duffy, C.I.; Hill, C.L.; McCague, K.; Mi, X.; Patterson, J.H.; et al. Medical Therapy for Heart Failure with Reduced Ejection Fraction: The CHAMP-HF Registry. J. Am. Coll. Cardiol. 2018, 72, 351–366. [Google Scholar] [CrossRef]
- DeVore, A.D.; Bosworth, H.B.; Granger, B.B. Improving implementation of evidence-based therapies for heart failure. Clin. Cardiol. 2022, 45 (Suppl. S1), S52–S59. [Google Scholar] [CrossRef] [PubMed]
- Samsky, M.D.; Lin, L.; Greene, S.J.; Lippmann, S.J.; Peterson, P.N.; Heidenreich, P.A.; Laskey, W.K.; Yancy, C.W.; Greiner, M.A.; Hardy, N.C.; et al. Patient Perceptions and Familiarity with Medical Therapy for Heart Failure. JAMA Cardiol. 2020, 5, 292–299. [Google Scholar] [CrossRef]
- Ferrante, D.; Varini, S.; Macchia, A.; Soifer, S.; Badra, R.; Nul, D.; Grancelli, H.; Doval, H. Long-Term Results After a Telephone Intervention in Chronic Heart Failure: DIAL (Randomized Trial of Phone Intervention in Chronic Heart Failure) Follow-Up. J. Am. Coll. Cardiol. 2010, 56, 372–378. [Google Scholar] [CrossRef]
- Weintraub, A.; Gregory, D.; Patel, A.R.; Levine, D.; Venesy, D.; Perry, K.; Delano, C.; Konstam, M.A. A multicenter randomized controlled evaluation of automated home monitoring and telephonic disease management in patients recently hospitalized for congestive heart failure: The SPAN-CHF II trial. J. Card. Fail. 2010, 16, 285–292. [Google Scholar] [CrossRef]
- Rich, M.W.; Beckham, V.; Wittenberg, C.; Leven, C.L.; Freedland, K.E.; Carney, R.M. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N. Engl. J. Med. 1995, 333, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Inglis, S.C.; Clark, R.A.; Dierckx, R.; Prieto-Merino, D.; Cleland, J.G. Structured telephone support or non-invasive telemonitoring for patients with heart failure. Cochrane Database Syst. Rev. 2015, 2015, Cd007228. [Google Scholar] [PubMed]
- DeWalt, D.A.; Malone, R.M.; Bryant, M.E.; Kosnar, M.C.; Corr, K.E.; Rothman, R.L.; Sueta, C.A.; Pignone, M.P. A heart failure self-management program for patients of all literacy levels: A randomized, controlled trial [ISRCTN11535170]. BMC Health Serv. Res. 2006, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Koehler, F.; Winkler, S.; Schieber, M.; Sechtem, U.; Stangl, K.; Böhm, M.; Boll, H.; Baumann, G.; Honold, M.; Koehler, K.; et al. Impact of remote telemedical management on mortality and hospitalizations in ambulatory patients with chronic heart failure: The telemedical interventional monitoring in heart failure study. Circulation 2011, 123, 1873–1880. [Google Scholar] [CrossRef]
- Bozkurt, B.; Coats, A.J.S.; Tsutsui, H.; Abdelhamid, M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J. Card. Fail. 2021, 27, 387–413. [Google Scholar]
- Bozkurt, B. How to Initiate and Uptitrate GDMT in Heart Failure. JACC Heart Fail. 2022, 10, 992–995. [Google Scholar] [CrossRef]
- Krueger, R.A. Focus Groups: A Practical Guide for Applied Research; Sage Publications: Thousand Oaks, CA, USA, 2014. [Google Scholar]
- Santhosh, L.; Rojas, J.C.; Lyons, P.G. Zooming into Focus Groups: Strategies for Qualitative Research in the Era of Social Distancing. ATS Sch. 2021, 2, 176–184. [Google Scholar] [CrossRef]
- Yokota, T.; Fukushima, A.; Tsuchihashi-Makaya, M.; Abe, T.; Takada, S.; Furihata, T.; Ishimori, N.; Fujino, T.; Kinugawa, S.; Ohta, M.; et al. The AppCare-HF randomized clinical trial: A feasibility study of a novel self-care support mobile app for individuals with chronic heart failure. Eur. Heart J. Digit. Health 2023, ztad032. [Google Scholar] [CrossRef]
- Upshaw, J.N.; Parker, S.; Gregory, D.; Koethe, B.; Vest, A.R.; Patel, A.R.; Kiernan, M.S.; DeNofrio, D.; Davidson, E.; Mohanty, S.; et al. The effect of tablet computer-based telemonitoring added to an established telephone disease management program on heart failure hospitalizations: The Specialized Primary and Networked Care in Heart Failure (SPAN-CHF) III Randomized Controlled Trial. Am. Heart J. 2023, 260, 90–99. [Google Scholar] [CrossRef]
- Mebazaa, A.; Davison, B.; Chioncel, O.; Cohen-Solal, A.; Diaz, R.; Filippatos, G.; Metra, M.; Ponikowski, P.; Sliwa, K.; Voors, A.A.; et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): A multinational, open-label, randomised, trial. Lancet 2022, 400, 1938–1952. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.S.; Maclean, T.; Blood, A.J.; Bosque-Hamilton, J.; Dunning, J.; Fischer, C.; Fera, L.; Smith, K.V.; Wagholikar, K.; Zelle, D.; et al. Remote Optimization of Guideline-Directed Medical Therapy in Patients with Heart Failure with Reduced Ejection Fraction. JAMA Cardiol. 2020, 5, 1430–1434. [Google Scholar] [CrossRef] [PubMed]

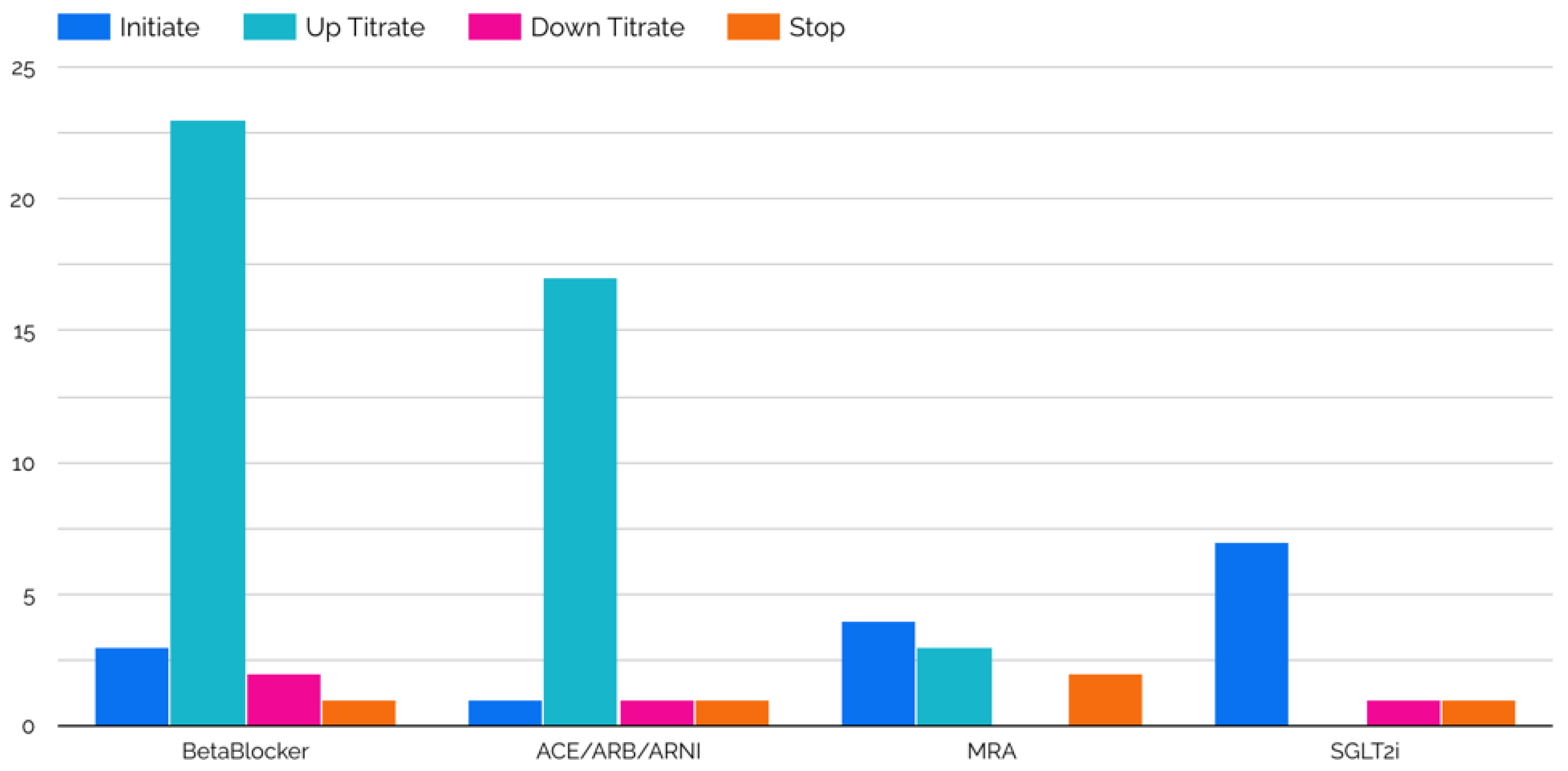
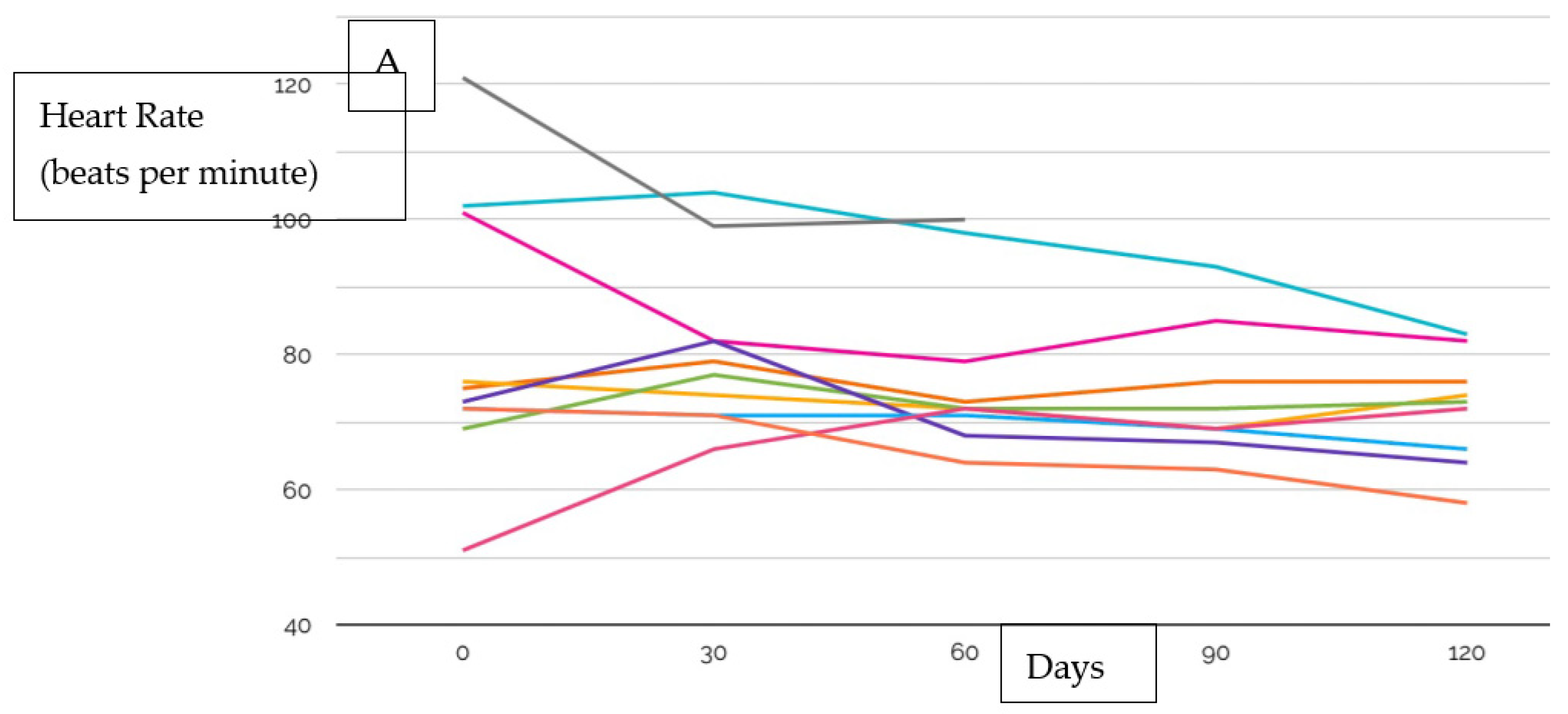
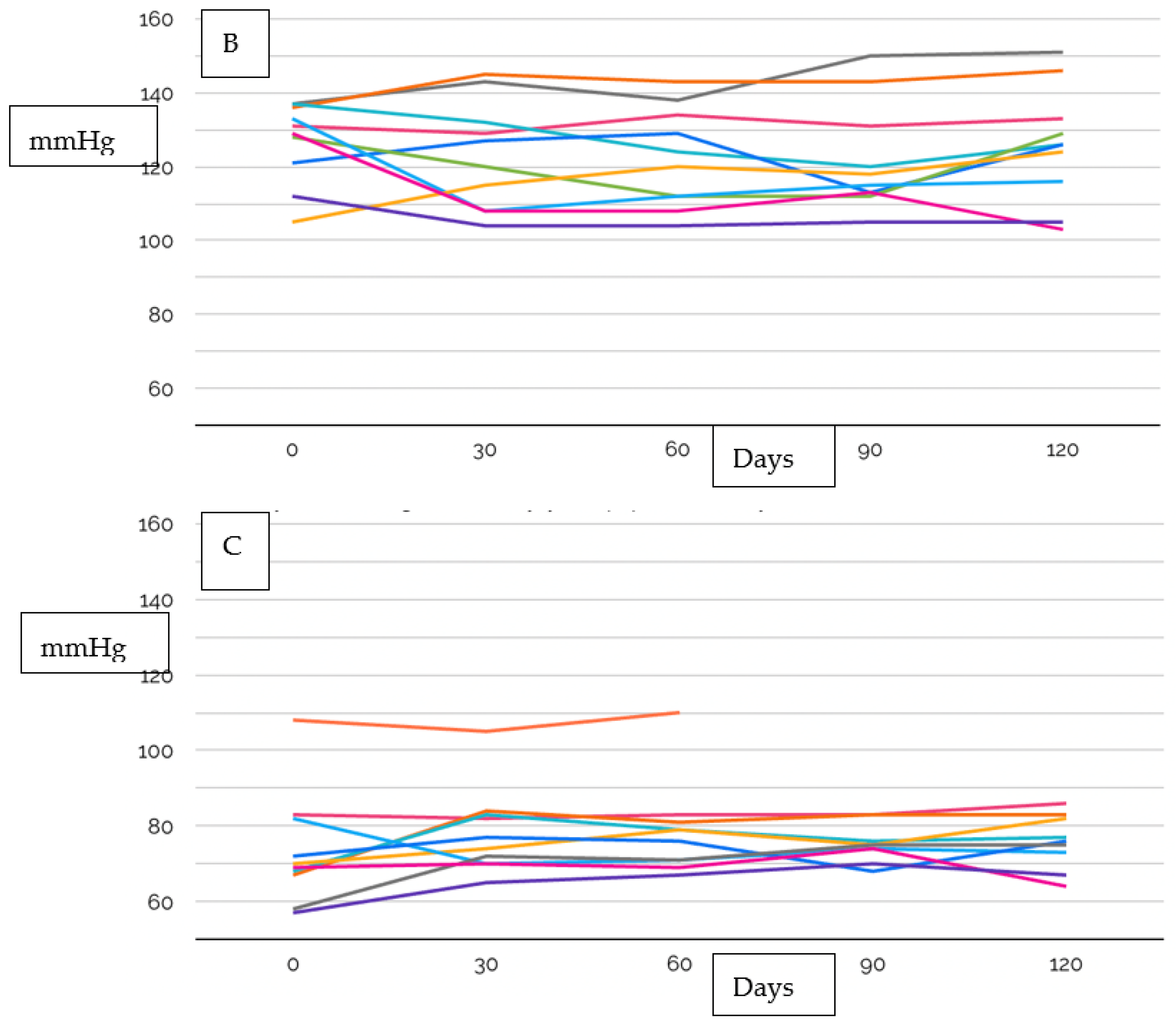
| Age, years (IQR) | 52.5 (46.5–63.5) |
| Left Ventricular Ejection Fraction (IQR) | 25% (22.5–35.5) |
| Female | 58.3% (7/12) |
| Black Race | 50.0% (6/12) |
| Systolic Blood Pressure, mmHg | 131 (118.8–136.3) |
| Diastolic Blood Pressure, mmHg | 69.5 (64.8–74.1) |
| Heart Rate | 72.5 (67.0–82.3) |
| Serum Potassium, mEq/L (IQR) | 4.2 (4.05–4.5) |
| Serum Creatinine mg/dL (IQR) | 1.3 (1.0–1.6) |
| Estimated glomerular filtration rate mL/min/1.73m2 (IQR) | 59 (39.5–79.5) |
| Baseline medication use | |
| ACEi/ARB/ARNI | 83.3% (10/12) |
| Beta-blocker | 91.7% (11/12) |
| Mineralocorticoid receptor antagonist | 66.7% (8/12) |
| SGLT2i | 50.0% (6/12) |
| Ivabradine | 0% (0/12) |
| Hydralazine | 16.7% (2/12) |
| Isosorbide dinitrate | 16.7% (2/12) |
| Domain/Themes | Examples |
|---|---|
| Regular oversight by a cohesive team | “I did feel better knowing that somebody was paying attention to and you know monitoring it did make me feel much safer” (Participant 1) “…I love that I was able to check my blood pressure at any time and knowing that somebody was on the other end if something went wrong…” (Participant 2) “Make sure that everything is looking right it was just a little feedback for me to know that yeah I’m on the right track and you know it also helped me remember to take my medication” (Participant 6) |
| Monitoring outside of the clinic | “it definitely makes you remember to take your medication and remember to take your blood pressure because somebody watching” (Participant 2) “my blood pressure would fluctuate quite a bit and I just went ahead and took the reading when I wasn’t feeling normal so that it would have a a document in there to say hey this is what my blood pressure was doing this time throughout the day” (Participant 6) |
| Frequent medication changes | “frustrating I had just filled my prescription and in my medication got changed so paying for it twice that’s all” (Participant 2) “…what I didn’t like was that my medications were changed so much that I would go from I feel OK and then all of a sudden medications get changed and my body has to get used to them and then I didn’t feel the greatest for a few days until my body got accustomed to the medications and once my body got accustomed to the medications and I was OK… (Participant 3) |
| Concerns about Information security | “I’ve been living with the thought you can die in your sleep in this and having to deal with that for over 20 years add take day by day and anybody that wants to know I’m glad to help and when this opportunity to do the research I was willing information more information where people get to know what’s going on with their heart” (Participant 1) “I was a little concerned ‘cause I know that you know text messages not secure at all but you know it’s it’s one of those things that’s helping me so I gave up a little bit of the privacy aspect of it to you know help myself to get better” (Participant 6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samsky, M.D.; Leverty, R.; Gray, J.M.; Davis, A.; Fisher, B.; Govil, A.; Stanis, T.; DeVore, A.D. Patient Perspectives on Digital Interventions to Manage Heart Failure Medications: The VITAL-HF Pilot. J. Clin. Med. 2023, 12, 4676. https://doi.org/10.3390/jcm12144676
Samsky MD, Leverty R, Gray JM, Davis A, Fisher B, Govil A, Stanis T, DeVore AD. Patient Perspectives on Digital Interventions to Manage Heart Failure Medications: The VITAL-HF Pilot. Journal of Clinical Medicine. 2023; 12(14):4676. https://doi.org/10.3390/jcm12144676
Chicago/Turabian StyleSamsky, Marc D., Renee Leverty, James M. Gray, Alexandra Davis, Brett Fisher, Ashul Govil, Tom Stanis, and Adam D. DeVore. 2023. "Patient Perspectives on Digital Interventions to Manage Heart Failure Medications: The VITAL-HF Pilot" Journal of Clinical Medicine 12, no. 14: 4676. https://doi.org/10.3390/jcm12144676
APA StyleSamsky, M. D., Leverty, R., Gray, J. M., Davis, A., Fisher, B., Govil, A., Stanis, T., & DeVore, A. D. (2023). Patient Perspectives on Digital Interventions to Manage Heart Failure Medications: The VITAL-HF Pilot. Journal of Clinical Medicine, 12(14), 4676. https://doi.org/10.3390/jcm12144676







