Bone Mass Changes Following Percutaneous Radiofrequency Ablation, Osteoplasty, Reinforcement, and Internal Fixation of Periacetabular Osteolytic Metastases
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
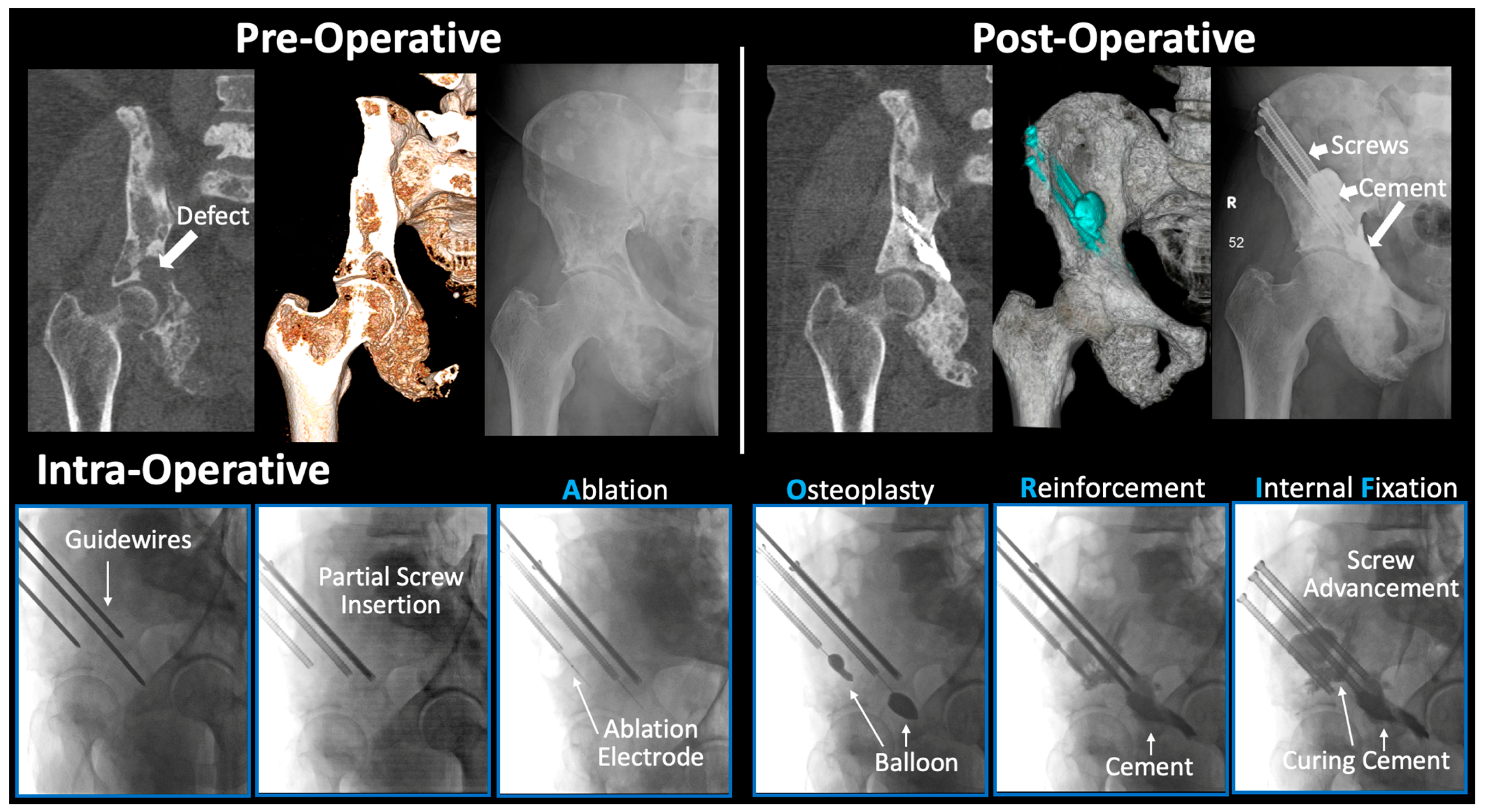
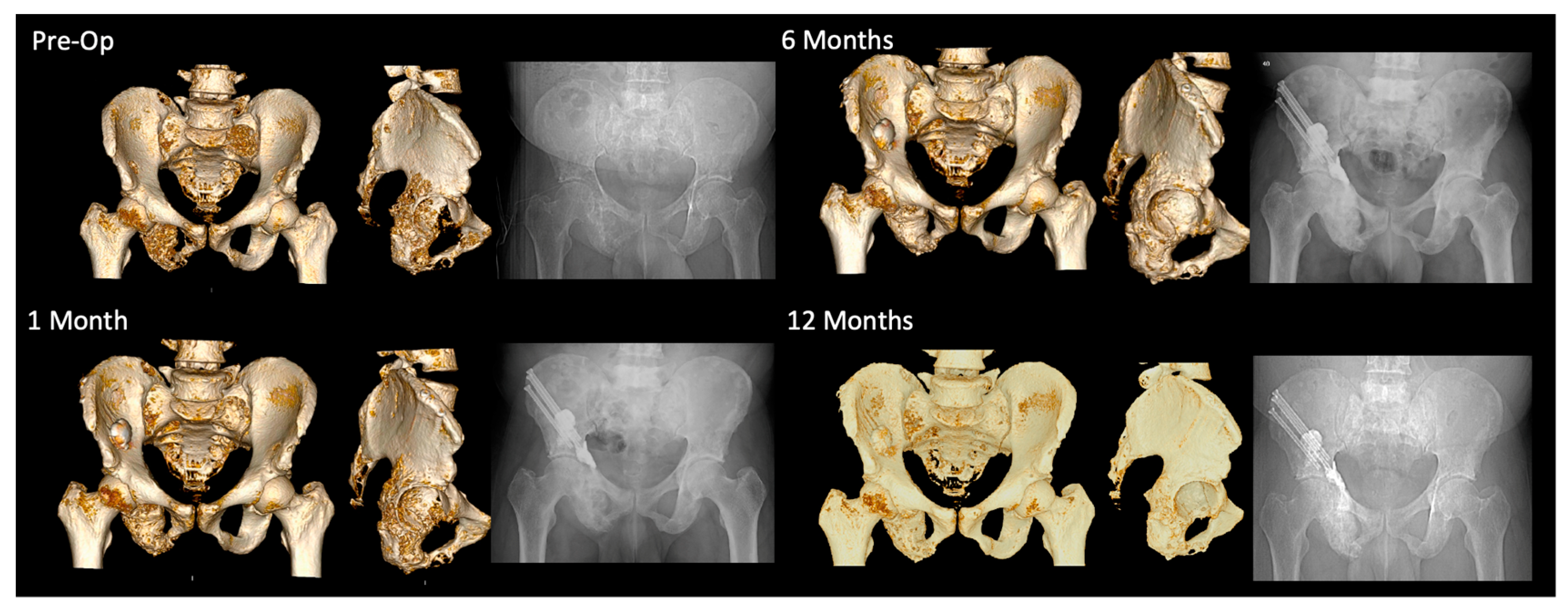
2.2. Surgical Technique
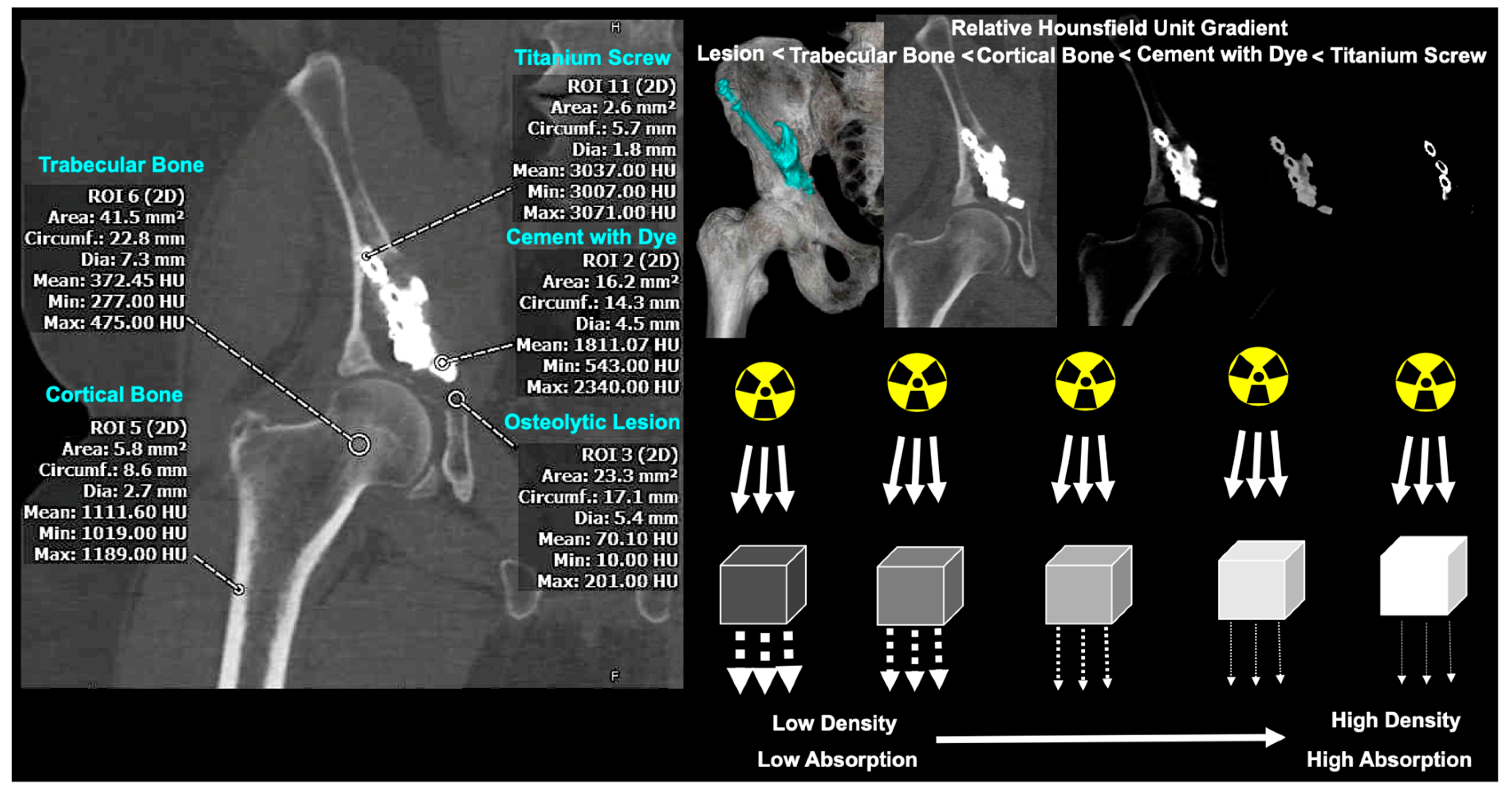
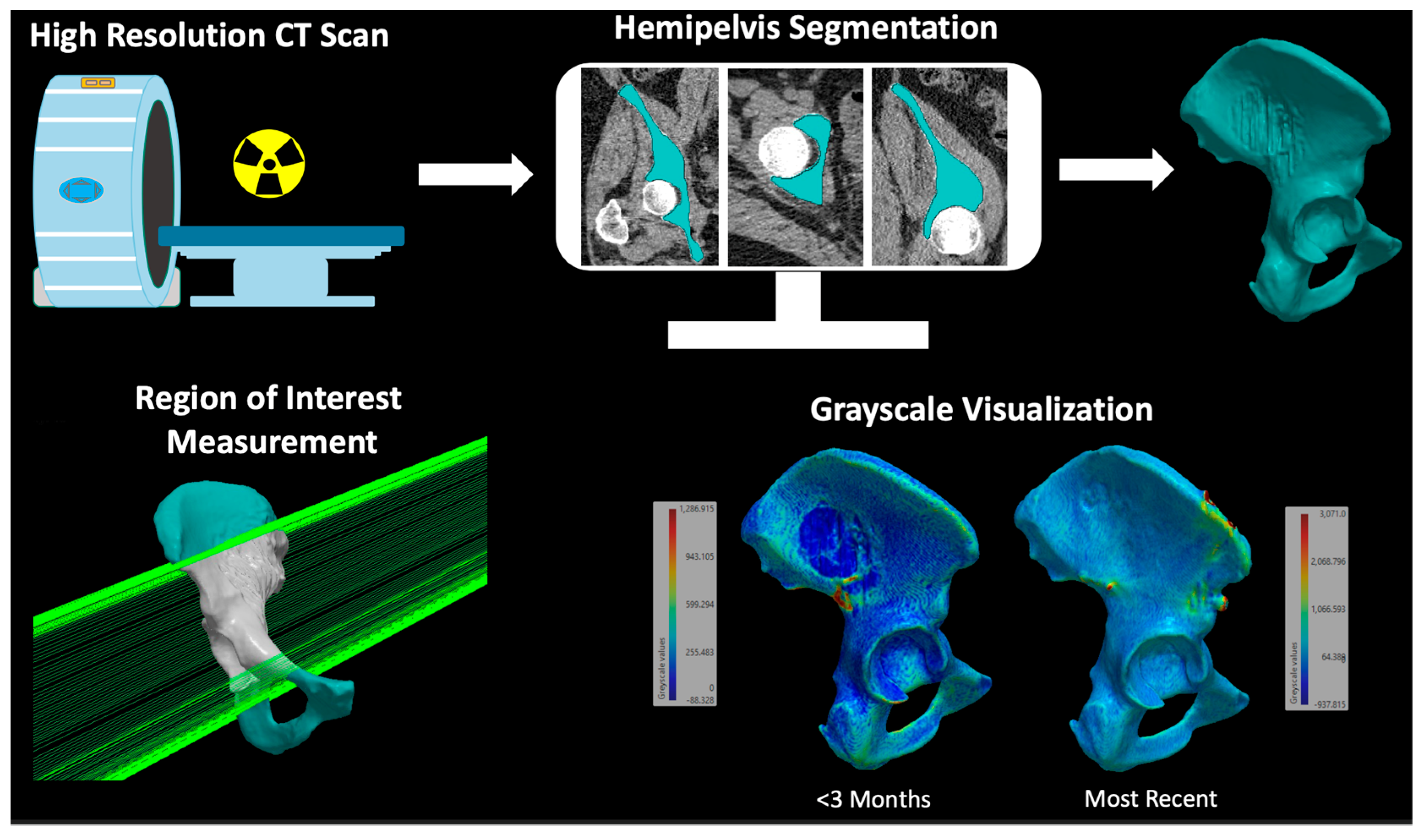
2.3. Two-Dimensional Assessment of Bone Mass
2.4. Three-Dimensional Assessment of Bone Mass
3. Results
3.1. Demographic and Oncologic Treatments
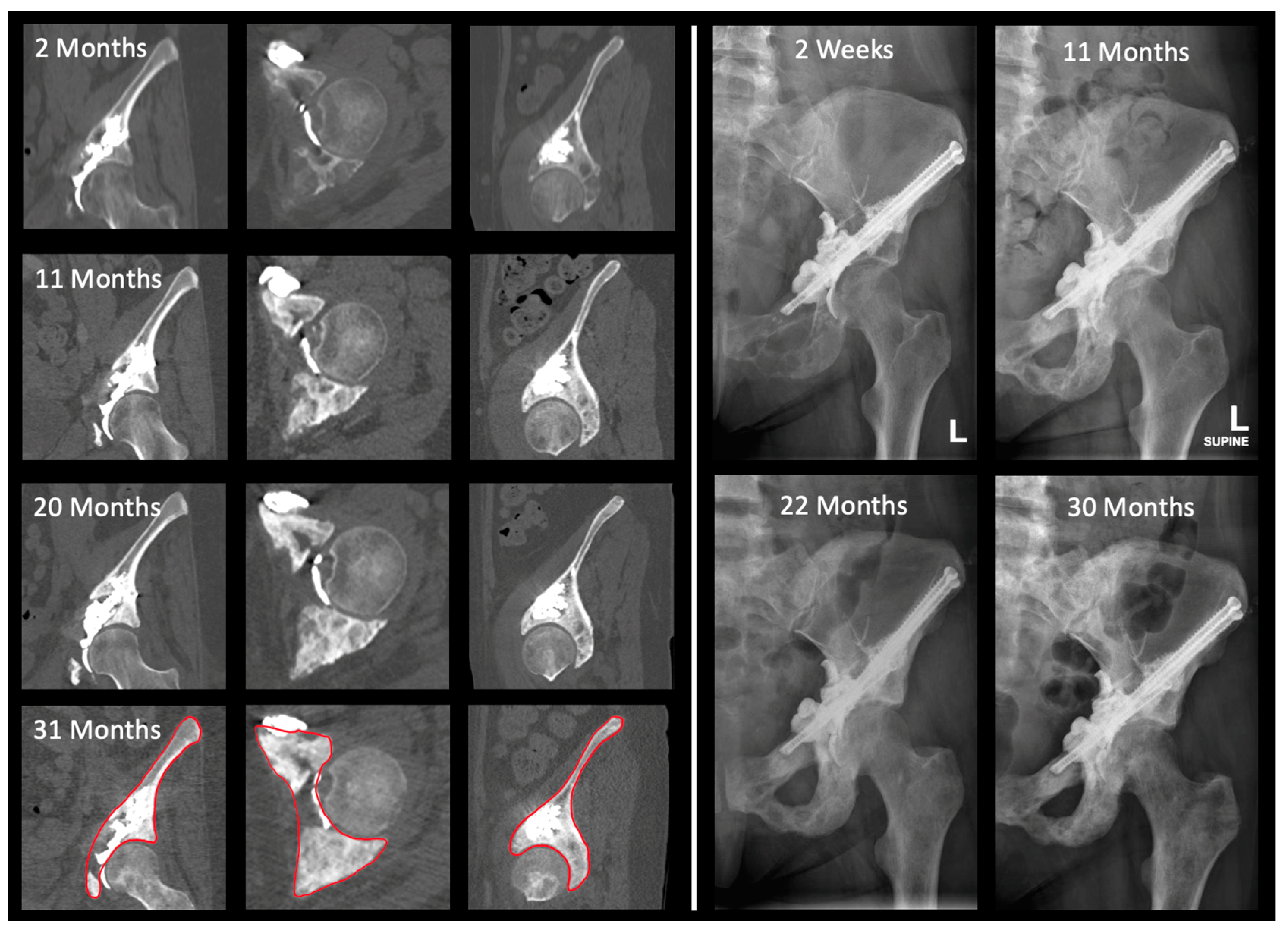
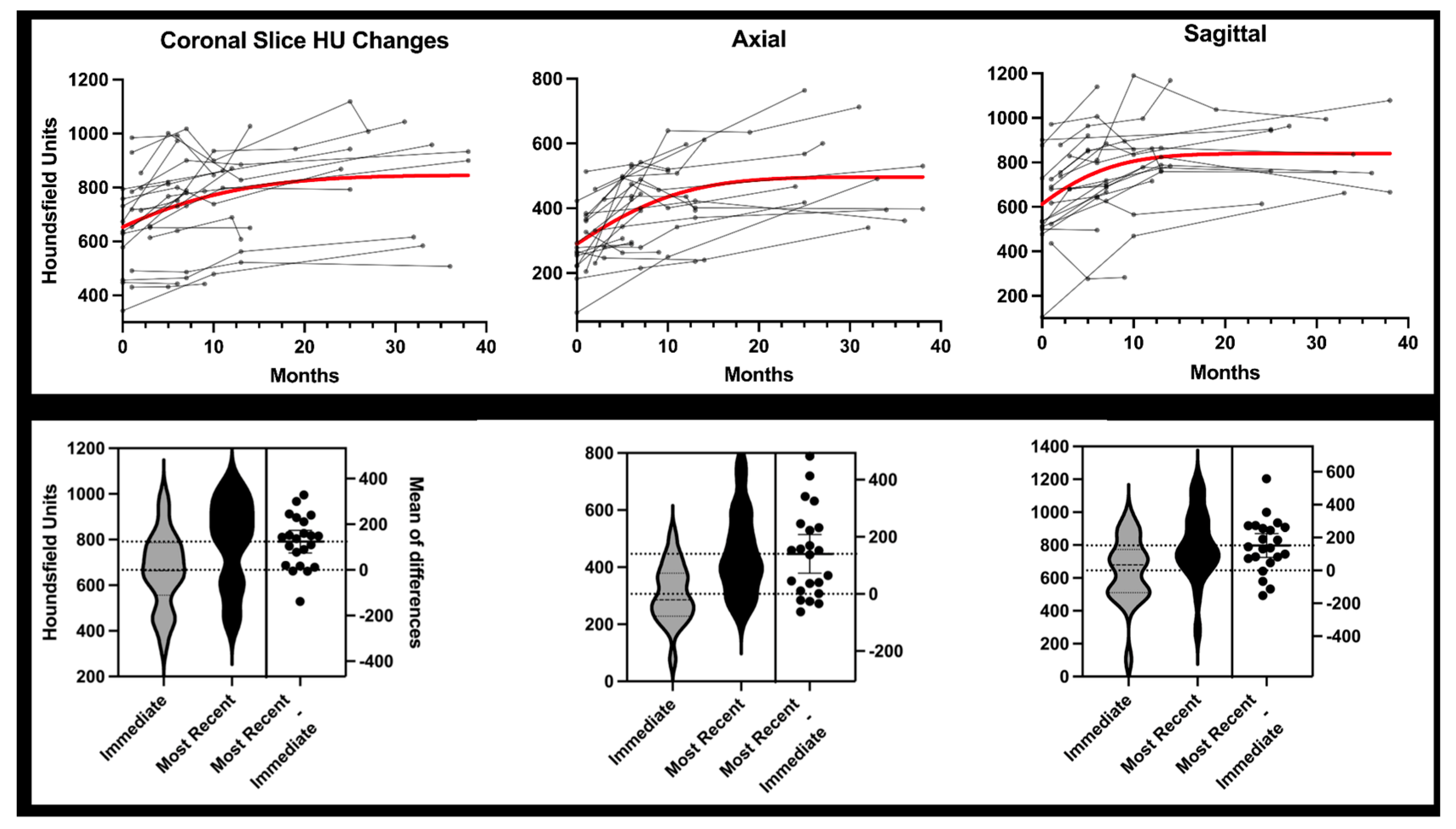
3.2. Two-Dimensional Analysis
3.3. Three-Dimensional Analysis
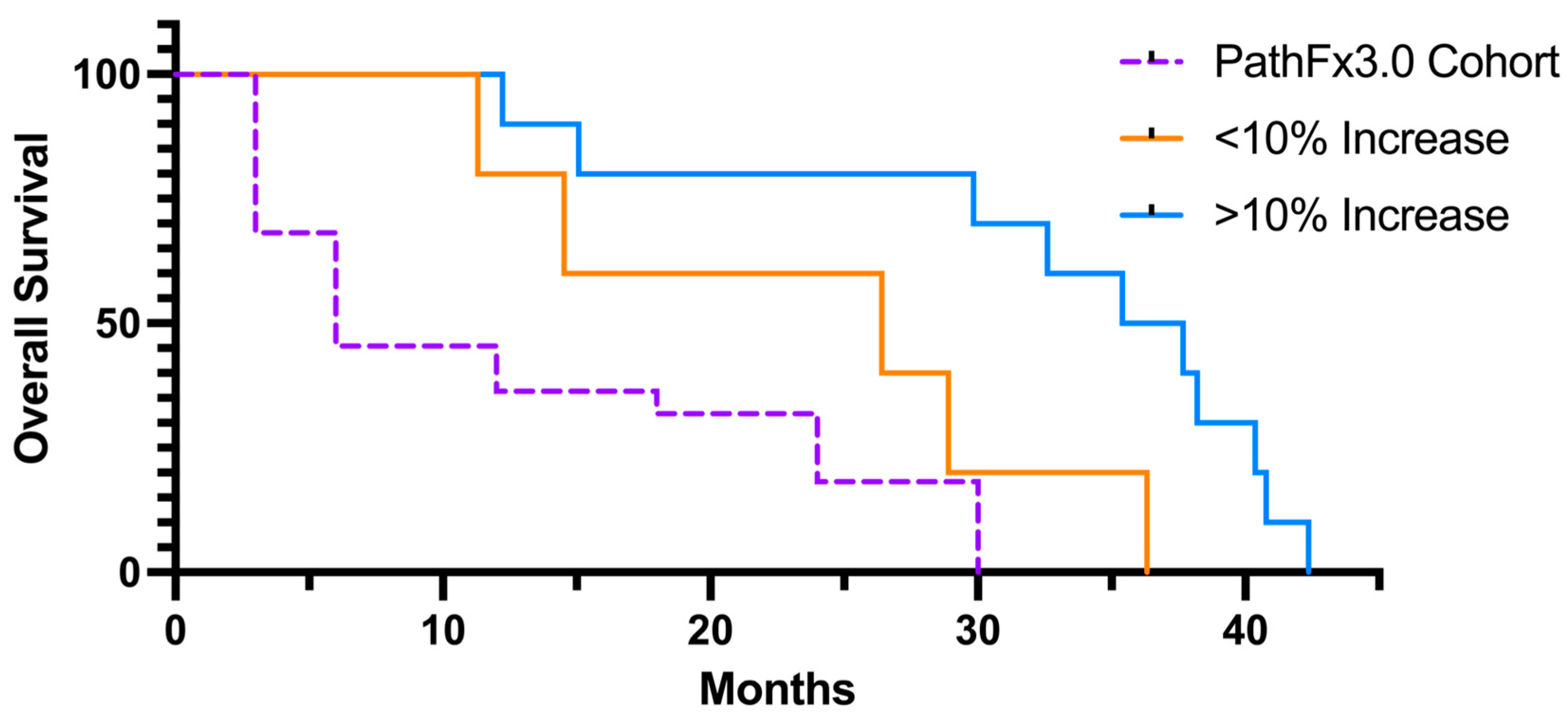
3.4. Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gazendam, A.; Axelrod, D.; Wilson, D.; Ghert, M. Emerging Concepts in the Surgical Management of Peri-Acetabular Metastatic Bone Disease. Curr. Oncol. 2021, 28, 2731–2740. [Google Scholar] [CrossRef] [PubMed]
- Kask, G.; Nieminen, J.; Van Iterson, V.; Naboistsikov, M.; Pakarinen, T.K.; Laitinen, M.K. Modified Harrington’s procedure for periacetabular metastases in 89 cases: A reliable method for cancer patients with good functional outcome, especially with long expected survival. Acta Orthop. 2020, 91, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.J.; Racano, A.; Yeung, H.; Farrokhyar, F.; Ghert, M.; Deheshi, B.M. Surgical Management of Bone Metastases: Quality of Evidence and Systematic Review. Ann. Surg. Oncol. 2014, 21, 4081–4089. [Google Scholar] [CrossRef] [PubMed]
- Assouline, J.; Tselikas, L.; Roux, C.; Yevich, S.; Delpla, A.; Najafi, A.; Al Ahmar, M.; Bijot, J.-C.; de Baère, T.; Deschamps, F. Prophylactic Percutaneous Consolidation of Large Osteolytic Tumors of the Pelvic Ring Using Fixation by Internal Cemented Screws. Radiol. Imaging Cancer 2021, 3, e200137. [Google Scholar] [CrossRef]
- Maccauro, G.; Liuzza, F.; Scaramuzzo, L.; Milani, A.; Muratori, F.; Rossi, B.; Waide, V.; Logroscino, G.; Logroscino, C.A.; Maffulli, N. Percutaneous acetabuloplasty for metastatic acetabular lesions. BMC Musculoskelet. Disord. 2008, 9, 66. [Google Scholar] [CrossRef]
- Dussik, C.M.; Toombs, C.; Alder, K.D.; Yu, K.E.; Berson, E.R.; Ibe, I.K.; Li, F.; Lindskog, D.M.; Friedlaender, G.E.; Latich, I.; et al. Percutaneous Ablation, Osteoplasty, Reinforcement, and Internal Fixation for Pain and Ambulatory Function in Periacetabular Osteolytic Malignancies. Radiology 2023, 307, e221401. [Google Scholar] [CrossRef]
- Guzik, G. Treatment of metastatic lesions localized in the acetabulum. J. Orthop. Surg. Res. 2016, 11, 54. [Google Scholar] [CrossRef]
- Morris, M.T.; Alder, K.D.; Moushey, A.; Munger, A.M.; Milligan, K.; Toombs, C.; Conway, D.; Lee, I.; Chen, F.; Tommasini, S.M.; et al. Biomechanical restoration of metastatic cancer-induced peri-acetabular bone defects by ablation-osteoplasty-reinforcement-internal fixation technique (AORIF): To screw or not to screw? Clin. Biomech. 2022, 92, 105565. [Google Scholar] [CrossRef]
- Jiang, W.; Friedlaender, G.; Lindskog, D.; Latich, I.; Lee, F.Y. Comparison of Percutaneous Interventional Ablation-Osteoplasty-Reinforcement-Internal Fixation (AORIF), Long Intramedullary Nailing, and Hemiarthroplasty for the Treatment of Focal Metastatic Osteolytic Lesions in the Femoral Head and Neck. Cardiovasc. Interv. Radiol. 2023, 46, 649–657. [Google Scholar] [CrossRef]
- Thanos, L.; Mylona, S.; Galani, P.; Tzavoulis, D.; Kalioras, V.; Tanteles, S.; Pomoni, M. Radiofrequency ablation of osseous metastases for the palliation of pain. Skelet. Radiol. 2008, 37, 189–194. [Google Scholar] [CrossRef]
- Rhim, H.; Lim, H.K. Radiofrequency Ablation of Hepatocellular Carcinoma: Pros and Cons. Gut Liver 2010, 4 (Suppl. S1), S113–S118. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Pritzlaff, S.; Jung, M.J.; Ghosh, P.; Hagedorn, J.M.; Tate, J.; Scarfo, K.; Strand, N.; Chakravarthy, K.; Sayed, D.; et al. Latest Evidence-Based Application for Radiofrequency Neurotomy (LEARN): Best Practice Guidelines from the American Society of Pain and Neuroscience (ASPN). J. Pain Res. 2021, 14, 2807–2831. [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; Lippi, L.; Curci, C.; Calafiore, D.; Cisari, C.; Ammendolia, A.; Invernizzi, M. Effectiveness of Combined Treatment Using Physical Exercise and Ultrasound-Guided Radiofrequency Ablation of Genicular Nerves in Patients with Knee Osteoarthritis. Appl. Sci. 2021, 11, 4338. [Google Scholar] [CrossRef]
- Atay, M.; Altun, S. Outcomes of Radiofrequency Ablation Therapy of Great Saphenous Veins Insufficiency. J. Coll. Physicians Surg. Pak. 2022, 32, 1009–1013. [Google Scholar]
- Nielsen, J.C.; Johannessen, A.; Raatikainen, P.; Hindricks, G.; Walfridsson, H.; Kongstad, O.; Pehrson, S.; Englund, A.; Hartikainen, J.; Mortensen, L.S.; et al. Radiofrequency Ablation as Initial Therapy in Paroxysmal Atrial Fibrillation. N. Engl. J. Med. 2012, 367, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- da Costa, A.C.; Sodergren, M.; Jayant, K.; Santa Cruz, F.; Spalding, D.; Pai, M.; Habib, N. Radiofrequency combined with immunomodulation for hepatocellular carcinoma: State of the art and innovations. World J. Gastroenterol. 2020, 26, 2040–2048. [Google Scholar] [CrossRef]
- Back, J.; Nguyen, M.N.; Li, L.; Lee, S.; Lee, I.; Chen, F.; Gillinov, L.; Chung, Y.-H.; Alder, K.D.; Kwon, H.-K.; et al. Inflammatory conversion of quiescent osteoblasts by metastatic breast cancer cells through pERK1/2 aggravates cancer-induced bone destruction. Bone Res. 2021, 9, 43. [Google Scholar] [CrossRef]
- Coolens, C.; Childs, P.J. Calibration of CT Hounsfield units for radiotherapy treatment planning of patients with metallic hip prostheses: The use of the extended CT-scale. Phys. Med. Biol. 2003, 48, 1591–1603. [Google Scholar] [CrossRef]
- Yaprak, G.; Gemici, C.; Seseogullari, O.O.; Karabag, I.S.; Cini, N. CT Derived Hounsfield Unit: An Easy Way to Determine Osteoporosis and Radiation Related Fracture Risk in Irradiated Patients. Front. Oncol. 2020, 10, 742. [Google Scholar] [CrossRef]
- Batawil, N.; Sabiq, S. Hounsfield unit for the diagnosis of bone mineral density disease: A proof of concept study. Radiography 2016, 22, e93–e98. [Google Scholar] [CrossRef]
- Elarjani, T.; Warner, T.; Nguyen, K.; Nguyen, S.; Urakov, T.M. Quantifying Bone Quality Using Computed Tomography Hounsfield Units in the Mid-sagittal View of the Lumbar Spine. World Neurosurg. 2021, 151, e418–e425. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.K.; Kim, S.M.; Lim, J.K. Diagnostic efficacy of Hounsfield units in spine CT for the assessment of real bone mineral density of degenerative spine: Correlation study between T-scores determined by DEXA scan and Hounsfield units from CT. Acta Neurochir. 2016, 158, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.B.; Wedin, R.; Fabbri, N.; Boland, P.; Healey, J.; Forsberg, J.A. External Validation of PATHFx Version 3.0 in Patients Treated Surgically and Nonsurgically for Symptomatic Skeletal Metastases. Clin. Orthop. Relat. Res. 2020, 478, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, D.; Lee, F. Bisphosphonate-loaded Bone Cement as a Local Adjuvant Therapy for Giant Cell Tumor of Bone: A 1 to 12-Year Follow-up Study. Am. J. Clin. Oncol. 2018, 42, 1. [Google Scholar] [CrossRef]
- Sandrasegaran, K.; Rydberg, J.; Tann, M.; Hawes, D.; Kopecky, K.; Maglinte, D. Benefits of routine use of coronal and sagittal reformations in multi-slice CT examination of the abdomen and pelvis. Clin. Radiol. 2007, 62, 340–347. [Google Scholar] [CrossRef]
- Ji, T.; Guo, W.; Yang, R.-L.; Tang, S.; Sun, X. Clinical outcome and quality of life after surgery for peri-acetabular metastases. J. Bone Jt. Surg. Br. 2011, 93, 1104–1110. [Google Scholar] [CrossRef]
- Araneta, K.T.S.; Rizkallah, M.; Boucher, L.-M.; Turcotte, R.E.; Aoude, A. Joint-sparing reconstruction for extensive periacetabular metastases: Literature review and a novel minimally invasive surgical technique. J. Bone Oncol. 2022, 34, 100428. [Google Scholar] [CrossRef]
- Khorana, A.A.; Tullio, K.; Elson, P.; Pennell, N.A.; Grobmyer, S.R.; Kalady, M.F.; Raymond, D.; Abraham, J.; Klein, E.A.; Walsh, R.M.; et al. Time to initial cancer treatment in the United States and association with survival over time: An observational study. PLoS ONE 2019, 14, e0213209. [Google Scholar]
- Inagaki, N.; Tanaka, T.; Udaka, J.; Akiyama, S.; Matsuoka, T.; Saito, M. Distribution of hounsfield unit values in the pelvic bones: A comparison between young men and women with traumatic fractures and older men and women with fragility fractures: A retrospective cohort study. BMC Musculoskelet. Disord. 2022, 23, 305. [Google Scholar] [CrossRef]
- Simion, G.; Eckardt, N.; Senft, C.; Schwarz, F. Bone density of the axis (C2) measured using Hounsfield units of computed tomography. J. Orthop. Surg. Res. 2023, 18, 93. [Google Scholar] [CrossRef]
- Free, J.; Eggermont, F.; Derikx, L.; van Leeuwen, R.; van der Linden, Y.; Jansen, W.; Raaijmakers, E.; Tanck, E.; Kaatee, R. The effect of different CT scanners, scan parameters and scanning setup on Hounsfield units and calibrated bone density: A phantom study. Biomed. Phys. Eng. Express 2018, 4, 055013. [Google Scholar] [CrossRef]
- Khan, M.A.; Williams, R.L.; Williams, D.F. In-vitro corrosion and wear of titanium alloys in the biological environment. Biomaterials 1996, 17, 2117–2126. [Google Scholar] [CrossRef] [PubMed]
- Cole, K.A.; Funk, G.A.; Rahaman, M.N.; McIff, T.E. Mechanical and degradation properties of poly(methyl methacrylate) cement/borate bioactive glass composites. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 108, 2765–2775. [Google Scholar] [CrossRef]
- Ayre, W.N.; Denyer, S.P.; Evans, S.L. Ageing and moisture uptake in polymethyl methacrylate (PMMA) bone cements. J. Mech. Behav. Biomed. Mater. 2014, 32, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Narod, S.A. Disappearing Breast Cancers. Curr. Oncol. 2012, 19, 59–60. [Google Scholar] [CrossRef] [PubMed]
- Ghomashchi, S.; Whyne, C.M.; Chinnery, T.; Habach, F.; Akens, M.K. Impact of radiofrequency ablation (RFA) on bone quality in a murine model of bone metastases. PLoS ONE 2021, 16, e0256076. [Google Scholar] [CrossRef]
- Wu, C.X.; Chen, M.-L.; Zhang, H.; Han, J.-J. Percutaneous Radiofrequency Ablation Combined With Chemotherapy Versus Chemotherapy Only for Ovarian Cancer Liver Metastasis. Front. Oncol. 2021, 11, 793024. [Google Scholar] [CrossRef]
- Walma, M.S.; for the Dutch Pancreatic Cancer Group; Rombouts, S.J.; Brada, L.J.H.; Rinkes, I.H.B.; Bosscha, K.; Bruijnen, R.C.; Busch, O.R.; Creemers, G.J.; Daams, F.; et al. Radiofrequency ablation and chemotherapy versus chemotherapy alone for locally advanced pancreatic cancer (PELICAN): Study protocol for a randomized controlled trial. Trials 2021, 22, 313. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, J.J.; Jang, I.G. Patient-Specific Phantomless Estimation of Bone Mineral Density and Its Effects on Finite Element Analysis Results: A Feasibility Study. Comput. Math. Methods Med. 2019, 2019, 4102410. [Google Scholar] [CrossRef]
| Age (u ± SD) | 60.7 ± 13.5 |
| Sex | |
| Male | 9 (41%) |
| Female | 13 (59%) |
| Deceased | 10 (45%) |
| Surgery Side | |
| Right | 8 (36%) |
| Left | 14 (64%) |
| Primary Malignancy | |
| Breast | 8 (36%) |
| Lung | 4 (18%) |
| Multiple Myeloma | 4 (18%) |
| Other | 6 (27%) |
| Bisphosphonates | 15 (68%) |
| Chemotherapy | 18 (82%) |
| Radiation Therapy | 12 (55%) |
| Progression | |
| Visceral | 20 (91%) |
| Skeletal | 18 (82%) |
| Baseline (u) | Longest Follow-Up (u) | Difference | p-Value | |
| Coronal | 666.9 ± 164.3 | 790.9 ± 189.0 | 124.0 ± 112.3 | <0.0001 |
| Axial | 306.3 ± 102.1 | 446.6 ± 143.6 | 140.3 ± 153.0 | 0.0003 |
| Sagittal | 645.6 ± 197.0 | 797.5 ± 207.6 | 151.9 ± 162.4 | 0.0003 |
| 3D Grayscale | 472.0 ± 128.1 | 645.5 ± 128.1 | 173.4 ± 166.4 | <0.0001 |
| wbHU | ||||
| Baseline | Comparison | Difference | p-Value | |
| 6 Months Comparison (n = 20) | 1336.7 ± 271.9 | 1502.0 ± 351.1 | 165.3 ± 164.0 | 0.0002 |
| 12 Months Comparison (n = 15) | 1338.2 ± 346.9 | 1569.1 ± 279.2 | 230.8 ± 214.0 | 0.0009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; Caruana, D.L.; Dussik, C.M.; Conway, D.; Latich, I.; Chapiro, J.; Lindskog, D.M.; Friedlaender, G.E.; Lee, F.Y. Bone Mass Changes Following Percutaneous Radiofrequency Ablation, Osteoplasty, Reinforcement, and Internal Fixation of Periacetabular Osteolytic Metastases. J. Clin. Med. 2023, 12, 4613. https://doi.org/10.3390/jcm12144613
Jiang W, Caruana DL, Dussik CM, Conway D, Latich I, Chapiro J, Lindskog DM, Friedlaender GE, Lee FY. Bone Mass Changes Following Percutaneous Radiofrequency Ablation, Osteoplasty, Reinforcement, and Internal Fixation of Periacetabular Osteolytic Metastases. Journal of Clinical Medicine. 2023; 12(14):4613. https://doi.org/10.3390/jcm12144613
Chicago/Turabian StyleJiang, Will, Dennis L. Caruana, Christopher M. Dussik, Devin Conway, Igor Latich, Julius Chapiro, Dieter M. Lindskog, Gary E. Friedlaender, and Francis Y. Lee. 2023. "Bone Mass Changes Following Percutaneous Radiofrequency Ablation, Osteoplasty, Reinforcement, and Internal Fixation of Periacetabular Osteolytic Metastases" Journal of Clinical Medicine 12, no. 14: 4613. https://doi.org/10.3390/jcm12144613
APA StyleJiang, W., Caruana, D. L., Dussik, C. M., Conway, D., Latich, I., Chapiro, J., Lindskog, D. M., Friedlaender, G. E., & Lee, F. Y. (2023). Bone Mass Changes Following Percutaneous Radiofrequency Ablation, Osteoplasty, Reinforcement, and Internal Fixation of Periacetabular Osteolytic Metastases. Journal of Clinical Medicine, 12(14), 4613. https://doi.org/10.3390/jcm12144613





