Impact of Alcohol Dehydrogenase 7 Polymorphism and Alcohol Consumption on Risk of Head and Neck Squamous Cell Carcinoma: A Korean Case-Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Methods
2.2.1. DNA Extraction
2.2.2. Genetic Analysis
2.2.3. Haplotype Analysis
2.2.4. Statistics
3. Results
3.1. ADH7 Allele Frequency
3.2. ADH7 Polymorphism and Risk of Head and Neck Squamous Cell Carcinoma
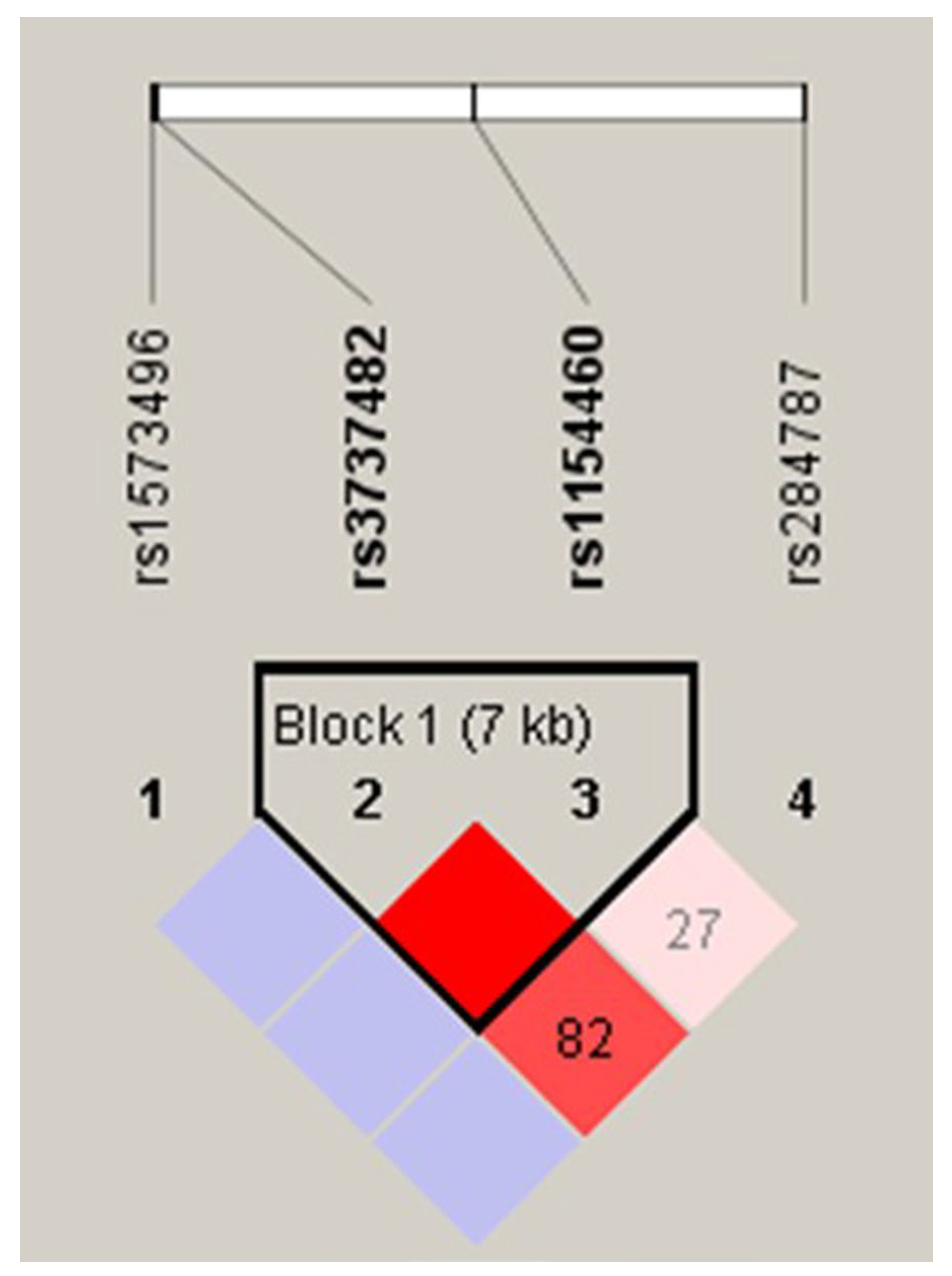
3.3. Linkage Disequilibrium and Haplotype of ADH7
3.4. ADH7 rs3737482T>C and rs1154460G>A and Risk of Head and Neck Squamous Cell Carcinoma According to Alcohol Consumption
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marziliano, A.; Teckie, S.; Diefenbach, M.A. Alcohol-related head and neck cancer: Summary of the literature. Head Neck 2020, 42, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.Y.; Chang, S.-C.; Hashibe, M.; La Vecchia, C.; Zhang, Z.-F. Alcohol consumption and cancers of the oral cavity and pharynx from 1988 to 2009: An update. Eur. J. Cancer Prev. 2010, 19, 431–465. [Google Scholar] [CrossRef] [PubMed]
- Turati, F.; Garavello, W.; Tramacere, I.; Bagnardi, V.; Rota, M.; Scotti, L.; Islami, F.; Corrao, G.; Boffetta, P.; La Vecchia, C.; et al. A meta-analysis of alcohol drinking and oral and pharyngeal cancers. Part 2: Results by subsites. Oral Oncol. 2010, 46, 720–726. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Alcohol consumption and ethyl carbamate. IARC Monogr. Eval. Carcinog. Risks Hum. 2010, 96, 3–1383. [Google Scholar]
- Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Bouvard, V.; Altieri, A.; Cogliano, V. Carcinogenicity of alcoholic beverages. Lancet Oncol. 2007, 8, 292–293. [Google Scholar] [CrossRef] [PubMed]
- Muto, M.; Nakane, M.; Hitomi, Y.; Yoshida, S.; Sasaki, S.; Ohtsu, A.; Yoshida, S.; Ebihara, S.; Esumi, H. Association between aldehyde dehydrogenase gene polymorphisms and the phenomenon of field cancerization in patients with head and neck cancer. Carcinogenesis 2002, 23, 1759–1766. [Google Scholar] [CrossRef]
- Druesne-Pecollo, N.; Tehard, B.; Mallet, Y.; Gerber, M.; Norat, T.; Hercberg, S.; Latino-Martel, P. Alcohol and genetic polymorphisms: Effect on risk of alcohol-related cancer. Lancet Oncol. 2009, 10, 173–180. [Google Scholar] [CrossRef]
- Yokoyama, A.; Mizukami, T.; Yokoyama, T. Genetic polymorphisms of alcohol dehydrogense-1B and aldehyde dehydrogenase-2, alcohol flushing, mean corpuscular volume, and aerodigestive tract neoplasia in Japanese drinkers. Biol. Basis Alcohol-Induc. Cancer 2015, 815, 265–279. [Google Scholar] [CrossRef]
- Homann, N.; Jousimies-Somer, H.; Jokelainen, K.; Heine, R.; Salaspuro, M. High acetaldehyde levels in saliva after ethanol consumption: Methodological aspects and pathogenetic implications. Carcinogenesis 1997, 18, 1739–1743. [Google Scholar] [CrossRef]
- Tae, K.; Lee, H.S.; Park, B.J.; Park, C.W.; Kim, K.R.; Cho, H.Y.; Kim, L.H.; Park, B.L.; Shin, H.D. Association of DNA repair geneXRCC1 polymorphisms with head and neck cancer in Korean population. Int. J. Cancer 2004, 111, 805–808. [Google Scholar] [CrossRef]
- Ji, Y.B.; Tae, K.; Lee, Y.S.; Lee, S.H.; Kim, K.R.; Park, C.W.; Park, B.L.; Shin, H.D. XPD Polymorphisms and risk of squamous cell carcinoma of the head and neck in a Korean sample. Clin. Exp. Otorhinolaryngol. 2010, 3, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Hashibe, M.; Boffetta, P.; Zaridze, D.; Shangina, O.; Szeszenia-Dabrowska, N.; Mates, D.; Janout, V.; Fabiánová, E.; Bencko, V.; Moullan, N.; et al. Evidence for an important role of alcohol- and aldehyde-metabolizing genes in cancers of the upper aerodigestive tract. Cancer Epidemiol. Biomark. Prev. 2006, 15, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Hashibe, M.; Boffetta, P.; Zaridze, D.; Shangina, O.; Szeszenia-Dabrowska, N.; Mates, D.; Janout, V.; Fabiánová, E.; Bencko, V.; Moullan, N.; et al. Impact of the Alcohol-Dehydrogenase (ADH) 1C and ADH1B polymorphisms on drinking behavior in nonalcoholic Japanese. Hum. Mutat. 2007, 28, 506–510. [Google Scholar]
- Peters, E.S.; McClean, M.D.; Liu, M.; Eisen, E.A.; Mueller, N.; Kelsey, K.T. The ADH1C polymorphism modifies the risk of squamous cell carcinoma of the head and neck associated with alcohol and tobacco use. Cancer Epidemiol. Biomark. Prev. 2005, 14, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-X.; Matsuo, K.; Ito, H.; Hirose, K.; Wakai, K.; Saito, T.; Shinoda, M.; Hatooka, S.; Mizutani, K.; Tajima, K. Esophageal cancer risk by ALDH2 and ADH2 polymorphisms and alcohol consumption: Exploration of gene-environment and gene-gene interactions. Asian Pac. J. Cancer Prev. 2005, 6, 256–262. [Google Scholar] [PubMed]
- Osier, M.V.; Pakstis, A.J.; Soodyall, H.; Comas, D.; Goldman, D.; Odunsi, A.; Okonofua, F.; Parnas, J.; Schulz, L.O.; Bertranpetit, J.; et al. A global perspective on genetic variation at the ADH genes reveals unusual patterns of linkage disequilibrium and diversity. Am. J. Hum. Genet. 2002, 71, 84–99. [Google Scholar] [CrossRef]
- Ji, Y.B.; Lee, S.H.; Kim, K.R.; Park, C.W.; Song, C.M.; Park, B.L.; Shin, H.D.; Tae, K. Association between ADH1B and ADH1C polymorphisms and the risk of head and neck squamous cell carcinoma. Tumor Biol. 2015, 36, 4387–4396. [Google Scholar] [CrossRef]
- Ji, Y.B.; Tae, K.; Ahn, T.H.; Lee, S.H.; Kim, K.R.; Park, C.W.; Park, B.L.; Shin, H.D. ADH1B and ALDH2 polymorphisms and their associations with increased risk of squamous cell carcinoma of the head and neck in the Korean population. Oral Oncol. 2011, 47, 583–587. [Google Scholar] [CrossRef]
- Hakenewerth, A.M.; Millikan, R.C.; Rusyn, I.; Herring, A.H.; Weissler, M.C.; Funkhouser, W.K.; North, K.E.; Barnholtz-Sloan, J.S.; Olshan, A.F. Effects of polymorphisms in alcohol metabolism and oxidative stress genes on survival from head and neck cancer. Cancer Epidemiol. 2013, 37, 479–491. [Google Scholar] [CrossRef]
- Wei, S.; Liu, Z.; Zhao, H.; Niu, J.; Wang, L.-E.; El-Naggar, A.K.; Sturgis, E.M.; Wei, Q. A single nucleotide polymorphism in the alcohol dehydrogenase 7 gene (alanine to glycine substitution at amino acid 92) is associated with the risk of squamous cell carcinoma of the head and neck. Cancer 2010, 116, 2984–2992. [Google Scholar] [CrossRef]
- Hakenewerth, A.M.; Millikan, R.C.; Rusyn, I.; Herring, A.H.; North, K.E.; Barnholtz-Sloan, J.S.; Funkhouser, W.F.; Weissler, M.C.; Olshan, A.F. Joint effects of alcohol consumption and polymorphisms in alcohol and oxidative stress metabolism genes on risk of head and neck cancer. Cancer Epidemiol. Biomark. Prev. 2011, 20, 2438–2449. [Google Scholar] [CrossRef]
- Oze, I.; Matsuo, K.; Suzuki, T.; Kawase, T.; Watanabe, M.; Hiraki, A.; Ito, H.; Hosono, S.; Ozawa, T.; Hatooka, S.; et al. Impact of multiple alcohol dehydrogenase gene polymorphisms on risk of upper aerodigestive tract cancers in a Japanese population. Cancer Epidemiol. Biomark. Prev. 2009, 18, 3097–3102. [Google Scholar] [CrossRef] [PubMed]
- Anantharaman, D.; Chabrier, A.; Gaborieau, V.; Franceschi, S.; Herrero, R.; Rajkumar, T.; Samant, T.; Mahimkar, M.B.; Brennan, P.; McKay, J.D. Genetic variants in nicotine addiction and alcohol metabolism genes, oral cancer risk and the propensity to smoke and drink alcohol: A replication study in India. PLoS ONE 2014, 9, e88240. [Google Scholar] [CrossRef] [PubMed]
- Hashibe, M.; McKay, J.D.; Curado, M.P.; Oliveira, J.C.; Koifman, S.; Koifman, R.; Zaridze, D.; Shangina, O.; Wünsch-Filho, V.; Eluf-Neto, J.; et al. Multiple ADH genes are associated with upper aerodigestive cancers. Nat. Genet. 2008, 40, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Cadoni, G.; Boccia, S.; Petrelli, L.; Di Giannantonio, P.; Arzani, D.; Giorgio, A.; De Feo, E.; Pandolfini, M.; Gallì, P.; Paludetti, G.; et al. A review of genetic epidemiology of head and neck cancer related to polymorphisms in metabolic genes, cell cycle control and alcohol metabolism. Acta Otorhinolaryngol. Ital. 2012, 32, 1–11. [Google Scholar]
- Wang, J.; Wei, J.; Xu, X.; Pan, W.; Ge, Y.; Zhou, C.; Liu, C.; Gao, J.; Yang, M.; Mao, W. Replication study of ESCC susceptibility genetic polymorphisms locating in the ADH1B-ADH1C-ADH7 cluster identified by GWAS. PLoS ONE 2014, 9, e94096. [Google Scholar] [CrossRef]
| Variables | Case | Control | p-Value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Age (year) | <0.001 | ||||
| ≤45 | 23 | (9.2) | 197 | (61.2) | |
| 45–60 | 73 | (29.2) | 88 | (27.3) | |
| ≥60 | 154 | (61.6) | 37 | (11.5) | |
| Sex | 0.002 | ||||
| Male | 219 | (87.6) | 305 | (94.7) | |
| Female | 31 | (12.4) | 17 | (5.3) | |
| Smoking | <0.001 | ||||
| None | 43 | (17.2) | 1 | (0.3) | |
| Light * | 44 | (17.6) | 82 | (25.5) | |
| Heavy † | 163 | (65.2) | 239 | (74.2) | |
| Alcohol status | <0.001 | ||||
| None | 93 | (37.2) | 89 | (27.6) | |
| Social ‡ | 53 | (21.2) | 149 | (46.3) | |
| Heavy § | 104 | (41.6) | 84 | (26.1) | |
| 250 | 322 | ||||
| Gene | Locus | Primer Name | Primer Sequence |
|---|---|---|---|
| ADH7 | rs1573496_CG | rs1573496_CG_F | TCGCTCCTAATGCAAAGGTT |
| rs1573496_CG_R | CAGATTTTGGCCACAGGAAT | ||
| rs1573496_CG_AM21 | CCTGGTTTCACTGTAGTCACT | ||
| rs3737482 _TC | rs3737482 _TC_F | TCGCTCCTAATGCAAAGGTT | |
| rs3737482 _TC_R | CAGATTTTGGCCACAGGAAT | ||
| rs3737482 _TC_AM27 | TCTAAGGTTTATAAACACCGTAAGGTT | ||
| rs1154460_GA | rs1154460_GA_F | TTTGGTCCAGTGAACCTGCT | |
| rs1154460_GA_R | ATTGGGCATCTTGAAACCAT | ||
| rs1154460_GA_AM38 | ATAATAAAGTTATAGACAATTAAATTTACCTTGTGCAT | ||
| rs284787_TC | rs284787_TC_F | ACAAGGCTATTTGCCAGCAT | |
| rs284788_TC_R | AAAAACACTTTTTATTAAATGGAGTCA | ||
| rs284789_TC_AM32 | TGTTCAATTTGATACAGTAGAATTGCAAGTCC |
| Gene | Locus | Amino Acid Change | Genotype | Frequency | HWE * | |||
|---|---|---|---|---|---|---|---|---|
| ADH7 | rs1573496C>G | Gly92Ala | C | CG | G | N | 0.001 | 0.983 |
| 571 | 1 | 0 | 572 | |||||
| rs3737482T>C | T | CT | C | N | 0.451 | 0.783 | ||
| 174 | 280 | 118 | 572 | |||||
| rs1154460G>A | G | AG | A | N | 0.356 | 0.177 | ||
| 230 | 277 | 65 | 572 | |||||
| rs284787T>C | T | CT | C | N | 0.344 | 0.690 | ||
| 248 | 254 | 70 | 572 | |||||
| Loci | GenoType | Distribution | Referent Analysis | Codominant Analysis | Dominant Analysis | Recessive Analysis | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Case (%) | Control (%) | OR * (95% CI †) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | ||
| rs1573496C>G | CC | 250 (100.0) | 321 (99.7) | 1 | |||||||
| CG | 0 (0.0) | 1 (0.3) | |||||||||
| GG | 0 (0.0) | 0 (0.0) | |||||||||
| rs3737482T>C | TT | 93 (37.2) | 81 (25.2) | 1 | |||||||
| CT | 111 (44.4) | 169 (52.5) | 0.48 (0.29–0.78) | 0.003 | 0.66 (0.49–0.90) | 0.008 | 0.47 (0.30–0.75) | 0.002 | 0.76 (0.44–1.29) | 0.31 | |
| CC | 46 (18.4) | 72 (22.4) | 0.69 (0.49–0.96) | 0.03 | |||||||
| rs1154460G>A | GG | 94 (37.6) | 136 (42.2) | 1 | |||||||
| AG | 120 (48.0) | 157 (48.8) | 1.31 (0.83–2.06) | 0.25 | 1.47 (1.06–2.04) | 0.02 | 1.49 (0.96–2.32) | 0.08 | 2.03 (1.05–3.92) | 0.04 | |
| AA | 36 (14.4) | 29 (9.0) | 1.63 (1.11–2.40) | 0.01 | |||||||
| rs284787T>C | TT | 101 (40.4) | 147 (45.7) | 1 | |||||||
| CT | 118 (47.2) | 136 (42.2) | 1.43 (0.90–2.28) | 0.13 | 1.32 (0.97–1.80) | 0.08 | 1.47 (0.95–2.28) | 0.08 | 1.38 (0.74–2.58) | 0.32 | |
| CC | 31 (12.4) | 39 (12.1) | 1.24 (0.87–1.74) | 0.23 | |||||||
| Gene | Alcohol | Genotype | Cancer | Normal | OR * (95% CI †) | p |
|---|---|---|---|---|---|---|
| ADH7 rs3737482T>C | Non-drinker (n = 182) | TT | 26 (28.0%) | 21 (23.6%) | 1 | |
| CT | 44 (47.3%) | 42 (47.2%) | 0.59 (0.21–1.68) | 0.322 | ||
| CC | 23 (24.7%) | 26 (29.2%) | 0.75 (0.23–2.42) | 0.628 | ||
| Drinker (n = 390) | TT | 67 (42.7%) | 60 (25.8%) | 1 | ||
| CT | 67 (42.7%) | 127 (54.5%) | 0.43 (0.42–0.77) | 0.004 | ||
| CC | 23 (14.6%) | 46 (19.7%) | 0.42 (0.19–0.94) | 0.034 | ||
| ADH7 rs1154460G>A | Non-drinker (n = 182) | GG | 43 (46.2) | 41 (46.1) | 1 | |
| AG | 39 (41.9) | 39 (43.8) | 0.80 (0.32–2.01) | 0.631 | ||
| AA | 11 (11.8) | 9 (10.1) | 1.25 (0.30–5.27) | 0.760 | ||
| Drinker (n = 390) | GG | 51 (32.5%) | 95 (40.8%) | 1 | ||
| AG | 81 (51.6%) | 118 (50.6%) | 1.55 (0.87–2.75) | 0.136 | ||
| AA | 25 (15.9%) | 20 (8.6%) | 3.55 (1.51–8.33) | 0.004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.-W.; Ji, Y.-B.; Song, C.-M.; Kim, J.-K.; Lee, S.-H.; Tae, K. Impact of Alcohol Dehydrogenase 7 Polymorphism and Alcohol Consumption on Risk of Head and Neck Squamous Cell Carcinoma: A Korean Case-Control Study. J. Clin. Med. 2023, 12, 4653. https://doi.org/10.3390/jcm12144653
Lee D-W, Ji Y-B, Song C-M, Kim J-K, Lee S-H, Tae K. Impact of Alcohol Dehydrogenase 7 Polymorphism and Alcohol Consumption on Risk of Head and Neck Squamous Cell Carcinoma: A Korean Case-Control Study. Journal of Clinical Medicine. 2023; 12(14):4653. https://doi.org/10.3390/jcm12144653
Chicago/Turabian StyleLee, Dong-Won, Yong-Bae Ji, Chang-Myeon Song, Jeong-Kyu Kim, Seung-Hwan Lee, and Kyung Tae. 2023. "Impact of Alcohol Dehydrogenase 7 Polymorphism and Alcohol Consumption on Risk of Head and Neck Squamous Cell Carcinoma: A Korean Case-Control Study" Journal of Clinical Medicine 12, no. 14: 4653. https://doi.org/10.3390/jcm12144653
APA StyleLee, D.-W., Ji, Y.-B., Song, C.-M., Kim, J.-K., Lee, S.-H., & Tae, K. (2023). Impact of Alcohol Dehydrogenase 7 Polymorphism and Alcohol Consumption on Risk of Head and Neck Squamous Cell Carcinoma: A Korean Case-Control Study. Journal of Clinical Medicine, 12(14), 4653. https://doi.org/10.3390/jcm12144653





