Novel Biomarkers of Gastric Cancer: Current Research and Future Perspectives
Abstract
1. Introduction
2. Biomarkers to Predict Responses to Cytotoxic Antitumor Drugs for Gastric Cancer
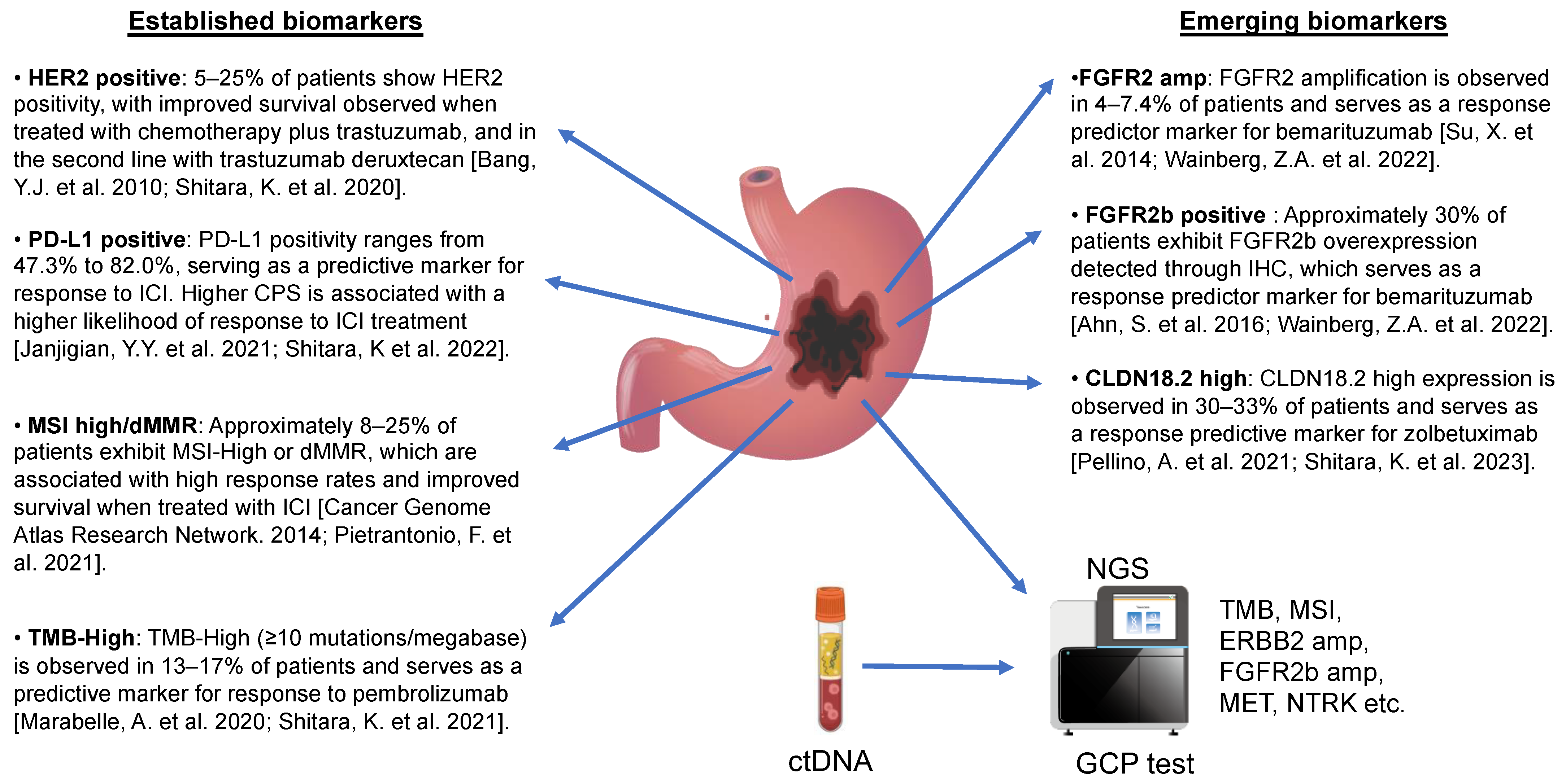
3. Established Molecular Biomarkers in Gastric Cancer
3.1. Human Epidermal Growth Factor Receptor 2 (HER2)
3.1.1. Molecular Characteristics of HER2
3.1.2. HER2 Expression in Gastric Cancer
3.1.3. HER2-Targeted Therapy
3.2. Biomarkers in the Treatment of ICIs in Advanced Gastric Cancer
3.2.1. PD-L1
Molecular Characteristics of PD-L1
PD-L1 Expression in Gastric Cancer
PD-1-Targeted Therapy
3.2.2. MSI-H/dMMR
Detection of MSI-H/dMMR
Clinical Significance of MSI-H/dMMR
3.2.3. Tumor Mutational Burden
Clinical Significance of TMB
Assessment of TMB
3.2.4. Immune-Related Adverse Events
4. Emerging Biomarkers
4.1. Fibroblast Growth Factor Receptor 2 (FGFR2)
4.1.1. Molecular Characteristics of FGFR2
4.1.2. Assessment of FGFR2 Status
4.1.3. FGFR2-Targeted Therapy
4.2. Claudin 18.2
4.2.1. Molecular Characteristics of Claudin 18.2
4.2.2. Clinical Significance of Claudin 18.2
4.2.3. Claudin 18.2-Targeted Therapy
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- GBD 2017 Stomach Cancer Collaborators. The global, regional, and national burden of stomach cancer in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 42–54. [Google Scholar] [CrossRef]
- Wroblewski, L.E.; Peek, R.M.J.; Wilson, K.T. Helicobacter pylori and gastric cancer: Factors that modulate disease risk. Clin. Microbiol. Rev. 2010, 23, 713–739. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Barsouk, A. Epidemiology of gastric cancer: Global trends, risk factors and prevention. Prz. Gastroenterol. 2019, 14, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Obermannová, R.; Alsina, M.; Cervantes, A.; Leong, T.; Lordick, F.; Nilsson, M.; van Grieken, N.C.T.; Vogel, A.; Smyth, E.C.; ESMO Guidelines Committee. Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 992–1004. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.S.; Choi, W.; Eom, B.W.; Park, S.H.; Kim, S.J.; Il Kim, Y.I.; Yoon, H.M.; Lee, J.Y.; Kim, C.G.; Kim, H.K.; et al. A comprehensive and comparative review of global gastric cancer treatment guidelines. J. Gastric Cancer 2022, 22, 3–23. [Google Scholar] [CrossRef]
- Parker, J.L.; Kuzulugil, S.S.; Pereverzev, K.; Mac, S.; Lopes, G.; Shah, Z.; Weerasinghe, A.; Rubinger, D.; Falconi, A.; Bener, A.; et al. Does biomarker use in oncology improve clinical trial failure risk? A large—Scale analysis. Cancer Med. 2021, 10, 1955–1963. [Google Scholar] [CrossRef]
- Henry, N.L.; Hayes, D.F. Cancer biomarkers. Mol. Oncol. 2012, 6, 140–146. [Google Scholar] [CrossRef]
- Dancey, J.E.; Dobbin, K.K.; Groshen, S.; Jessup, J.M.; Hruszkewycz, A.H.; Koehler, M.; Parchment, R.; Ratain, M.J.; Shankar, L.K.; Stadler, W.M.; et al. Guidelines for the development and incorporation of biomarker studies in early clinical trials of novel agents. Clin. Cancer Res. 2010, 16, 1745–1755. [Google Scholar] [CrossRef]
- Janiaud, P.; Serghiou, S.; Ioannidis, J.P.A. New clinical trial designs in the era of precision medicine: An overview of de fi nitions, strengths, weaknesses, and current use in oncology. Cancer Treat. Rev. 2019, 73, 20–30. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Sohn, B.H.; Hwang, J.E.; Jang, H.J.; Lee, H.S.; Oh, S.C.; Shim, J.J.; Lee, K.W.; Kim, E.H.; Yim, S.Y.; Lee, S.H.; et al. Clinical significance of four molecular subtypes of gastric cancer identified by the cancer genome atlas project. Clin. Cancer Res. 2017, 23, 4441–4449. [Google Scholar] [CrossRef] [PubMed]
- Derks, S.; de Klerk, L.K.; Xu, X.; Fleitas, T.; Liu, K.X.; Liu, Y.; Dietlein, F.; Margolis, C.; Chiaravalli, A.M.; Da Silva, A.C.; et al. Characterizing diversity in the tumor-immune microenvironment of distinct subclasses of gastroesophageal adenocarcinomas. Ann. Oncol. 2020, 31, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Kawazoe, A.; Lordick, F.; Janjigian, Y.Y.; Shitara, K. Biomarker-targeted therapies for advanced-stage gastric and gastro-oesophageal junction cancers: An emerging paradigm. Nat. Rev. Clin. Oncol. 2021, 18, 473–487. [Google Scholar] [CrossRef]
- Catenacci, D.V.; Tesfaye, A.; Tejani, M.; Cheung, E.; Eisenberg, P.; Scott, A.J.; Eng, C.; Hnatyszyn, J.; Marina, N.; Powers, J.; et al. Bemarituzumab with modified FOLFOX6 for advanced FGFR2-positive gastroesophageal cancer: FIGHT Phase III study design. Future Oncol. 2019, 15, 2073–2082. [Google Scholar] [CrossRef]
- Sahin, U.; Türeci, Ö.; Manikhas, G.; Lordick, F.; Rusyn, A.; Vynnychenko, I.; Dudov, A.; Bazin, I.; Bondarenko, I.; Melichar, B.; et al. FAST: A randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann. Oncol. 2021, 32, 609–619. [Google Scholar] [CrossRef]
- Metzger, R.; Leichman, C.G.; Danenberg, K.D.; Danenberg, P.V.; Lenz, H.J.; Hayashi, K.; Groshen, S.; Salonga, D.; Cohen, H.; Laine, L.; et al. ERCC1 mRNA levels complement thymidylate synthase mRNA levels in predicting response and survival for gastric cancer patients receiving combination cisplatin and fluorouracil chemotherapy. J. Clin. Oncol. 1998, 16, 309–316. [Google Scholar] [CrossRef]
- Wei, J.; Zou, Z.; Qian, X.; Ding, Y.; Xie, L.; Sanchez, J.J.; Zhao, Y.; Feng, J.; Ling, Y.; Liu, Y.; et al. ERCC1 mRNA levels and survival of advanced gastric cancer patients treated with a modified FOLFOX regimen. Br. J. Cancer 2008, 98, 1398–1402. [Google Scholar] [CrossRef]
- Fareed, K.R.; Al-Attar, A.; Soomro, I.N.; Kaye, P.V.; Patel, J.; Lobo, D.N.; Parsons, S.L.; Madhusudan, S. Tumour regression and ERCC1 nuclear protein expression predict clinical outcome in patients with gastro-oesophageal cancer treated with neoadjuvant chemotherapy. Br. J. Cancer 2010, 102, 1600–1607. [Google Scholar] [CrossRef]
- Hirakawa, M.; Sato, Y.; Ohnuma, H.; Takayama, T.; Sagawa, T.; Nobuoka, T.; Harada, K.; Miyamoto, H.; Sato, Y.; Takahashi, Y.; et al. A phase II study of neoadjuvant combination chemotherapy with docetaxel, cisplatin, and S-1 for locally advanced resectable gastric cancer: Nucleotide excision repair (NER) as potential chemoresistance marker. Cancer Chemother. Pharmacol. 2013, 71, 789–797. [Google Scholar] [CrossRef]
- Diasio, R.B. Oral DPD-inhibitory fluoropyrimidine drugs. Oncology 2000, 14 (Suppl. S9), 19–23. [Google Scholar] [PubMed]
- Terashima, M.; Irinoda, T.; Fujiwara, H.; Nakaya, T.; Takagane, A.; Abe, K.; Yonezawa, H.; Oyama, K.; Inaba, T.; Saito, K.; et al. Roles of thymidylate synthase and dihydropyrimidine dehydrogenase in tumor progression and sensitivity to 5-fluorouracil in human gastric cancer. Anticancer. Res. 2002, 22, 761–768. [Google Scholar] [PubMed]
- Napieralski, R.; Ott, K.; Kremer, M.; Specht, K.; Vogelsang, H.; Becker, K.; Müller, M.; Lordick, F.; Fink, U.; Rüdiger Siewert, J.; et al. Combined GADD45A and thymidine phosphorylase expression levels predict response and survival of neoadjuvant-treated gastric cancer patients. Clin. Cancer Res. 2005, 11, 3025–3031. [Google Scholar] [CrossRef]
- Wang, W.; Cassidy, J.; O’Brien, V.; Ryan, K.M.; Collie-Duguid, E. Mechanistic and predictive profiling of 5-fluorouracil resistance in human cancer cells. Cancer Res. 2004, 64, 8167–8176. [Google Scholar] [CrossRef]
- Wang, D.; Yu, X.; Wang, X. High/positive expression of 5-fluorouracil metabolic enzymes predicts better response to S-1 in patients with gastric cancer: A meta-analysis. Int. J. Biol. Markers 2016, 31, e101–e109. [Google Scholar] [CrossRef]
- Akiyama, T.; Sudo, C.; Ogawara, H.; Toyoshima, K.; Yamamoto, T. The product of the human c-erbB-2 gene: A 185-kilodalton glycoprotein with tyrosine kinase activity. Science 1986, 232, 1644–1646. [Google Scholar] [CrossRef]
- Rubin, I.; Yarden, Y. The basic biology of HER2. Ann. Oncol. 2001, 12 (Suppl. S1), S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Werner, D.; Pauligk, C.; Steinmetz, K.; Kelsen, D.P.; Jäger, E.; Altmannsberger, H.M.; Robinson, E.; Tafe, L.J.; Tang, L.H.; et al. Prognosis of metastatic gastric and gastroesophageal junction cancer by HER2 status: A European and USA International collaborative analysis. Ann. Oncol. 2012, 23, 2656–2662. [Google Scholar] [CrossRef]
- Abrahao-Machado, L.F.; Scapulatempo-Neto, C. HER2 testing in gastric cancer: An update. World J. Gastroenterol. 2016, 22, 4619–4625. [Google Scholar] [CrossRef]
- Yu, D.H.; Tang, L.; Dong, H.; Dong, Z.; Zhang, L.; Fu, J.; Su, X.; Zhang, T.; Fu, H.; Han, L.; et al. Oncogenic HER2 fusions in gastric cancer. J. Transl. Med. 2015, 13, 116. [Google Scholar] [CrossRef]
- Cho, E.Y.; Srivastava, A.; Park, K.; Kim, J.; Lee, M.H.; Do, I.; Lee, J.; Kim, K.M.; Sohn, T.S.; Kang, W.K.; et al. Comparison of four immunohistochemical tests and FISH for measuring HER2 expression in gastric carcinomas. Pathology 2012, 44, 216–220. [Google Scholar] [CrossRef]
- Matsumoto, T.; Sasako, M.; Mizusawa, J.; Hirota, S.; Ochiai, A.; Kushima, R.; Katai, H.; Tanaka, Y.; Fukushima, N.; Nashimoto, A.; et al. HER2 expression in locally advanced gastric cancer with extensive lymph node (bulky N2 or paraaortic) metastasis (JCOG1005-A trial). Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2015, 18, 467–475. [Google Scholar] [CrossRef]
- Alessandrini, L.; Manchi, M.; De Re, V.; Dolcetti, R.; Canzonieri, V. Proposed molecular and miRNA classification of gastric cancer. Int. J. Mol. Sci. 2018, 19, 1683. [Google Scholar] [CrossRef]
- Bartley, A.N.; Washington, M.K.; Ismaila, N.; Ajani, J.A. HER2 testing and clinical decision making in gastroesophageal adenocarcinoma: Guideline summary from the College of American Pathologists, American Society for Clinical Pathology, and American Society of Clinical Oncology. J. Oncol. Pract. 2017, 13, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Rüschoff, J.; Hanna, W.; Bilous, M.; Hofmann, M.; Osamura, R.Y.; Penault-Llorca, F.; van de Vijver, M.; Viale, G. HER2 testing in gastric cancer: A practical approach. Mod. Pathol. 2012, 25, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.Y.; Park, K.; Do, I.; Cho, J.; Kim, J.; Lee, J.; Kim, S.; Kim, K.M.; Sohn, T.S.; Kang, W.K.; et al. Heterogeneity of ERBB2 in gastric carcinomas: A study of tissue microarray and matched primary and metastatic carcinomas. Mod. Pathol. 2013, 26, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Ahn, S.; Van Vrancken, M.; Lee, M.; Ha, S.Y.; Lee, H.; Min, B.H.; Lee, J.H.; Kim, J.J.; Choi, S.; et al. Ideal number of biopsy tumor fragments for predicting HER2 status in gastric carcinoma resection specimens. Oncotarget 2015, 6, 38372–38380. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Chu, J.; Kim, B.; Ha, S.Y.; Kim, S.T.; Lee, J.; Kang, W.K.; Han, H.; Sohn, I.; Kim, K.M. Tumor heterogeneity index to detect human epidermal growth factor Receptor 2 amplification by next-generation sequencing: A direct comparison study with immunohistochemistry. J. Mol. Diagn. 2019, 21, 612–622. [Google Scholar] [CrossRef]
- Maron, S.B.; Chase, L.M.; Lomnicki, S.; Kochanny, S.; Moore, K.L.; Joshi, S.S.; Landron, S.; Johnson, J.; Kiedrowski, L.A.; Nagy, R.J.; et al. Circulating tumor DNA sequencing analysis of gastroesophageal adenocarcinoma. Clin. Cancer Res. 2019, 25, 7098–7112. [Google Scholar] [CrossRef]
- Cheng, M.L.; Pectasides, E.; Hanna, G.J.; Parsons, H.A.; Choudhury, A.D.; Oxnard, G.R. Circulating tumor DNA in advanced solid tumors: Clinical relevance and future directions. CA Cancer J. Clin. 2021, 71, 176–190. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Maron, S.B.; Chatila, W.K.; Millang, B.; Chavan, S.S.; Alterman, C.; Chou, J.F.; Segal, M.F.; Simmons, M.Z.; Momtaz, P.; et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: An open-label, single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 821–831. [Google Scholar] [CrossRef]
- Shitara, K.; Yatabe, Y.; Matsuo, K.; Sugano, M.; Kondo, C.; Takahari, D.; Ura, T.; Tajika, M.; Ito, S.; Muro, K. Prognosis of patients with advanced gastric cancer by HER2 status and trastuzumab treatment. Gastric Cancer 2013, 16, 261–267. [Google Scholar] [CrossRef]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastrooesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Kurokawa, Y.; Matsuura, N.; Kimura, Y.; Adachi, S.; Fujita, J.; Imamura, H.; Kobayashi, K.; Yokoyama, Y.; Shaker, M.N.; Takiguchi, S.; et al. Multicenter large-scale study of prognostic impact of HER2 expression in patients with resectable gastric cancer. Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2015, 18, 691–697. [Google Scholar] [CrossRef]
- Kim, K.C.; Koh, Y.W.; Chang, H.M.; Kim, T.H.; Yook, J.H.; Kim, B.S.; Jang, S.J.; Park, Y.S. Evaluation of HER2 protein expression in gastric carcinomas: Comparative analysis of 1,414 cases of whole-tissue sections and 595 cases of tissue microarrays. Ann. Surg. Oncol. 2011, 18, 2833–2840. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, X.; Wei, X.; Tang, C.; Zhang, W. HER2-targeted therapies in gastric cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188549. [Google Scholar] [CrossRef]
- Mitani, S.; Kawakami, H. Emerging targeted therapies for HER2 positive gastric cancer that can overcome trastuzumab resistance. Cancers 2020, 12, 400. [Google Scholar] [CrossRef] [PubMed]
- Pietrantonio, F.; Caporale, M.; Morano, F.; Scartozzi, M.; Gloghini, A.; De Vita, F.; Giommoni, E.; Fornaro, L.; Aprile, G.; Melisi, D.; et al. HER2 loss in HER2-positive gastric or gastroesophageal cancer after trastuzumab therapy: Implication for further clinical research. Int. J. Cancer 2016, 139, 2859–2864. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Ryu, M.H.; Park, Y.S.; Ahn, J.Y.; Park, Y.; Park, S.R.; Ryoo, B.Y.; Lee, G.H.; Jung, H.Y.; Kang, Y.K. Loss of HER2 positivity after anti-HER2 chemotherapy in HER2-positive gastric cancer patients: Results of the GASTric cancer HER2 reassessment study 3 (GASTHER3). Gastric Cancer Off. J. Int. Gastric Cancer Assoc. Jpn. Gastric Cancer Assoc. 2019, 22, 527–535. [Google Scholar] [CrossRef]
- Makiyama, A.; Sukawa, Y.; Kashiwada, T.; Kawada, J.; Hosokawa, A.; Horie, Y.; Tsuji, A.; Moriwaki, T.; Tanioka, H.; Shinozaki, K.; et al. Randomized, Phase II study of trastuzumab beyond progression in patients with HER2-positive advanced gastric or gastroesophageal junction cancer: WJOG7112G (T-ACT study). J. Clin. Oncol. 2020, 38, 1919–1927. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Sanchez-Vega, F.; Jonsson, P.; Chatila, W.K.; Hechtman, J.F.; Ku, G.Y.; Riches, J.C.; Tuvy, Y.; Kundra, R.; Bouvier, N.; et al. Genetic predictors of response to systemic therapy in esophagogastric cancer. Cancer Discov. 2018, 8, 49–58. [Google Scholar] [CrossRef]
- Shitara, K.; Bang, Y.J.; Iwasa, S.; Sugimoto, N.; Ryu, M.H.; Sakai, D.; Chung, H.C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab deruxtecan in previously treated HER2-positive gastric cancer. N. Engl. J. Med. 2020, 382, 2419–2430. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Bang, Y.J.; Iwasa, S.; Sugimoto, N.; Ryu, M.H.; Sakai, D.; Chung, H.C.; Kawakami, H.; Yabusaki, H.; Lee, J.; et al. Trastuzumab deruxtecan in anti–human epidermal growth factor Receptor 2 treatment–naive patients with human epidermal growth factor Receptor 2–low gastric or gastroesophageal junction adenocarcinoma: Exploratory cohort results in a Phase II trial. J. Clin. Oncol. 2023, 41, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Beeram, M.; Hamilton, E.; Oh, D.Y.; Hanna, D.L.; Kang, Y.K.; Elimova, E.; Chaves, J.; Goodwin, R.; Lee, J.; et al. Zanidatamab, a novel bispecific antibody, for the treatment of locally advanced or metastatic HER2-expressing or HER2-amplified cancers: A phase 1, dose-escalation and expansion study. Lancet Oncol. 2022, 23, 1558–1570. [Google Scholar] [CrossRef] [PubMed]
- Weisser, N.E.; Sanches, M.; Escobar-Cabrera, E.; O’Toole, J.; Whalen, E.; Chan, P.W.Y.; Wickman, G.; Abraham, L.; Choi, K.; Harbourne, B.; et al. An anti-HER2 biparatopic antibody that induces unique HER2 clustering and complement-dependent cytotoxicity. Nat. Commun. 2023, 14, 1394. [Google Scholar] [CrossRef]
- Catenacci, D.V.T.; Kang, Y.K.; Park, H.; Uronis, H.E.; Lee, K.W.; Ng, M.C.H.; Enzinger, P.C.; Park, S.H.; Gold, P.J.; Lacy, J.; et al. CP-MGAH22-5 study group. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastrooesophageal adenocarcinoma (CP-MGAH22-05): A single-arm, phase 1b–2 trial. Lancet Oncol. 2020, 21, 1066–1076. [Google Scholar] [CrossRef]
- Yoshioka, T.; Shien, K.; Namba, K.; Torigoe, H.; Sato, H.; Tomida, S.; Yamamoto, H.; Asano, H.; Soh, J.; Tsukuda, K.; et al. Antitumor activity of pan-HER inhibitors in HER2-positive gastric cancer. Cancer Sci. 2018, 109, 1166–1176. [Google Scholar] [CrossRef]
- Chan, A.; Delaloge, S.; Holmes, F.A.; Moy, B.; Iwata, H.; Harvey, V.J.; Robert, N.J.; Silovski, T.; Gokmen, E.; von Minckwitz, G.; et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016, 17, 367–377. [Google Scholar] [CrossRef]
- Kulukian, A.; Lee, P.; Taylor, J.; Rosler, R.; de Vries, P.; Watson, D.; Forero-Torres, A.; Peterson, S. Preclinical activity of HER2-selective tyrosine kinase inhibitor tucatinib as a single agent or in combination with trastuzumab or docetaxel in solid tumor models. Mol. Cancer Ther. 2020, 19, 976–987. [Google Scholar] [CrossRef]
- Oh, D.-Y.; Chung, H.C.; Im, Y.H.; Yen, C.J.; Chao, Y.; Li, Z.; Wang, X.; Wang, J.; Li, H.; Kang, Y.-K. ZW25, an anti-HER2 bispecific antibody, plus chemotherapy with/without tislelizumab as first-line treatment for patients with advanced HER2-positive breast cancer or gastric/gastroesophageal junction adenocarcinoma: A phase 1B/2 trial-in-progress. J. Clin. Oncol. 2020, 38, TPS3145. [Google Scholar] [CrossRef]
- Tabernero, J.; Shen, L.; Elimova, E.; Ku, G.; Liu, T.; Shitara, K.; Lin, X.; Boyken, L.; Li, H.; Grim, J.; et al. HERIZON-GEA-01: Zanidatamab + chemo ± tislelizumab for 1L treatment of HER2-positive gastroesophageal adenocarcinoma. Future Oncol. 2022, 18, 3255–3266. [Google Scholar] [CrossRef]
- Chaganty, B.K.R.; Qiu, S.; Gest, A.; Lu, Y.; Ivan, C.; Calin, G.A.; Weiner, L.M.; Fan, Z. Trastuzumab upregulates PD-L1 as a potential mechanism of trastuzumab resistance through engagement of immune effector cells and stimulation of IFNγ secretion. Cancer Lett. 2018, 430, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Janjigian, Y.Y.; Kawazoe, A.; Yañez, P.; Li, N.; Lonardi, S.; Kolesnik, O.; Barajas, O.; Bai, Y.; Shen, L.; Tang, Y.; et al. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature 2021, 600, 727–730. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef]
- Yi, M.; Niu, M.; Xu, L.; Luo, S.; Wu, K. Regulation of PD-L1 expression in the tumor microenvironment. J. Hematol. Oncol. 2021, 14, 10. [Google Scholar] [CrossRef]
- Thompson, E.D.; Zahurak, M.; Murphy, A.; Cornish, T.; Cuka, N.; Abdelfatah, E.; Yang, S.; Duncan, M.; Ahuja, N.; Taube, J.M.; et al. Patterns of PD-L1 expression and CD8 T cell infiltration in gastric adenocarcinomas and associated immune stroma. Gut 2017, 66, 794–801. [Google Scholar] [CrossRef]
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef]
- Böger, C.; Behrens, H.-M.; Mathiak, M.; Krüger, S.; Kalthoff, H.; Röcken, C. PD-L1 is an independent prognostic predictor in gastric cancer of Western patients. Oncotarget 2016, 7, 24269–24283. [Google Scholar] [CrossRef]
- Fashoyin-Aje, L.; Donoghue, M.; Chen, H.; He, K.; Veeraraghavan, J.; Goldberg, K.B.; Keegan, P.; McKee, A.E.; Pazdur, R.F.D.A. FDA Approval summary: Pembrolizumab for recurrent locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma expressing PD-L1. Oncologist 2019, 24, 103–109. [Google Scholar] [CrossRef]
- Phillips, T.; Simmons, P.; Inzunza, H.D.; Cogswell, J.; Novotny, J.J.; Taylor, C.; Zhang, X. Development of an automated PD-L1 immunohistochemistry (IHC) assay for non-small cell lung cancer. Appl. Immunohistochem. Mol. Morphol. 2015, 23, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Cristescu, R.; Bass, A.J.; Kim, K.M.; Odegaard, J.I.; Kim, K.; Liu, X.Q.; Sher, X.; Jung, H.; Lee, M.; et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat. Med. 2018, 24, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.S.; Doi, T.; Jang, R.W.; Muro, K.; Satoh, T.; Machado, M.; Sun, W.; Jalal, S.I.; Shah, M.A.; Metges, J.P.; et al. Safety and efficacy of pembrolizumab monotherapy in patients with previously treated advanced gastric and gastroesophageal junction cancer: Phase 2 clinical KEYNOTE-059 trial. JAMA Oncol. 2018, 4, e180013. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Özgüroğlu, M.; Bang, Y.J.; Di Bartolomeo, M.; Mandalà, M.; Ryu, M.H.; Fornaro, L.; Olesiński, T.; Caglevic, C.; Chung, H.C.; et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): A randomised, open-label, controlled, phase 3 trial. Lancet 2018, 392, 123–133. [Google Scholar] [CrossRef]
- Shitara, K.; Ajani, J.A.; Moehler, M.; Garrido, M.; Gallardo, C.; Shen, L.; Yamaguchi, K.; Wyrwicz, L.; Skoczylas, T.; Bragagnoli, A.C.; et al. Nivolumab plus chemotherapy or ipilimumab in gastro-oesophageal cancer. Nature 2022, 603, 942–948. [Google Scholar] [CrossRef]
- Ahn, S.; Kim, K.-M. PD-L1 expression in gastric cancer: Interchangeability of 22C3 and 28-8 pharmDx assays for responses to immunotherapy. Mod. Pathol. Off. J. US Can. Acad. Pathol. Inc. 2021, 34, 1719–1727. [Google Scholar] [CrossRef]
- Park, Y.; Koh, J.; Na, H.Y.; Kwak, Y.; Lee, K.-W.; Ahn, S.-H.; Park, D.J.; Kim, H.-H.; Lee, H.S. PD-L1 testing in gastric cancer by the combined positive score of the 22C3 PharmDx and SP263 assay with clinically relevant cut-offs. Cancer Res. Treat. 2020, 52, 661–670. [Google Scholar] [CrossRef]
- Narita, Y.; Sasaki, E.; Masuishi, T.; Taniguchi, H.; Kadowaki, S.; Ito, S.; Yatabe, Y.; Muro, K. PD-L1 immunohistochemistry comparison of 22C3 and 28–8 assays for gastric cancer. J. Gastrointest. Oncol. 2021, 12, 2696–2705. [Google Scholar] [CrossRef]
- Yamashita, K.; Iwatsuki, M.; Harada, K.; Koga, Y.; Kiyozumi, Y.; Eto, K.; Hiyoshi, Y.; Ishimoto, T.; Iwagami, S.; Baba, Y.; et al. Can PD-L1 expression evaluated by biopsy sample accurately reflect its expression in the whole tumour in gastric cancer? Br. J. Cancer 2019, 121, 278–280. [Google Scholar] [CrossRef]
- Ye, M.; Huang, D.; Zhang, Q.; Weng, W.; Tan, C.; Qin, G.; Jiang, W.; Sheng, W.; Wang, L. Heterogeneous programmed death-ligand 1 expression in gastric cancer: Comparison of tissue microarrays and whole sections. Cancer Cell Int. 2020, 20, 186. [Google Scholar] [CrossRef]
- Zhou, K.I.; Peterson, B.; Serritella, A.; Thomas, J.; Reizine, N.; Moya, S.; Tan, C.; Wang, Y.; Catenacci, D.V.T. Spatial and temporal heterogeneity of PD-L1 expression and tumor mutational burden in gastroesophageal adenocarcinoma at baseline diagnosis and after chemotherapy. Clin. Cancer Res. 2020, 26, 6453–6463. [Google Scholar] [CrossRef]
- Inge, L.J.; Dennis, E. Development and applications of computer image analysis algorithms for scoring of PD-L1 immunohistochemistry. Immunooncol. Technol. 2020, 6, 2–8. [Google Scholar] [CrossRef]
- Yoon, H.H.; Jin, Z.; Kour, O.; Kankeu Fonkoua, L.A.; Shitara, K.; Gibson, M.K.; Prokop, L.J.; Moehler, M.; Kang, Y.K.; Shi, Q.; et al. Association of PD-L1 expression and other variables with benefit from immune checkpoint inhibition in advanced gastroesophageal cancer: Systematic Review and Meta-analysis of 17 Phase 3 Randomized Clinical Trials. JAMA Oncol. 2022, 8, 1456–1465. [Google Scholar] [CrossRef]
- Shitara, K.; Van Cutsem, E.; Bang, Y.J.; Fuchs, C.; Wyrwicz, L.; Lee, K.W.; Kudaba, I.; Garrido, M.; Chung, H.C.; Lee, J.; et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: The KEYNOTE-062 Phase 3 randomized clinical trial. JAMA Oncol. 2020, 6, 1571–1580. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Fuchs, C.S.; Tabernero, J.; Shitara, K.; Muro, K.; Van Cutsem, E.; Bang, Y.J.; Chung, H.C.; Yamaguchi, K.; Varga, E.; et al. Efficacy of pembrolizumab monotherapy for advanced gastric/gastroesophageal junction cancer with programmed death ligand 1 combined positive score ≥ 10. Clin. Cancer Res. 2021, 27, 1923–1931. [Google Scholar] [CrossRef]
- Zhao, J.J.; Yap, D.W.T.; Chan, Y.H.; Tan, B.K.J.; Teo, C.B.; Syn, N.L.; Smyth, E.C.; Soon, Y.Y.; Sundar, R. Low programmed death-ligand 1–expressing subgroup outcomes of first-line immune checkpoint inhibitors in gastric or esophageal adenocarcinoma. J. Clin. Oncol. 2022, 40, 392–402. [Google Scholar] [CrossRef]
- Baretti, M.; Le, D.T. DNA mismatch repair in cancer. Pharmacol. Ther. 2018, 189, 45–62. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, T.A.; Erie, D.A. DNA MISMATCH REPAIR. Annu. Rev. Biochem. 2005, 74, 681–710. [Google Scholar] [CrossRef] [PubMed]
- Vilar, E.; Gruber, S.B. Microsatellite instability in colorectal cancer-the stable evidence. Nat. Rev. Clin. Oncol. 2010, 7, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Mathiak, M.; Warneke, V.S.; Behrens, H.M.; Haag, J.; Böger, C.; Krüger, S.; Röcken, C. Clinicopathologic characteristics of microsatellite instable gastric carcinomas revisited: Urgent need for standardization. Appl. Immunohistochem. Mol. Morphol. 2017, 25, 12–24. [Google Scholar] [CrossRef]
- Li, K.; Luo, H.; Huang, L.; Luo, H.; Zhu, X. Microsatellite instability: A review of what the oncologist should know. Cancer Cell Int. 2020, 20, 16. [Google Scholar] [CrossRef]
- Smyth, E.C.; Wotherspoon, A.; Peckitt, C.; Gonzalez, D.; Hulkki-Wilson, S.; Eltahir, Z.; Fassan, M.; Rugge, M.; Valeri, N.; Okines, A.; et al. Mismatch repair deficiency, microsatellite instability, and survival: An exploratory analysis of the Medical Research Council adjuvant gastric infusional chemotherapy (MAGIC) trial. JAMA Oncol. 2017, 3, 1197–1203. [Google Scholar] [CrossRef]
- Kim, J.Y.; Shin, N.R.; Kim, A.; Lee, H.J.; Park, W.Y.; Kim, J.Y.; Lee, C.H.; Huh, G.Y.; Park, D.Y. Microsatellite instability status in gastric cancer: A reappraisal of its clinical significance and relationship with mucin phenotypes. Korean J. Pathol. 2013, 47, 28–35. [Google Scholar] [CrossRef]
- Cristescu, R.; Lee, J.; Nebozhyn, M.; Kim, K.M.; Ting, J.C.; Wong, S.S.; Liu, J.; Yue, Y.G.; Wang, J.; Yu, K.; et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat. Med. 2015, 21, 449–456. [Google Scholar] [CrossRef]
- Zubarayev, M.; Min, E.K.; Son, T. Clinical and molecular prognostic markers of survival after surgery for gastric cancer: Tumor-node-metastasis staging system and beyond. Transl. Gastroenterol. Hepatol. 2019, 4, 59. [Google Scholar] [CrossRef]
- Smyth, E.C.; Nilsson, M.; Grabsch, H.I.; van Grieken, N.C.; Lordick, F. Gastric cancer. Lancet 2020, 396, 635–648. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Raimondi, A.; Choi, Y.Y.; Kang, W.; Langley, R.E.; Kim, Y.W.; Kim, K.-M.; Nankivell, M.G.; Perrone, F.; Kook, M.-C.; et al. MSI-GC-01: Individual patient data (IPD) meta-analysis of microsatellite instability (MSI) and gastric cancer (GC) from four randomized clinical trials (RCTs). J. Clin. Oncol. 2019, 37, 66. [Google Scholar] [CrossRef]
- Al-Batran, S.-E.; Lorenzen, S.; Homann, N.; Thuss-Patience, P.C.; Schenk, M.; Lindig, U.; Kretzschmar, A.; Heuer, V.; Goekkurt, E.; Haag, G.M.; et al. Pathological regression in patients with microsatellite instability (MSI) receiving perioperative atezolizumab in combination with FLOT vs. FLOT alone for resectable esophagogastric adenocarcinoma: Results from the Dante trial of the German Gastric. Ann. Oncol. 2021, 32. [Google Scholar] [CrossRef]
- Andre, T.; Tougeron, D.; Piessen, G.; De La Fouchardiere, C.; Louvet, C.; Adenis, A.; Jary, M.; Tournigand, C.; Aparicio, T.; Desrame, J.; et al. Neoadjuvant nivolumab plus ipilimumab and adjuvant nivolumab in patients (pts) with localized microsatellite instability-high (MSI)/mismatch repair deficient (dMMR) oeso-gastric adenocarcinoma (OGA): The GERCOR NEONIPIGA phase II study. J. Clin. Oncol. 2022, 40, 244. [Google Scholar] [CrossRef]
- Pietrantonio, F.; Randon, G.; Di Bartolomeo, M.; Luciani, A.; Chao, J.; Smyth, E.C.; Petrelli, F. Predictive role of microsatellite instability for PD-1 blockade in patients with advanced gastric cancer: A meta-analysis of randomized clinical trials. ESMO Open 2021, 6, 100036. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Campos Bragagnoli, A.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef]
- Yarchoan, M.; Johnson III, B.A.; Lutz, E.R.; Laheru, D.A.; Jaffee, E.M. Targeting neoantigens to augment antitumour immunity. Nat. Rev. Cancer 2017, 17, 209–222. [Google Scholar] [CrossRef]
- Yarchoan, M.; Albacker, L.A.; Hopkins, A.C.; Montesion, M.; Murugesan, K.; Vithayathil, T.T.; Zaidi, N.; Azad, N.S.; Laheru, D.A.; Frampton, G.M.; et al. PD-L1 expression and tumor mutational burden are independent biomarkers in most cancers. JCI Insight 2019, 4, e126908. [Google Scholar] [CrossRef] [PubMed]
- Sha, D.; Jin, Z.; Budczies, J.; Kluck, K.; Stenzinger, A.; Sinicrope, F.A. Tumor mutational burden as a predictive biomarker in solid tumors. Cancer Discov. 2020, 10, 1808–1825. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; Fakih, M.; Lopez, J.; Shah, M.; Shapira-Frommer, R.; Nakagawa, K.; Chung, H.C.; Kindler, H.L.; Lopez-Martin, J.A.; Miller, W.H.J.; et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: Prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020, 21, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Özgüroğlu, M.; Bang, Y.J.; Di Bartolomeo, M.; Mandalà, M.; Ryu, M.H.; Caglevic, C.; Chung, H.C.; Muro, K.; Van Cutsem, E.; et al. Molecular determinants of clinical outcomes with pembrolizumab versus paclitaxel in a randomized, open-label, phase III trial in patients with gastroesophageal adenocarcinoma. Ann. Oncol. 2021, 32, 1127–1136. [Google Scholar] [CrossRef]
- Caputo, V.; Ciardiello, F.; Della Corte, C.M.D.; Martini, G.; Troiani, T.; Napolitano, S. Diagnostic value of liquid biopsy in the era of precision medicine: 10 years of clinical evidence in cancer. Explor. Target. Antitumor Ther. 2023, 4, 102–138. [Google Scholar] [CrossRef]
- Wang, Z.; Duan, J.; Cai, S.; Han, M.; Dong, H.; Zhao, J.; Zhu, B.; Wang, S.; Zhuo, M.; Sun, J.; et al. Assessment of blood tumor mutational burden as a potential biomarker for immunotherapy in patients with non-small cell lung cancer with use of a next-generation sequencing cancer gene panel. JAMA Oncol. 2019, 5, 696–702. [Google Scholar] [CrossRef]
- Merino, D.M.; McShane, L.M.; Fabrizio, D.; Funari, V.; Chen, S.J.; White, J.R.; Wenz, P.; Baden, J.; Barrett, J.C.; Chaudhary, R.; et al. Establishing guidelines to harmonize tumor mutational burden (TMB): In silico assessment of variation in TMB quantification across diagnostic platforms: Phase I of the Friends of Cancer Research TMB Harmonization Project. J. Immunother. Cancer 2020, 8, e000147. [Google Scholar] [CrossRef]
- Garcia-Diaz, A.; Shin, D.S.; Moreno, B.H.; Saco, J.; Escuin-Ordinas, H.; Rodriguez, G.A.; Zaretsky, J.M.; Sun, L.; Hugo, W.; Wang, X.; et al. Interferon receptor signaling pathways regulating PD-L1 and PD-L2 expression. Cell Rep. 2017, 19, 1189–1201. [Google Scholar] [CrossRef]
- Wang, J.; Xiu, J.; Farrell, A.; Baca, Y.; Arai, H.; Battaglin, F.; Kawanishi, N.; Soni, S.; Zhang, W.; Millstein, J.; et al. Mutational analysis of microsatellite-stable gastrointestinal cancer with high tumour mutational burden: A retrospective cohort study. Lancet Oncol. 2023, 24, 151–161. [Google Scholar] [CrossRef]
- Das, S.; Johnson, D.B. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 306. [Google Scholar] [CrossRef]
- Masuda, K.; Shoji, H.; Nagashima, K.; Yamamoto, S.; Ishikawa, M.; Imazeki, H.; Aoki, M.; Miyamoto, T.; Hirano, H.; Honma, Y.; et al. Correlation between immune-related adverse events and prognosis in patients with gastric cancer treated with nivolumab. BMC Cancer 2019, 19, 974. [Google Scholar] [CrossRef]
- Katoh, M. Fibroblast growth factor receptors as treatment targets in clinical oncology. Nat. Rev. Clin. Oncol. 2019, 16, 105–122. [Google Scholar] [CrossRef]
- Hur, J.Y.; Chao, J.; Kim, K.; Kim, S.T.; Kim, K.M.; Klempner, S.J.; Lee, J. High-level FGFR2 amplification is associated with poor prognosis and Lower response to chemotherapy in gastric cancers. Pathol. Res. Pract. 2020, 216, 152878. [Google Scholar] [CrossRef]
- Su, X.; Zhan, P.; Gavine, P.R.; Morgan, S.; Womack, C.; Ni, X.; Shen, D.; Bang, Y.J.; Im, S.A.; Ho Kim, W.; et al. FGFR2 amplification has prognostic significance in gastric cancer: Results from a large international multicentre study. Br. J. Cancer 2014, 110, 967–975. [Google Scholar] [CrossRef]
- Deng, N.; Goh, L.K.; Wang, H.; Das, K.; Tao, J.; Tan, I.B.; Zhang, S.; Lee, M.; Wu, J.; Lim, K.H.; et al. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut 2012, 61, 673–684. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.H.; Jang, H.J.; Han, B.; Zang, D.Y. Pathological and prognostic impacts of FGFR2 overexpression in gastric cancer: A meta-analysis. J. Cancer 2019, 10, 20–27. [Google Scholar] [CrossRef]
- Ahn, S.; Lee, J.; Hong, M.; Kim, S.T.; Park, S.H.; Choi, M.G.; Lee, J.H.; Sohn, T.S.; Bae, J.M.; Kim, S.; et al. FGFR2 in gastric cancer: Protein overexpression predicts gene amplification and high H-index predicts poor survival. Mod. Pathol. 2016, 29, 1095–1103. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Enzinger, P.C.; Kang, Y.-K.; Yamaguchi, K.; Qin, S.; Lee, K.-W.; Oh, S.C.; Li, J.; Turk, H.M.; Teixeira, A.C.; et al. Randomized double-blind placebo-controlled phase 2 study of bemarituzumab combined with modified FOLFOX6 (mFOLFOX6) in first-line (1L) treatment of advanced gastric/gastroesophageal junction adenocarcinoma (FIGHT). J. Clin. Oncol. 2021, 39, 160. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Enzinger, P.C.; Kang, Y.K.; Qin, S.; Yamaguchi, K.; Kim, I.H.; Saeed, A.; Oh, S.C.; Li, J.; Turk, H.M.; et al. Bemarituzumab in patients with FGFR2b-selected gastric or gastro-oesophageal junction adenocarcinoma (FIGHT): A randomised, double-blind, placebo-controlled, phase 2 study. Lancet Oncol. 2022, 23, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Smyth, E.C.; Chao, J.; Muro, K.; Yen, P.; Yanes, R.E.; Zahlten-Kumeli, A.; Rha, S.Y. Trial in progress: Phase 3 study of bemarituzumab + mFOLFOX6 versus placebo + mFOLFOX6 in previously untreated advanced gastric or gastroesophageal junction (GEJ) cancer with FGFR2b overexpression (FORTITUDE-101). J. Clin. Oncol. 2022, 40, TPS4164. [Google Scholar] [CrossRef]
- Tsukita, S.; Furuse, M. The structure and function of claudins, cell adhesion molecules at tight junctions. Ann. N. Y. Acad. Sci. 2000, 915, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Morin, P.J. Claudin proteins in human cancer: Promising new targets for diagnosis and therapy. Cancer Res. 2005, 65, 9603–9606. [Google Scholar] [CrossRef]
- Niimi, T.; Nagashima, K.; Ward, J.M.; Minoo, P.; Zimonjic, D.B.; Popescu, N.C.; Kimura, S. Claudin-18, a novel downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor, encodes lung- and stomach-specific isoforms through alternative splicing. Mol. Cell Biol. 2001, 21, 7380–7390. [Google Scholar] [CrossRef]
- Sahin, U.; Koslowski, M.; Dhaene, K.; Usener, D.; Brandenburg, G.; Seitz, G.; Huber, C.; Türeci, O. Claudin-18 splice variant 2 is a pan-cancer target suitable for therapeutic antibody development. Clin. Cancer Res. 2008, 14, 7624–7634. [Google Scholar] [CrossRef]
- Pellino, A.; Brignola, S.; Riello, E.; Niero, M.; Murgioni, S.; Guido, M.; Nappo, F.; Businello, G.; Sbaraglia, M.; Bergamo, F.; et al. Association of CLDN18 protein expression with clinicopathological features and prognosis in advanced gastric and gastroesophageal junction adenocarcinomas. J. Pers. Med. 2021, 11, 1095. [Google Scholar] [CrossRef]
- Kubota, Y.; Kawazoe, A.; Mishima, S.; Nakamura, Y.; Kotani, D.; Kuboki, Y.; Bando, H.; Kojima, T.; Doi, T.; Yoshino, T.; et al. Comprehensive clinical and molecular characterization of claudin 18.2 expression in advanced gastric or gastroesophageal junction cancer. ESMO Open 2023, 8, 100762. [Google Scholar] [CrossRef]
- Türeci, O.; Sahin, U.; Schulze-Bergkamen, H.; Zvirbule, Z.; Lordick, F.; Koeberle, D.; Thuss-Patience, P.; Ettrich, T.; Arnold, D.; Bassermann, F.; et al. A multicentre, phase IIa study of zolbetuximab as a single agent in patients with recurrent or refractory advanced adenocarcinoma of the stomach or lower oesophagus: The MONO study. Ann. Oncol. 2019, 30, 1487–1495. [Google Scholar] [CrossRef]
- Shitara, K.; Lordick, F.; Bang, Y.; Enzinger, P.; Ilson, D.; Shah, M.A.; Van Cutsem, E.; Xu, R.; Aprile, G. Articles Zolbetuximab plus mFOLFOX6 in patients with advanced unresectable or metastatic gastric or gastrooesophageal junction adenocarcinoma (SPOTLIGHT): A multicentre, randomised, double-blind, phase 3 trial. Lancet 2023, 6736, 1655–1668. [Google Scholar] [CrossRef]
- Xu, R.; Shitara, K.; Ajani, J.A.; Bang, Y.-J.; Enzinger, P.C.; Ilson, D.H.; Lordick, F.; Van Cutsem, E.; Gallego Plazas, J.; Huang, J.; et al. Zolbetuximab + CAPOX in 1L claudin-18.2+ (CLDN18.2+)/HER2− locally advanced (LA) or metastatic gastric or gastroesophageal junction (mG/GEJ) adenocarcinoma: Primary phase 3 results from GLOW. J. Clin. Oncol. 2023, 41, 405736. [Google Scholar] [CrossRef]
- Ma, S.; Zhou, M.; Xu, Y.; Gu, X.; Zou, M.; Abudushalamu, G.; Yao, Y.; Fan, X.; Wu, G. Clinical application and detection techniques of liquid biopsy in gastric cancer. Mol. Cancer 2023, 22, 7. [Google Scholar] [CrossRef]
- Wang, D.S.; Liu, Z.X.; Lu, Y.X.; Bao, H.; Wu, X.; Zeng, Z.L.; Liu, Z.; Zhao, Q.; He, C.Y.; Lu, J.H.; et al. Liquid biopsies to track trastuzumab resistance in metastatic HER2-positive gastric cancer. Gut 2019, 68, 1152–1161. [Google Scholar] [CrossRef]
- Yang, J.; Gong, Y.; Lam, V.K.; Shi, Y.; Guan, Y.; Zhang, Y.; Ji, L.; Chen, Y.; Zhao, Y.; Qian, F.; et al. Deep sequencing of circulating tumor DNA detects molecular residual disease and predicts recurrence in gastric cancer. Cell Death Dis. 2020, 11, 346. [Google Scholar] [CrossRef]
- Nakamura, Y.; Taniguchi, H.; Ikeda, M.; Bando, H.; Kato, K.; Morizane, C.; Esaki, T.; Komatsu, Y.; Kawamoto, Y.; Takahashi, N.; et al. Clinical utility of circulating tumor DNA sequencing in advanced gastrointestinal cancer: SCRUM-Japan GI-SCREEN and GOZILA studies. Nat. Med. 2020, 26, 1859–1864. [Google Scholar] [CrossRef]
- Onoyama, T.; Ishikawa, S.; Isomoto, H. Gastric cancer and genomics: Review of literature. J. Gastroenterol. 2022, 57, 505–516. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, Y.; Okamoto, K.; Kawano, Y.; Kasai, A.; Kawaguchi, T.; Sagawa, T.; Sogabe, M.; Miyamoto, H.; Takayama, T. Novel Biomarkers of Gastric Cancer: Current Research and Future Perspectives. J. Clin. Med. 2023, 12, 4646. https://doi.org/10.3390/jcm12144646
Sato Y, Okamoto K, Kawano Y, Kasai A, Kawaguchi T, Sagawa T, Sogabe M, Miyamoto H, Takayama T. Novel Biomarkers of Gastric Cancer: Current Research and Future Perspectives. Journal of Clinical Medicine. 2023; 12(14):4646. https://doi.org/10.3390/jcm12144646
Chicago/Turabian StyleSato, Yasushi, Koichi Okamoto, Yutaka Kawano, Akinari Kasai, Tomoyuki Kawaguchi, Tamotsu Sagawa, Masahiro Sogabe, Hiroshi Miyamoto, and Tetsuji Takayama. 2023. "Novel Biomarkers of Gastric Cancer: Current Research and Future Perspectives" Journal of Clinical Medicine 12, no. 14: 4646. https://doi.org/10.3390/jcm12144646
APA StyleSato, Y., Okamoto, K., Kawano, Y., Kasai, A., Kawaguchi, T., Sagawa, T., Sogabe, M., Miyamoto, H., & Takayama, T. (2023). Novel Biomarkers of Gastric Cancer: Current Research and Future Perspectives. Journal of Clinical Medicine, 12(14), 4646. https://doi.org/10.3390/jcm12144646






