Predictors of Changes in Quality of Life of Patients with Major Depressive Disorder—A Prospective Naturalistic 3-Month Follow-Up Study
Abstract
1. Introduction
2. Materials and Methods
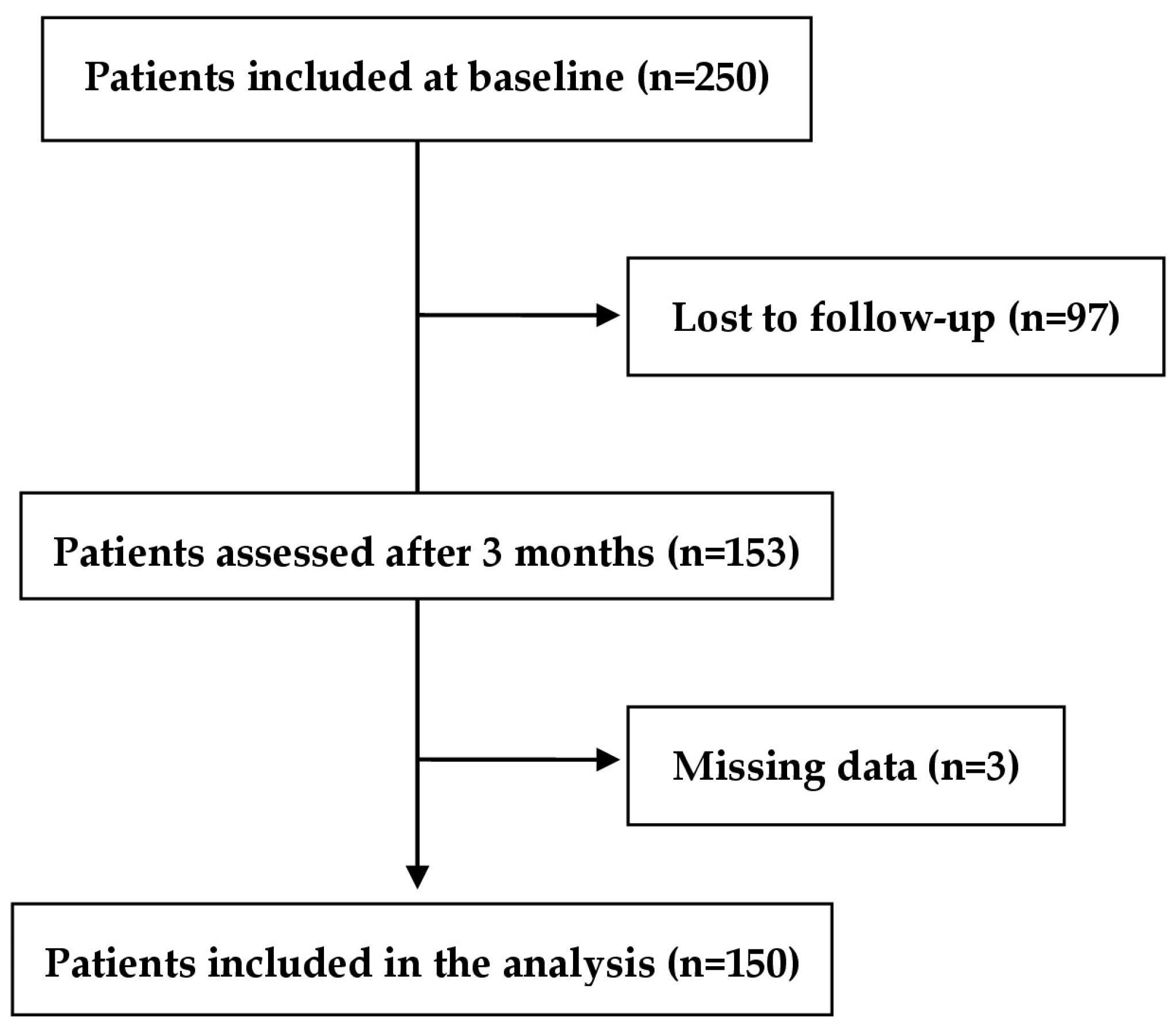
2.1. Study Design and Population
2.2. Measures
2.2.1. Sociodemographic, Personal, and Clinical Variables
2.2.2. Instruments
2.2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990–2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Cho, Y.; Lee, J.K.; Kim, D.-H.; Park, J.-H.; Choi, M.; Kim, H.-J.; Nam, M.-J.; Lee, K.-U.; Han, K.; Park, Y.-G. Factors Associated with Quality of Life in Patients with Depression: A Nationwide Population-Based Study. PLoS ONE 2019, 14, e0219455. [Google Scholar] [CrossRef]
- Suciu, B.D.; Păunescu, R.L.; Micluţia, I.V. Implications of Cognitive Impairment on Several Aspects of Functionality and Quality of Life in Major Depressed Patients. J. Evid. Based Psychother. 2022, 22, 141–154. [Google Scholar] [CrossRef]
- Hohls, J.K.; König, H.-H.; Quirke, E.; Hajek, A. Anxiety, Depression and Quality of Life—A Systematic Review of Evidence from Longitudinal Observational Studies. Int. J. Environ. Res. Public Health 2021, 18, 12022. [Google Scholar] [CrossRef] [PubMed]
- Saarijärvi, S.; Salminen, J.K.; Toikka, T.; Raitasalo, R. Health-Related Quality of Life among Patients with Major Depression. Nord. J. Psychiatry 2002, 56, 261–264. [Google Scholar] [CrossRef]
- Bonicatto, S.C.; Dew, M.A.; Zaratiegui, R.; Lorenzo, L.; Pecina, P. Adult Outpatients with Depression: Worse Quality of Life than in Other Chronic Medical Diseases in Argentina. Soc. Sci. Med. 2001, 52, 911–919. [Google Scholar] [CrossRef] [PubMed]
- IsHak, W.W.; Balayan, K.; Bresee, C.; Greenberg, J.M.; Fakhry, H.; Christensen, S.; Rapaport, M.H. A Descriptive Analysis of Quality of Life Using Patient-Reported Measures in Major Depressive Disorder in a Naturalistic Outpatient Setting. Qual. Life Res. 2013, 22, 585–596. [Google Scholar] [CrossRef] [PubMed]
- IsHak, W.W.; Greenberg, J.M.; Balayan, K.; Kapitanski, N.; Jeffrey, J.; Fathy, H.; Fakhry, H.; Rapaport, M.H. Quality of Life: The Ultimate Outcome Measure of Interventions in Major Depressive Disorder. Harv. Rev. Psychiatry 2011, 19, 229–239. [Google Scholar] [CrossRef]
- The Lancet Psychiatry. Measuring Success: The Problem with Primary Outcomes. Lancet Psychiatry 2020, 7, 1. [Google Scholar] [CrossRef]
- Zimmerman, M.; Martinez, J.H.; Young, D.; Chelminski, I.; Dalrymple, K. Severity Classification on the Hamilton Depression Rating Scale. J. Affect. Disord. 2013, 150, 384–388. [Google Scholar] [CrossRef]
- Zimmerman, M.; Chelminski, I.; Posternak, M. A Review of Studies of the Hamilton Depression Rating Scale in Healthy Controls. J. Nerv. Ment. Dis. 2004, 192, 595–601. [Google Scholar] [CrossRef]
- Cummergen, K.; Hannah, L.; Jopling, L.; Cameron, R.; Walsh, C.; Perez, J. What Outcomes Matter to Service Users Who Experience Persistent Depression: A Mixed-Method Narrative Review and Synthesis. J. Affect. Disord. Rep. 2022, 10, 100431. [Google Scholar] [CrossRef]
- Rosenblat, J.D.; Simon, G.E.; Sachs, G.S.; Deetz, I.; Doederlein, A.; De Peralta, D.; Dean, M.M.; McIntyre, R.S. Treatment Effectiveness and Tolerability Outcomes That Are Most Important to Individuals with Bipolar and Unipolar Depression. J. Affect. Disord. 2019, 243, 116–120. [Google Scholar] [CrossRef]
- The WHOQOL Group. Development of the World Health Organization WHOQOL-Bref Quality of Life Assessment. Psychol. Med. 1998, 28, 551–558. [Google Scholar] [CrossRef]
- Hofmann, S.G.; Curtiss, J.; Carpenter, J.K.; Kind, S. Effect of Treatments for Depression on Quality of Life: A Meta-Analysis. Cogn. Behav. Ther. 2017, 46, 265–286. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, T.; Næss, S. To Measure Quality of Life: Relevance and Use in the Psychiatric Domain. Nord. J. Psychiatry 1996, 50 (Suppl. S37), 29–39. [Google Scholar] [CrossRef]
- Daly, E.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Gaynes, B.N.; Warden, D.; Morris, D.W.; Luther, J.F.; Farabaugh, A.; Cook, I.; et al. Health-Related Quality Of Life In Depression: A STAR*D Report. Ann. Clin. Psychiatry 2010, 22, 43–55. [Google Scholar] [PubMed]
- Cohen, R.M.; Greenberg, J.M.; IsHak, W.W. Incorporating Multidimensional Patient-Reported Outcomes of Symptom Severity, Functioning, and Quality of Life in the Individual Burden of Illness Index for Depression to Measure Treatment Impact and Recovery in MDD. JAMA Psychiatry 2013, 70, 343. [Google Scholar] [CrossRef] [PubMed]
- Rapaport, M.H.; Clary, C.; Fayyad, R.; Endicott, J. Quality-Of-Life Impairment in Depressive and Anxiety Disorders. Am. J. Psychiatry 2005, 162, 1171–1178. [Google Scholar] [CrossRef]
- Rubio, J.M.; Olfson, M.; Villegas, L.; Pérez-Fuentes, G.; Wang, S.; Blanc, C. Quality of Life Following Remission of Mental Disorders. J. Clin. Psychiatry 2013, 74, e445–e450. [Google Scholar] [CrossRef]
- Papakostas, G.I.; Petersen, T.; Mahal, Y.; Mischoulon, D.; Nierenberg, A.A.; Fava, M. Quality of Life Assessments in Major Depressive Disorder: A Review of the Literature. Gen. Hosp. Psychiatry 2004, 26, 13–17. [Google Scholar] [CrossRef]
- Jha, M.K.; Greer, T.L.; Grannemann, B.D.; Carmody, T.; Rush, A.J.; Trivedi, M.H. Early Normalization of Quality of Life Predicts Later Remission in Depression: Findings from the CO-MED Trial. J. Affect. Disord. 2016, 206, 17–22. [Google Scholar] [CrossRef]
- Zimmerman, M.; Martinez, J.A.; Attiullah, N.; Friedman, M.; Toba, C.; Boerescu, D.A.; Rahgeb, M. Why Do Some Depressed Outpatients Who Are in Remission according to the Hamilton Depression Rating Scale Not Consider Themselves to Be in Remission? J. Clin. Psychiatry 2012, 73, 790–795. [Google Scholar] [CrossRef]
- IsHak, W.W.; Bonifay, W.; Collison, K.; Reid, M.; Youssef, H.; Parisi, T.; Cohen, R.M.; Cai, L. The Recovery Index: A Novel Approach to Measuring Recovery and Predicting Remission in Major Depressive Disorder. J. Affect. Disord. 2017, 208, 369–374. [Google Scholar] [CrossRef]
- Wiesinger, T.; Kremer, S.; Bschor, T.; Baethge, C. Antidepressants and Quality of Life in Patients with Major Depressive Disorder—Systematic Review and Meta-Analysis of Double-Blind, Placebo-Controlled RCTs. Acta Psychiatr. Scand. 2023, 147, 545–560. [Google Scholar] [CrossRef]
- Almohammed, O.A.; Alsalem, A.A.; Almangour, A.A.; Alotaibi, L.H.; Al Yami, M.S.; Lai, L. Antidepressants and Health-Related Quality of Life (HRQoL) for Patients with Depression: Analysis of the Medical Expenditure Panel Survey from the United States. PLoS ONE 2022, 17, e0265928. [Google Scholar] [CrossRef]
- Andrade, C. Antidepressant Drugs and Health-Related Quality of Life. J. Clin. Psychiatry 2022, 83, 22f14527. [Google Scholar] [CrossRef]
- Noto, S.; Wake, M.; Mishiro, I.; Hammer-Helmich, L.; Ren, H.; Moriguchi, Y.; Fujikawa, K.; Fernandez, J. Health-Related Quality of Life over 6 Months in Patients with Major Depressive Disorder Who Started Antidepressant Monotherapy. Value Health Reg. Issues 2022, 30, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-C.; Lin, C.-H.; Wang, F.-C.; Lu, M.-J. Factors Related to the Improvement in Quality of Life for Depressed Inpatients Treated with Fluoxetine. BMC Psychiatry 2017, 17, 309. [Google Scholar] [CrossRef] [PubMed]
- Morton, E.; Bhat, V.; Giacobbe, P.; Lou, W.; Michalak, E.E.; McInerney, S.; Chakrabarty, T.; Frey, B.N.; Milev, R.V.; Müller, D.J.; et al. Predictors of Quality of Life Improvement with Escitalopram and Adjunctive Aripiprazole in Patients with Major Depressive Disorder: A CAN-BIND Study Report. CNS Drugs 2021, 35, 439–450. [Google Scholar] [CrossRef] [PubMed]
- Wongprommate, D.; Wongpakaran, T.; Pinyopornpanish, M.; Lerttrakarnnon, P.; Jiraniramai, S.; Satthapisit, S.; Saisavoey, N.; Wannarit, K.; Nakawiro, D.; Tantrarungroj, T.; et al. Predictors for Quality of Life among Older Adults with Depressive Disorders: A Prospective 3-Month Follow-up Cohort Study. Perspect. Psychiatr. Care 2022, 58, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Moses, T.; Leuchter, A.F.; Cook, I.; Abrams, M. Does the Clinical Course of Depression Determine Improvement in Symptoms and Quality of Life? J. Nerv. Ment. Dis. 2006, 194, 241–248. [Google Scholar] [CrossRef]
- Jaracz, J.; Gattner, K.; Jaracz, K.; Górna, K. Unexplained Painful Physical Symptoms in Patients with Major Depressive Disorder: Prevalence, Pathophysiology and Management. CNS Drugs 2016, 30, 293–304. [Google Scholar] [CrossRef]
- Chopra, K.; Arora, V. An Intricate Relationship between Pain and Depression: Clinical Correlates, Coactivation Factors and Therapeutic Targets. Expert Opin. Ther. Targets 2013, 18, 159–176. [Google Scholar] [CrossRef]
- Muñoz, R.A.; McBride, M.E.; Brnabic, A.J.M.; López, C.J.; Hetem, L.A.B.; Secin, R.; Dueñas, H.J. Major Depressive Disorder in Latin America: The Relationship between Depression Severity, Painful Somatic Symptoms, and Quality of Life. J. Affect. Disord. 2005, 86, 93–98. [Google Scholar] [CrossRef]
- Novick, D.; Montgomery, W.; Kadziola, Z.; Moneta, V.; Peng, X.; Brugnoli, R.; Haro, J.M. Do Concomitant Pain Symptoms in Patients with Major Depression Affect Quality of Life Even When Taking into Account Baseline Depression Severity? Patient Prefer. Adherence 2013, 7, 463–470. [Google Scholar] [CrossRef]
- Chung, K.-F.; Tso, K.-C.; Yeung, W.-F.; Li, W.-H. Quality of Life in Major Depressive Disorder: The Role of Pain and Pain Catastrophizing Cognition. Compr. Psychiatry 2012, 53, 387–395. [Google Scholar] [CrossRef]
- Ma, Y.; Xiang, Q.; Yan, C.; Liao, H.; Wang, J. Relationship between Chronic Diseases and Depression: The Mediating Effect of Pain. BMC Psychiatry 2021, 21, 436. [Google Scholar] [CrossRef]
- Currie, S.R.; Wang, J. More Data on Major Depression as an Antecedent Risk Factor for First Onset of Chronic Back Pain. Psychol. Med. 2005, 35, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.K.Y.; Crane, C. Suicidality in Chronic Pain: A Review of the Prevalence, Risk Factors and Psychological Links. Psychol. Med. 2006, 36, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Elliott, T.E.; Renier, C.M.; Palcher, J.A. Chronic Pain, Depression, and Quality of Life: Correlations and Predictive Value of the SF-36. Pain Med. 2003, 4, 331–339. [Google Scholar] [CrossRef]
- Rapti, E.; Damigos, D.; Apostolara, P.; Roka, V.; Tzavara, C.; Lionis, C. Patients with Chronic Pain: Evaluating Depression and Their Quality of Life in a Single Center Study in Greece. BMC Psychol. 2019, 7, 86. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, J.; Feng, L.; Feng, Y.; Xiao, L.; Chen, X.; Yang, J.; Wang, G. The Associations between Depressive Symptoms, Functional Impairment, and Quality of Life, in Patients with Major Depression: Undirected and Bayesian Network Analyses. Psychol. Med. 2022, 1–13. [Google Scholar] [CrossRef]
- Sheehan, D.V.; Nakagome, K.; Asami, Y.; Pappadopulos, E.A.; Boucher, M. Restoring Function in Major Depressive Disorder: A Systematic Review. J. Affect. Disord. 2017, 215, 299–313. [Google Scholar] [CrossRef] [PubMed]
- Lam, R.W.; Parikh, S.V.; Michalak, E.E.; Dewa, C.S.; Kennedy, S.H. Canadian Network for Mood and Anxiety Treatments (CANMAT) consensus recommendations for functional outcomes in major depressive disorder. Ann. Clin. Psychiatry 2015, 27, 142–149. [Google Scholar]
- Bauer, M.; Pfennig, A.; Severus, E.; Whybrow, P.C.; Angst, J.; Möller, H.-J. World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for Biological Treatment of Unipolar Depressive Disorders, Part 1: Update 2013 on the Acute and Continuation Treatment of Unipolar Depressive Disorders. World J. Biol. Psychiatry 2013, 14, 334–385. [Google Scholar] [CrossRef]
- Lam, R.W.; McIntosh, D.; Wang, J.; Enns, M.W.; Kolivakis, T.; Michalak, E.E.; Sareen, J.; Song, W.-Y.; Kennedy, S.H.; MacQueen, G.M.; et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 Clinical Guidelines for the Management of Adults with Major Depressive Disorder: Section 1. Disease Burden and Principles of Care. Can. J. Psychiatry. Rev. Can. Psychiatr. 2016, 61, 510–523. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5); American Psychiatric Association Publishing: Arlington, VA, USA, 2013. [Google Scholar]
- Kriston, L.; von Wolff, A. Not as Golden as Standards Should Be: Interpretation of the Hamilton Rating Scale for Depression. J. Affect. Disord. 2011, 128, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M. A Rating Scale for Depression. J. Neurol. Neurosurg. Psychiatry 1960, 23, 56–62. [Google Scholar] [CrossRef]
- Obeid, S.; Abi Elias Hallit, C.; Haddad, C.; Hany, Z.; Hallit, S. Validation of the Hamilton Depression Rating Scale (HDRS) and Sociodemographic Factors Associated with Lebanese Depressed Patients. L’Encéphale 2018, 44, 397–402. [Google Scholar] [CrossRef]
- World Health Organization. WHO-BREF: Introduction, Administration, Scoring and Generic Version of the Assessment; WHO: Geneva, Switzerland, 1996; Available online: https://apps.who.int/iris/rest/bitstreams/59977/retrieve (accessed on 9 May 2023).
- Berlim, M.T.; Pavanello, D.P.; Caldieraro, M.A.K.; Fleck, M.P.A. Reliability and Validity of the WHOQOL BREF in a Sample of Brazilian Outpatients with Major Depression. Qual. Life Res. 2005, 14, 561–564. [Google Scholar] [CrossRef]
- Berlim, M.T.; Fleck, M.P.A. Quality of Life and Major Depression. In Quality of Life Impairment in Schizophrenia, Mood and Anxiety Disorders; New Perspectives on Research and Treatment; Ritsner, M.S., Awad, A.G., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 241–252. [Google Scholar]
- Skevington, S.M.; Lotfy, M.; O’Connell, K.A. The World Health Organization’s WHOQOL-BREF Quality of Life Assessment: Psychometric Properties and Results of the International Field Trial. A Report from the WHOQOL Group. Qual. Life Res. 2004, 13, 299–310. [Google Scholar] [CrossRef]
- Hawker, G.A.; Mian, S.; Kendzerska, T.; French, M. Measures of Adult Pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF). Arthritis Care Res. 2011, 63, S240–S252. [Google Scholar] [CrossRef] [PubMed]
- Romera, I.; Pérez, V.; Manuel Menchón, J.; Schacht, A.; Papen, R.; Neuhauser, D.; Abbar, M.; Picard, H.; Gilaberte, I. Early vs. Conventional Switching of Antidepressants in Patients with MDD and Moderate to Severe Pain: A Double-Blind Randomized Study. J. Affect. Disord. 2012, 143, 47–55. [Google Scholar] [CrossRef]
- Perahia, D.G.S.; Quail, D.; Desaiah, D.; Montejo, A.L.; Schatzberg, A.F. Switching to Duloxetine in Selective Serotonin Reuptake Inhibitor Non- and Partial-Responders: Effects on Painful Physical Symptoms of Depression. J. Psychiatr. Res. 2009, 43, 512–518. [Google Scholar] [CrossRef]
- Leon, A.C.; Olfson, M.; Portera, L.; Farber, L.; Sheehan, D.V. Assessing Psychiatric Impairment in Primary Care with the Sheehan Disability Scale. Int. J. Psychiatry Med. 1997, 27, 93–105. [Google Scholar] [CrossRef]
- Sheehan, D.V.; Harnett-Sheehan, K.; Raj, B.A. The Measurement of Disability. Int. Clin. Psychopharmacol. 1996, 11 (Suppl. S3), 89–95. [Google Scholar] [CrossRef]
- Mancini, M.; Sheehan, D.V.; Demyttenaere, K.; Amore, M.; Deberdt, W.; Quail, D.; Sagman, D. Evaluation of the Effect of Duloxetine Treatment on Functioning as Measured by the Sheehan Disability Scale. Int. Clin. Psychopharmacol. 2012, 27, 298–309. [Google Scholar] [CrossRef]
- Sheehan, K.H.; Sheehan, D.V. Assessing Treatment Effects in Clinical Trials with the Discan Metric of the Sheehan Disability Scale. Int. Clin. Psychopharmacol. 2008, 23, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Berlim, M.T.; McGirr, A.; Fleck, M.P. Can Sociodemographic and Clinical Variables Predict the Quality of Life of Outpatients with Major Depression? Psychiatry Res. 2008, 160, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.; Pan, A.-W.; Hsiung, P.-C. Quality of Life for Patients with Major Depression in Taiwan:A Model-Based Study of Predictive Factors. Psychiatry Res. 2009, 168, 153–162. [Google Scholar] [CrossRef]
- Vancampfort, D.; Kimbowa, S.; Schuch, F.; Mugisha, J. Physical Activity, Physical Fitness and Quality of Life in Outpatients with Major Depressive Disorder versus Matched Healthy Controls: Data from a Low-Income Country. J. Affect. Disord. 2021, 294, 802–804. [Google Scholar] [CrossRef] [PubMed]
- Riihimäki, K.; Sintonen, H.; Vuorilehto, M.; Jylhä, P.; Saarni, S.; Isometsä, E. Health-Related Quality of Life of Primary Care Patients with Depressive Disorders. Eur. Psychiatry 2016, 37, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Pyne, J. Use of the Quality of Well-Being Self-Administered Version (QWB-SA) in Assessing Health-Related Quality of Life in Depressed Patients. J. Affect. Disord. 2003, 76, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.-W.; Chen, Y.-L.; Chung, L.-I.; Wang, J.-D.; Chen, T.-J.; Hsiung, P.-C. A Longitudinal Study of the Predictors of Quality of Life in Patients with Major Depressive Disorder Utilizing a Linear Mixed Effect Model. Psychiatry Res. 2012, 198, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cheng, H.; Huang, Y.; Liu, Z.; Luo, X. Depression Symptoms and Chronic Pain in the Community Population in Beijing, China. Psychiatry Res. 2012, 200, 313–317. [Google Scholar] [CrossRef]
- Garcia-Cebrian, A.; Gandhi, P.; Demyttenaere, K.; Peveler, R. The Association of Depression and Painful Physical Symptoms–a Review of the European Literature. Eur. Psychiatry 2006, 21, 379–388. [Google Scholar] [CrossRef]
- Razali, S.M.; Khalib, A.Q. Pain Symptoms in Malay Patients with Major Depression. Asian J. Psychiatry 2012, 5, 297–302. [Google Scholar] [CrossRef]
- Oon-Arom, A.; Likhitsathian, S.; Maneeton, B.; Sulaiman, A.H.; Shih-Yen, E.C.; Udomratn, P.; Chen, C.; Srisurapanont, M. Subjective Depressive Symptoms Associated with Pain in Patients with Major Depressive Disorder: Findings from the Study on the Aspect of Asian Depression. Perspect. Psychiatr. Care 2019, 56, 188–193. [Google Scholar] [CrossRef]
- Vietri, J.; Otsubo, T.; Montgomery, W.; Tsuji, T.; Harada, E. The Incremental Burden of Pain in Patients with Depression: Results of a Japanese Survey. BMC Psychiatry 2015, 15, 104. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.J.; Lu, Y.; Detke, M.J.; Hudson, J.; Iyengar, S.; Demitrack, M.A. Effects of Duloxetine on Painful Physical Symptoms Associated with Depression. Psychosomatics 2004, 45, 17–28. [Google Scholar] [CrossRef]
- Lee, M.G.; Yum, S.Y.; Hong, J.T.; Yoon, C.W.; Noh, J.S.; Lee, K.H.; Kim, J.H.; Lee, S.Y.; Singh, P.; Treuer, T.; et al. Association between Painful Physical Symptoms and Clinical Outcomes in Korean Patients with Major Depressive Disorder: A Three-Month Observational Study. Psychiatry Investig. 2009, 6, 255–263. [Google Scholar] [CrossRef]
- Lenox-Smith, A.; Macdonald, M.T.B.; Reed, C.; Tylee, A.; Peveler, R.; Quail, D.; Wildgust, H.J. Quality of Life in Depressed Patients in UK Primary Care: The FINDER Study. Neurol. Ther. 2013, 2, 25–42. [Google Scholar] [CrossRef]
- Spindler, M.; Koch, K.; Borisov, E.; Özyurt, J.; Sörös, P.; Thiel, C.; Bantel, C. The Influence of Chronic Pain and Cognitive Function on Spatial-Numerical Processing. Front. Behav. Neurosci. 2018, 12, 165. [Google Scholar] [CrossRef] [PubMed]
- Majedi, H.; Mohammadi, M.; Tafakhori, A.; Khazaeipour, Z. The Influence of Chronic Pain on Number Sense and Numeric Rating Scale: A Prospective Cohort Study. Anesthesiol. Pain Med. 2020, 10, e103532. [Google Scholar] [CrossRef] [PubMed]
- Wolrich, J.; Poots, A.J.; Kuehler, B.M.; Rice, A.S.C.; Rahman, A.; Bantel, C. Is Number Sense Impaired in Chronic Pain Patients? Br. J. Anaesth. 2014, 113, 1024–1031. [Google Scholar] [CrossRef]
- Suciu, B.D.; Păunescu, R.L.; Micluţia, I.V. Assessment of Cognitive Performances in Major Depressed Patients: A 6-Month Follow-up Study. Int. J. Psychiatry Clin. Pract. 2020, 25, 378–384. [Google Scholar] [CrossRef]
- Euasobhon, P.; Atisook, R.; Bumrungchatudom, K.; Zinboonyahgoon, N.; Saisavoey, N.; Jensen, M.P. The Reliability and Responsivity of Pain Intensity Scales in Individuals with Chronic Pain. Pain 2022, 163, e1184–e1191. [Google Scholar] [CrossRef]
- Nitzan, U.; Hecht, M.; Braw, Y.; Maoz, H.; Levkovitz, Y.; Yarnitsky, D.; Granovsky, Y.; Bloch, Y. Initial Evaluation of Pain Intensity among Depressed Patients as a Possible Mediator between Depression and Pain Complaints. Front. Psychiatry 2019, 10, 48. [Google Scholar] [CrossRef]
- Citrome, L.; Jain, R.; Tung, A.; Landsman-Blumberg, P.B.; Kramer, K.; Ali, S. Prevalence, Treatment Patterns, and Stay Characteristics Associated with Hospitalizations for Major Depressive Disorder. J. Affect. Disord. 2019, 249, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wu, Z.; Cao, L.; Wang, Y.; Su, Y.; Huang, J.; Fang, M.; Yao, Z.; Wang, Z.; Wang, F.; et al. Predictors and Moderators of Quality of Life in Patients with Major Depressive Disorder: An AGTs-MDD Study Report. J. Psychiatr. Res. 2021, 138, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Claassen, C.A.; Trivedi, M.H.; Rush, A.J.; Husain, M.M.; Zisook, S.; Young, E.; Leuchter, A.; Wisniewski, S.R.; Balasubramani, G.K.; Alpert, J. Clinical Differences among Depressed Patients with and without a History of Suicide Attempts: Findings from the STAR⁎D Trial. J. Affect. Disord. 2007, 97, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wu, Z.; Zhang, H.; Mellor, D.; Ding, L.; Wu, H.; Wu, C.; Huang, J.; Hong, W.; Peng, D.; et al. Somatic Symptoms Vary in Major Depressive Disorder in China. Compr. Psychiatry 2018, 87, 32–37. [Google Scholar] [CrossRef] [PubMed]
| Patient Characteristics | |
|---|---|
| Age (years), mean ± SD | 50.77 ± 10.17 |
| Gender, N (%) | |
| Males | 45 (30.0%) |
| Females | 105 (70.0%) |
| Level of education, N (%) | |
| ≤8 years | 30 (20.0%) |
| 9–12 years | 98 (65.3%) |
| >12 years | 22 (14.7%) |
| Marital Status, N (%) | |
| With Partner | 47 (31.3%) |
| Single | 103 (68.7%) |
| Place of Residence, N (%) | |
| Rural | 52 (34.7%) |
| Urban | 98 (65.3%) |
| Professional Status, N (%) | |
| Employed | 52 (34.7%) |
| Retired or Unemployed | 98 (65.3%) |
| Income, N (%) | |
| Low | 35 (23.3%) |
| Medium | 92 (61.3%) |
| High | 23 (15.3%) |
| Age at first diagnosis of depressive disorder, mean ± SD | 42.21 ± 12.37 |
| Illness Duration (years), mean ± SD | 8.65 ± 9.35 |
| History of Suicide Attempts, N (%) | |
| No | 114 (76.0%) |
| Yes | 36 (24.0%) |
| Family Psychiatric History, N (%) | |
| Yes | 50 (33.3%) |
| No | 100 (66.7%) |
| Number of psychiatric hospitalizations, mean ± SD | 5.84 ± 11.53 |
| Treatment before study enrolment, N (%) | |
| Yes | 122 (81.3%) |
| No | 28 (18.7%) |
| Level of depressive symptoms severity, N (%) | |
| Mild | 42 (28%) |
| Moderate | 63 (42%) |
| Severe | 45 (30%) |
| Measure | T0 | T1 | p * | Change in Score (Difference between T1 and T0) |
|---|---|---|---|---|
| HAM-D | 19.73 ± 5.64 | 11.47 ± 6.05 | 0.000 | −8.25 ± 5.93 |
| WHOQOL-BREF domain | ||||
| Physical health | 43.94 ± 18.56 | 59.33 ± 19.56 | 0.000 | 15.39 ± 17.00 |
| Psychological | 49.27 ± 21.21 | 60.86 ± 20.86 | 0.000 | 11.58 ± 17.79 |
| Social relationships | 52.64 ± 23.01 | 58.11 ± 20.89 | 0.000 | 5.47 ± 16.86 |
| Environmental | 58.95 ± 16.67 | 64.65 ± 15.14 | 0.000 | 5.71 ± 8.73 |
| SDS | 19.45 ± 9.14 | 8.43 ± 9.30 | 0.000 | −11.02 ± 9.21 |
| VAS for pain | 4.74 ± 3.45 | 4.12 ± 3.49 | 0.020 | −0.63 ± 3.63 |
| Days Lost | 2.46 ± 2.79 | 0.49 ± 1.31 | 0.000 | −1.97 ± 2.66 |
| Days Unproductive | 2.38 ± 2.53 | 1.97 ± 2.64 | 0.121 | −0.41 ±3.10 |
| BMI | 27.16 ± 5.35 | 27.87 ± 5.42 | 0.000 | 0.70 ± 1.73 |
| Predictor | B | t | p | Adjusted R2 |
|---|---|---|---|---|
| Physical health | ||||
| Number of psychiatric hospitalizations | −0.313 | −2.630 | 0.009 | 0.038 |
| Change in BMI score | 2.109 | 2.679 | 0.008 | 0.040 |
| Change in VAS for pain score | −1.731 | −4.832 | 0.000 | 0.130 |
| Change in HAM-D score | −1.934 | −11.119 | 0.000 | 0.451 |
| Change in SDS score | −1.149 | −9.681 | 0.000 | 0.384 |
| Change in days lost score | −2.302 | −4.707 | 0.000 | 0.124 |
| Change in days unproductive score | −1.660 | −3.868 | 0.000 | 0.086 |
| Psychological | ||||
| Age | −0.351 | −2.495 | 0.014 | 0.034 |
| Marital status | 6.861 | 2.219 | 0.028 | 0.026 |
| Number of psychiatric hospitalizations | −0.285 | −2.270 | 0.025 | 0.027 |
| Change in BMI score | 2.172 | 2.634 | 0.009 | 0.038 |
| Change in HAM-D score | −1.722 | −8.530 | 0.000 | 0.325 |
| Change in SDS score | −1.099 | −8.424 | 0.000 | 0.320 |
| Change in days lost score | −2.014 | −3.851 | 0.000 | 0.085 |
| Change in days unproductive score | −1.735 | −3.860 | 0.000 | 0.085 |
| Social relationships | ||||
| History of suicide attempts | 6.879 | 2.160 | 0.032 | 0.024 |
| Change in HAM-D score | −1.049 | −4.830 | 0.000 | 0.130 |
| Change in SDS score | −0.673 | −4.814 | 0.000 | 0.130 |
| Change in days lost score | −1.255 | −2.462 | 0.015 | 0.033 |
| Environment | ||||
| Level of education | −2.489 | −2.069 | 0.040 | 0.022 |
| Marital status | 3.495 | 2.306 | 0.023 | 0.028 |
| Change in HAM-D score | −0.466 | −4.059 | 0.000 | 0.094 |
| Change in SDS score | −0.293 | −3.952 | 0.000 | 0.089 |
| Change in days lost score | −0.898 | −3.466 | 0.001 | 0.069 |
| Model | R | R2 | Adjusted R2 | R2 Change | F Change | p F Change |
|---|---|---|---|---|---|---|
| Physical health | ||||||
| (Constant), change in HAM-D score, change in SDS score, change in VAS score | 0.746 | 0.556 | 0.547 | 0.038 | 12.398 | 0.001 |
| Psychological | ||||||
| (Constant), change in HAM-D score, change in SDS score, marital status | 0.651 | 0.424 | 0.412 | 0.025 | 6.206 | 0.014 |
| Social relationships | ||||||
| (Constant), change in HAM-D score, change in SDS score, history of suicide attempts | 0.440 | 0.194 | 0.177 | 0.026 | 4.638 | 0.033 |
| Environment | ||||||
| (Constant), change in HAM-D score, educational level, change in days lost score | 0.399 | 0.160 | 0.142 | 0.027 | 4.598 | 0.034 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dionisie, V.; Puiu, M.G.; Manea, M.; Pacearcă, I.A. Predictors of Changes in Quality of Life of Patients with Major Depressive Disorder—A Prospective Naturalistic 3-Month Follow-Up Study. J. Clin. Med. 2023, 12, 4628. https://doi.org/10.3390/jcm12144628
Dionisie V, Puiu MG, Manea M, Pacearcă IA. Predictors of Changes in Quality of Life of Patients with Major Depressive Disorder—A Prospective Naturalistic 3-Month Follow-Up Study. Journal of Clinical Medicine. 2023; 12(14):4628. https://doi.org/10.3390/jcm12144628
Chicago/Turabian StyleDionisie, Vlad, Maria Gabriela Puiu, Mirela Manea, and Ioana Anca Pacearcă. 2023. "Predictors of Changes in Quality of Life of Patients with Major Depressive Disorder—A Prospective Naturalistic 3-Month Follow-Up Study" Journal of Clinical Medicine 12, no. 14: 4628. https://doi.org/10.3390/jcm12144628
APA StyleDionisie, V., Puiu, M. G., Manea, M., & Pacearcă, I. A. (2023). Predictors of Changes in Quality of Life of Patients with Major Depressive Disorder—A Prospective Naturalistic 3-Month Follow-Up Study. Journal of Clinical Medicine, 12(14), 4628. https://doi.org/10.3390/jcm12144628







