Clinical Outcomes of Total En Bloc Spondylectomy for Previously Irradiated Spinal Metastases: A Retrospective Propensity Score-Matched Comparative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Participants
2.3. Variables and Outcomes
2.4. Statistical Analysis
3. Results
3.1. Patients and Surgical Characteristics
3.2. Postoperative Complication Rate
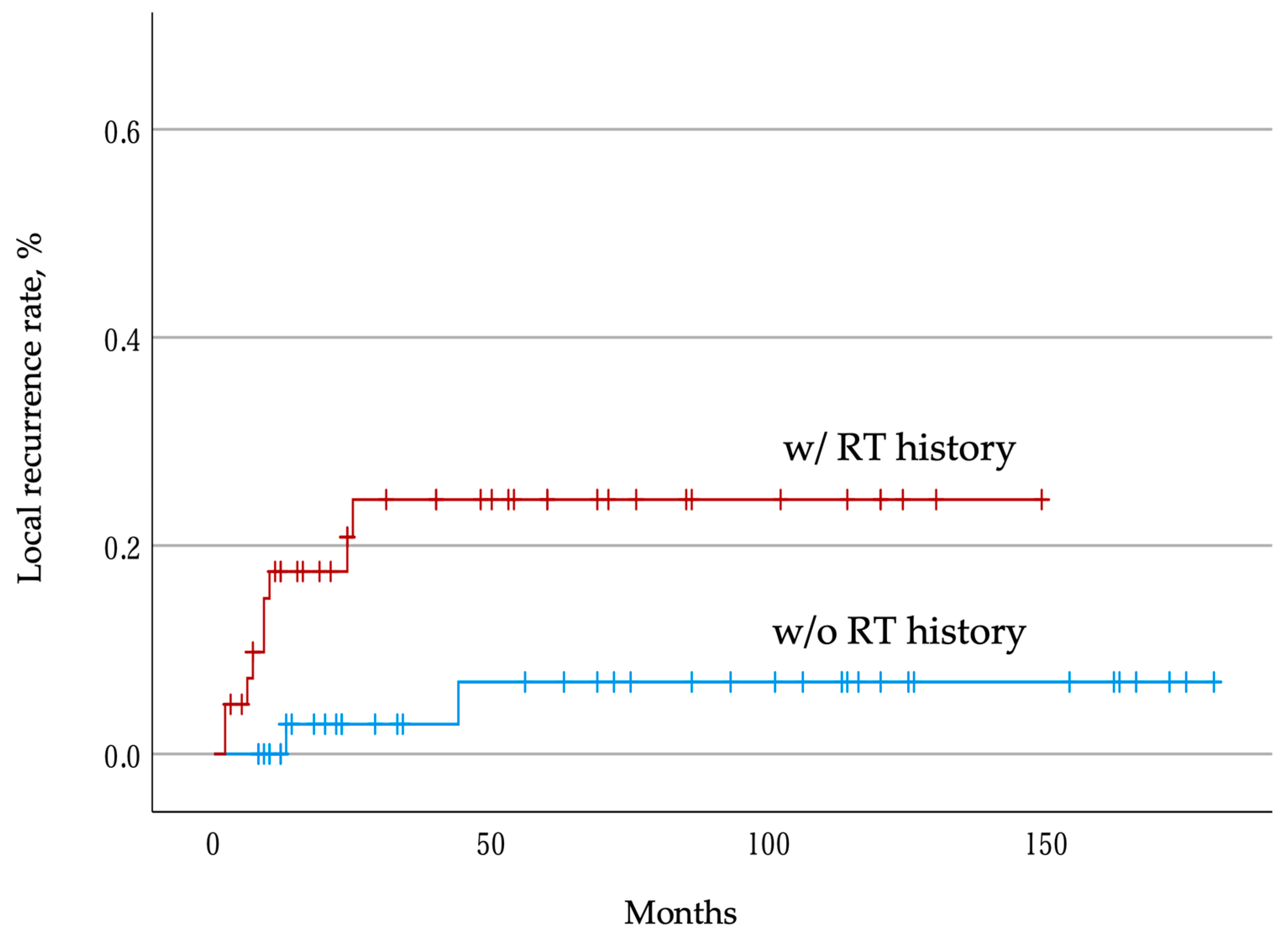
3.3. Postoperative Local Recurrence Rate
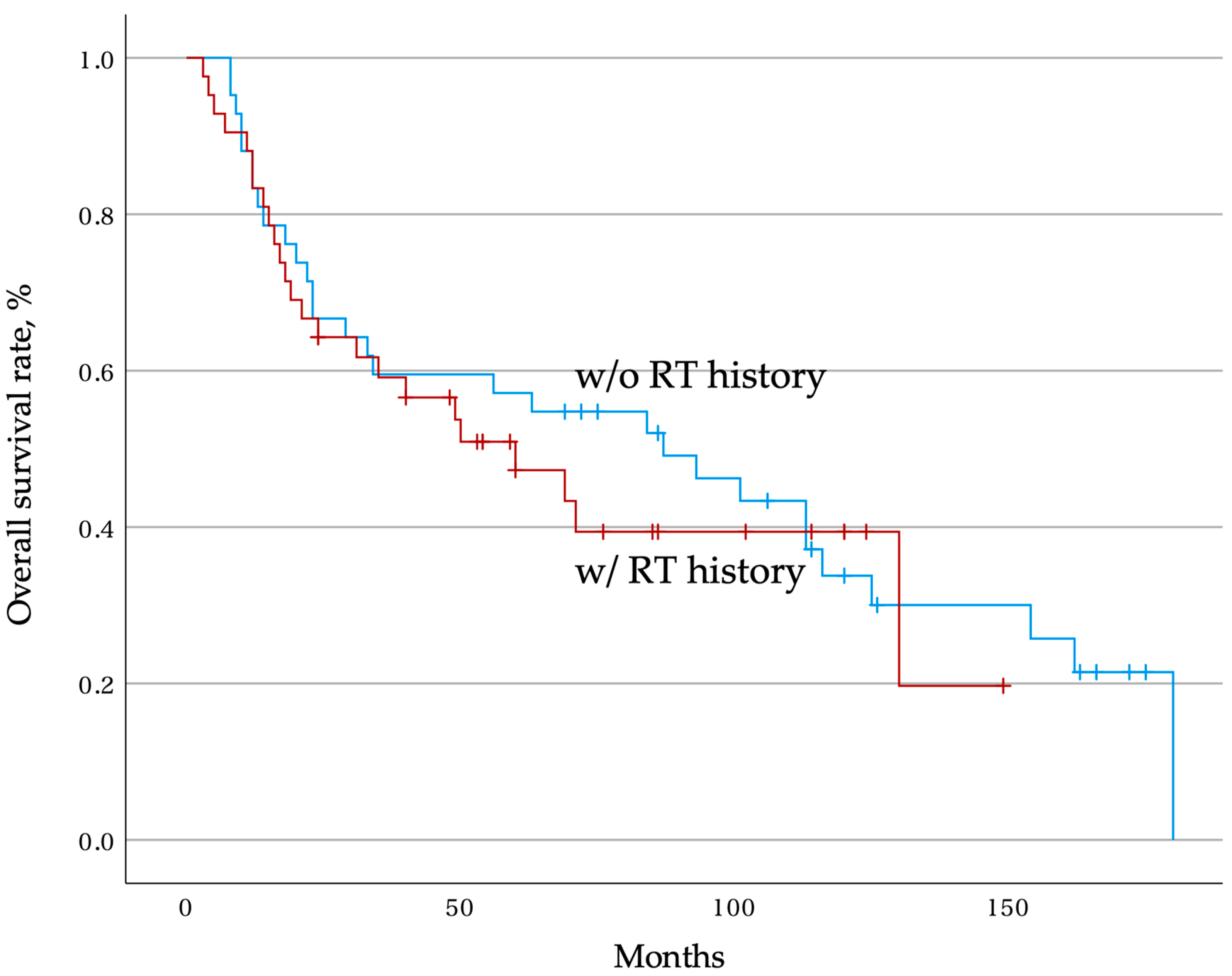
3.4. Overall Postoperative Survival Rate
3.5. Prognostic Factor
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Böhm, P.; Huber, J. The surgical treatment of bony metastases of the spine and limbs. J. Bone Jt. Surg. Br. 2002, 84, 521–529. [Google Scholar] [CrossRef]
- Klimo, P., Jr.; Schmidt, M.H. Surgical management of spinal metastases. Oncologist 2004, 9, 188–196. [Google Scholar] [CrossRef]
- Bilsky, M.H.; Lis, E.; Raizer, J.; Lee, H.; Boland, P. The diagnosis and treatment of metastatic spinal tumor. Oncologist 1999, 4, 459–469. [Google Scholar] [CrossRef]
- Byrne, T.N. Spinal cord compression from epidural metastases. N. Engl. J. Med. 1992, 327, 614–619. [Google Scholar] [CrossRef]
- Laufer, I.; Bilsky, M.H. Advances in the treatment of metastatic spine tumors: The future is not what it used to be. J. Neurosurg. Spine 2019, 30, 299–307. [Google Scholar] [CrossRef]
- Tomita, K.; Kawahara, N.; Baba, H.; Tsuchiya, H.; Nagata, S.; Toribatake, Y. Total en bloc spondylectomy for solitary spinal metastases. Int. Orthop. 1994, 18, 291–298. [Google Scholar] [CrossRef]
- Tomita, K.; Kawahara, N.; Baba, H.; Tsuchiya, H.; Fujita, T.; Toribatake, Y. Total en bloc spondylectomy. A new surgical technique for primary malignant vertebral tumors. Spine 1997, 22, 324–333. [Google Scholar] [CrossRef]
- Tomita, K.; Kawahara, N.; Murakami, H.; Demura, S. Total en bloc spondylectomy for spinal tumors: Improvement of the technique and its associated basic background. J. Orthop. Sci. 2006, 11, 3–12. [Google Scholar] [CrossRef]
- Kawahara, N.; Tomita, K.; Murakami, H.; Demura, S. Total en bloc spondylectomy for spinal tumors: Surgical techniques and related basic background. Orthop. Clin. N. Am. 2009, 40, 47–63. [Google Scholar] [CrossRef]
- Murakami, H.; Demura, S.; Kato, S.; Nishida, H.; Yoshioka, K.; Hayashi, H.; Inoue, K.; Ota, T.; Shinmura, K.; Yokogawa, N.; et al. Increase of IL-12 following reconstruction for total en bloc spondylectomy using frozen autografts treated with liquid nitrogen. PLoS ONE 2013, 8, e64818. [Google Scholar] [CrossRef]
- Motzer, R.J.; Jonasch, E.; Agarwal, N.; Bhayani, S.; Bro, W.P.; Chang, S.S.; Choueiri, T.K.; Costello, B.A.; Derweesh, I.H.; Fishman, M.; et al. Kidney cancer, version 2.2017, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2017, 15, 804–834. [Google Scholar] [CrossRef] [PubMed]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Van Cann, T.; Cornillie, J.; Wozniak, A.; Debiec-Rychter, M.; Sciot, R.; Hompes, D.; Vergote, I.; Schöffski, P. Retrospective A nalysisof Outcome of Patients with Metastatic leiomyosarcoma in a Tertiary Referral Center. Oncol. Res. Treat. 2018, 41, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Demura, S.; Murakami, H.; Yoshioka, K.; Shinmura, K.; Yokogawa, N.; Shimizu, T.; Kawahara, N.; Tsuchiya, H. Clinical outcomes and prognostic factors following the surgical resection of renal cell carcinoma spinal metastases. Cancer Sci. 2021, 112, 2416–2425. [Google Scholar] [CrossRef]
- Kato, S.; Murakami, H.; Demura, S.; Fujimaki, Y.; Yoshioka, K.; Yokogawa, N.; Tsuchiya, H. The impact of complete surgical resection of spinal metastases on the survival of patients with thyroid cancer. Cancer Med. 2016, 5, 2343–2349. [Google Scholar] [CrossRef]
- Kato, S.; Demura, S.; Shinmura, K.; Yokogawa, N.; Yonezawa, N.; Shimizu, T.; Oku, N.; Kitagawa, R.; Murakami, H.; Kawahara, N.; et al. Clinical outcomes and survivals after total en bloc spondylectomy for metastatic leiomyosarcoma in the spine. Eur. Spine J. 2020, 29, 3237–3244. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.C.; Boriani, S.; Gokaslan, Z.L.; Sundaresan, N. En bloc spondylectomy for spinal metastases: A review of techniques. Neurosurg. Focus 2003, 15, E6. [Google Scholar] [CrossRef]
- Yokogawa, N.; Murakami, H.; Demura, S.; Kato, S.; Yoshioka, K.; Hayashi, H.; Ishii, T.; Igarashi, T.; Fang, X.; Tsuchiya, H. Perioperative complications of total en bloc spondylectomy: Adverse effects of preoperative irradiation. PLoS ONE 2014, 9, e98797. [Google Scholar] [CrossRef]
- Igarashi, T.; Murakami, H.; Demura, S.; Kato, S.; Yoshioka, K.; Yokogawa, N.; Tsuchiya, H. Risk factors for local recurrence after total en bloc spondylectomy for metastatic spinal tumors: A retrospective study. J. Orthop. Sci. 2018, 23, 459–463. [Google Scholar] [CrossRef]
- Tokuhashi, Y.; Matsuzaki, H.; Oda, H.; Oshima, M.; Ryu, J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine 2005, 30, 2186–2191. [Google Scholar] [CrossRef]
- Rosenbaum, P.R.; Rubin, D.B. The central role of the propensity score in observational studies for causal effects. Biometrika 1983, 70, 41–55. [Google Scholar] [CrossRef]
- Boriani, S.; Saravanja, D.; Yamada, Y.; Varga, P.P.; Biagini, R.; Fisher, C.G. Challenges of local recurrence and cure in low grademalignant tumors of the spine. Spine 2009, 34, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Demura, S.; Kitagawa, R.; Yokogawa, N.; Shimizu, T.; Kobayashi, M.; Yamada, Y.; Nagatani, S.; Murakami, H.; Kawahara, N.; et al. Clinical outcomes following total en bloc spondylectomy for spinal metastases from lung cancer. J. Orthop. Sci. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Leithner, A.; Radl, R.; Gruber, G.; Hochegger, M.; Leithner, K.; Welkerling, H.; Rehak, P.; Windhager, R. Predictive value of seven preoperative prognostic scoring systems for spinal metastases. Eur. Spine J. 2008, 17, 1488–1495. [Google Scholar] [CrossRef]
- Hussain, A.K.; Vig, K.S.; Cheung, Z.B.; Phan, K.; Lima, M.C.; Kim, J.S.; Kaji, D.A.; Arvind, V.; Cho, S.K.-W. The impact of metastatic spinal tumor location on 30-day perioperative mortality and morbidity after surgical decompression. Spine 2018, 43, E648–E655. [Google Scholar] [CrossRef]
- Demura, S.; Kato, S.; Shinmura, K.; Yokogawa, N.; Shimizu, T.; Handa, M.; Annen, R.; Kobayashi, M.; Yamada, Y.; Murakami, H.; et al. Perioperative complications of total en bloc spondylectomy for spinal tumours. Bone Jt. J. 2021, 103, 976–983. [Google Scholar] [CrossRef]
- Laufer, I.; Rubin, D.G.; Lis, E.; Cox, B.W.; Stubblefield, M.D.; Yamada, Y.; Bilsky, M.H. The NOMS framework: Approach to the treatment of spinal metastatic tumors. Oncologist 2013, 18, 744–751. [Google Scholar] [CrossRef]
- Mehta, N.; Zavitsanos, P.J.; Moldovan, K.; Oyelese, A.; Fridley, J.S.; Gokaslan, Z.; Kinsella, T.J.; Hepel, J.T. Local failure and vertebral body fracture risk using multi fraction stereotactic body radiation therapy for spine metastases. Adv. Radiat. Oncol. 2018, 3, 245–251. [Google Scholar] [CrossRef]
- Ito, K.; Ogawa, H.; Shimizuguchi, T.; Nihei, K.; Furuya, T.; Tanaka, H.; Karasawa, K. Stereotactic body radiotherapy for spinal metastases: Clinical experience in 134 cases from a single Japanese institution. Technol. Cancer Res. Treat. 2018, 17, 1533033818806472. [Google Scholar] [CrossRef]
- Tseng, C.L.; Soliman, H.; Myrehaug, S.; Lee, Y.K.; Ruschin, M.; Atenafu, E.G.; Campbell, M.; Maralani, P.J.; Yang, V.; Yee, A.; et al. Imaging-based outcomes for 24 Gy in 2 daily fractions for patients with de novo spinal metastases treated with spine stereotactic body radiation therapy (SBRT). Int. J. Radiat. Oncol. Biol. Phys. 2018, 102, 499–507. [Google Scholar] [CrossRef]
- Bishop, A.J.; Tao, R.; Rebueno, N.C.; Christensen, E.N.; Allen, P.K.; Wang, X.A.; Amini, B.; Tannir, N.M.; Tatsui, C.E.; Rhines, L.D.; et al. Outcomes for spine stereotactic body radiation therapy and an analysis of predictors of local recurrence. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 1016–1026. [Google Scholar] [CrossRef]
- Guckenberger, M.; Mantel, F.; Gerszten, P.C.; Flickinger, J.C.; Sahgal, A.; Létourneau, D.; Grills, I.S.; Jawad, M.; Fahim, D.K.; Shin, J.H.; et al. Safety and efficacy of stereotactic body radiotherapy as primary treatment for vertebral metastases: A multi institutional analysis. Radiat. Oncol. 2014, 9, 226. [Google Scholar] [CrossRef]
- Kato, S.; Demura, S.; Murakami, H.; Shinmura, K.; Yokogawa, N.; Annen, R.; Kobayashi, M.; Yamada, Y.; Nagatani, S.; Kawahara, N.; et al. Medium to long-term clinical outcomes of spinal metastasectomy. Cancers 2022, 14, 2852. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Demura, S.; Shinmura, K.; Yokogawa, N.; Shimizu, T.; Murakami, H.; Kawahara, N.; Tomita, K.; Tsuchiya, H. Surgical metastasectomy in the spine: A review article. Oncologist 2021, 26, e1833–e1843. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Gao, X.; Zhang, Y.; Wang, Y.; Wang, J.; Wang, T.; Liu, Y.; Hou, S.; Zhang, J.; Zhou, Y.; et al. A comparison of two different surgical procedures in the treatment of isolated spinal metastasis patients with metastatic spinal cord compression: A case-control study. Eur. Spine J. 2022, 31, 1583–1589. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, R.; Dea, N.; Detsky, J.S.; Sahgal, A. Management of recurrent or progressive spinal metastases: Reirradiation techniques and surgical principles. Neurooncol. Pract. 2020, 7, i45–i53. [Google Scholar] [CrossRef]
- Ghogawala, Z.; Mansfield, F.L.; Borges, L.F. Spinal radiation before surgical decompression adversely affects outcomes of surgery for symptomatic metastatic spinal cord compression. Spine 2001, 26, 818–824. [Google Scholar] [CrossRef]
- Dormand, E.L.; Banwell, P.E.; Goodacre, T.E. Radiotherapy and wound healing. Int. Wound J. 2005, 2, 112–127. [Google Scholar] [CrossRef]
- Yokogawa, N.; Murakami, H.; Demura, S.; Kato, S.; Yoshioka, K.; Tsuchiya, H. Incidental durotomy during total en bloc spondylectomy. Spine J. 2018, 18, 381–386. [Google Scholar] [CrossRef]
- Yokogawa, N.; Murakami, H.; Demura, S.; Kato, S.; Yoshioka, K.; Hayashi, H.; Ishii, T.; Igarashi, T.; Fang, X.; Tsuchiya, H. Postoperative cerebrospinal fluid leakage associated with total en bloc spondylectomy. Orthopedics 2015, 38, e561–e566. [Google Scholar] [CrossRef]
- Yokogawa, N.; Murakami, H.; Demura, S.; Kato, S.; Yoshioka, K.; Yamamoto, M.; Iseki, S.; Tsuchiya, H. Effects of radiation on spinal dura mater and surrounding tissue in mice. PLoS ONE 2015, 10, e0133806. [Google Scholar] [CrossRef] [PubMed]
- Demura, S.; Kawahara, N.; Murakami, H.; Nambu, K.; Kato, S.; Yoshioka, K.; Okayama, T.; Tomita, K. Surgical site infection in spinal metastasis: Risk factors and countermeasures. Spine 2009, 34, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Blyth, B.J.; Cole, A.J.; MacManus, M.P.; Martin, O.A. Radiation therapy-induced metastasis: Radiobiology and clinical implications. Clin. Exp. Metastasis 2018, 35, 223–236. [Google Scholar] [CrossRef]
- Ishihara, S.; Yasuda, M.; Nishioka, T.; Mizutani, T.; Kawabata, K.; Shirato, H.; Haga, H. Irradiation-tolerant lung cancer cells acquire invasive ability dependent on dephosphorylation of the myosin regulatory light chain. FEBS Lett. 2013, 587, 732–736. [Google Scholar] [CrossRef]
- Ishihara, S.; Haga, H.; Yasuda, M.; Mizutani, T.; Kawabata, K.; Shirato, H.; Nishioka, T. Integrin beta1-dependent invasive migration of irradiation-tolerant human lung adenocarcinoma cells in 3D collagen matrix. Biochem. Biophys. Res. Commun. 2010, 396, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T.; Ueda, Y.; Kawahara, N.; Baba, H.; Tomita, K. Local spread of metastatic vertebral tumors. A histologic study. Spine 1997, 22, 1905–1912. [Google Scholar] [CrossRef]
- Shimizu, T.; Demura, S.; Kato, S.; Shinmura, K.; Yokogawa, N.; Yonezawa, N.; Oku, N.; Kitagawa, R.; Handa, M.; Annen, R.; et al. Radiation disrupts the protective function of the spinal meninges in a mouse model of tumor-induced spinal cord compression. Clin. Orthop. Relat. Res. 2021, 479, 163–176. [Google Scholar] [CrossRef]
- Guckenberger, M.; Dahele, M.; Ong, W.L.; Sahgal, A. Stereotactic body radiation therapy for spinal metastases: Benefits and limitations. Semin. Radiat. Oncol. 2023, 33, 159–171. [Google Scholar] [CrossRef]
| w/ RT History (n = 46) | w/o RT History (n = 96) | p-Value | |
|---|---|---|---|
| Age at the time of surgery (years), mean ± SD | 56.3 ± 11.2 | 57.1 ± 10.4 | 0.7 |
| Sex: male, n (%) | 26 (56.5) | 54 (56.3) | 0.98 |
| Primary tumor | renal:17, thyroid:4, breast:4, lung:6, others:13, unknown:2 | renal:30, thyroid:13, breast:13, lung:7, others:30, unknown:3 | 0.72 |
| History of local surgery, n (%) | 5 (10.9) | 4 (4.2) | 0.15 |
| Level of spinal metastases: lumbar, n (%) | 12 (26.1) | 30 (31.3) | 0.53 |
| Epidural extension, n (%) | 40 (87.0) | 72 (75.0) | 0.1 |
| Frankel grade A–C, n (%) | 15 (32.6) | 18 (18.8) | 0.07 |
| ECOG PS score ≥ 3, n (%) | 16 (39.1) | 18 (18.8) | 0.04 * |
| Other bone metastases, n (%) | 21(45.7) | 32 (33.3) | 0.16 |
| Major organ metastases, n (%) | 12 (26.1) | 33 (34.4) | 0.32 |
| Revised Tokuhashi score, mean ± SD | 10.3 ± 2.2 | 11.3 ± 2.2 | 0.02 * |
| Number of resected vertebrae, mean ± SD | 1.8 ± 0.8 | 1.4 ± 0.7 | 0.02 * |
| Operative time (min), mean ± SD | 545.9 ± 182.6 | 496.0 ± 134.7 | 0.11 |
| Intraoperative bleeding (mL), mean ± SD | 869.2 ± 941.1 | 826.9 ± 942.1 | 0.81 |
| w/ RT History (n = 42) | w/o RT History (n = 42) | p-Value | |
|---|---|---|---|
| Age at the time of surgery (years), mean ± SD | 57.0 ± 10.4 | 55.5 ± 11.1 | 0.52 |
| Sex: male, n (%) | 23 (54.8) | 25 (59.5) | 0.66 |
| Primary tumor | renal:15, thyroid:3, breast:4, lung:6, others:12, unknown:2 | renal:18, thyroid:4, breast:4, lung:4, others:11, unknown:1 | 0.99 |
| History of local surgery, n (%) | 4 (9.5) | 2 (4.8) | 0.4 |
| Level of spinal metastases: lumbar, n (%) | 12 (28.6) | 18 (42.9) | 0.17 |
| Epidural extension, n (%) | 36 (85.7) | 37 (88.1) | 0.75 |
| Frankel grade A–C, n (%) | 12 (28.6) | 9 (21.4) | 0.45 |
| ECOG PS score ≥ 3, n (%) | 13 (31.0) | 10 (23.8) | 0.46 |
| Other bone metastases, n (%) | 17(40.5) | 15 (35.7) | 0.65 |
| Major organ metastases, n (%) | 11 (26.2) | 14 (33.3) | 0.47 |
| Revised Tokuhashi score, mean ± SD | 10.5 ± 2.0 | 10.8 ± 2.2 | 0.65 |
| Number of resected vertebrae, mean ± SD | 1.7 ± 0.8 | 1.6 ± 0.8 | 0.6 |
| Operative time (min), mean ± SD | 546.1 ± 189.0 | 573.3 ± 118.3 | 0.44 |
| Intraoperative bleeding (mL), mean ± SD | 903.0 ± 975.5 | 1144.6 ± 965.3 | 0.26 |
| w/ RT History (n = 42) | w/o RT History (n = 42) | p-Value | |
|---|---|---|---|
| Postoperative complication, n (%) | 24 (57.1) | 15 (35.7) | 0.04 * |
| Number of complications, mean ± SD | 1.3 ± 1.3 | 0.6 ± 0.8 | 0.02 * |
| Wound dehiscence, n (%) | 7 (16.7%) | 3 (7.1%) | 0.31 |
| Surgical site infection, n (%) | 5 (11.9%) | 1 (2.4%) | 0.2 |
| Cerebrospinal fluid leakage, n (%) | 8 (19.0%) | 2 (4.8%) | 0.09 |
| Respiratory, n (%) | 10 (23.8%) | 3 (7.1%) | 0.07 |
| Cardiovascular, n (%) | 4 (9.5%) | 2 (4.8%) | 0.68 |
| Gastrointestinal, n (%) | 3 (7.1%) | 1 (2.4%) | 0.62 |
| Neurological, n (%) | 9 (21.4%) | 6 (14.3%) | 0.39 |
| Variables | HR | 95%CI | p-Value |
|---|---|---|---|
| Age at the time of surgery (years) | 1.01 | 0.98–1.03 | 0.69 |
| Sex: male | 0.93 | 0.59–1.46 | 0.76 |
| History of radiotherapy | 1.18 | 0.72–1.91 | 0.51 |
| History of local surgery | 0.94 | 0.37–2.38 | 0.9 |
| ECOG PS score | 1.02 | 0.77–1.36 | 0.87 |
| Revised Tokuhashi score | 0.79 | 0.70–0.89 | <0.001 * |
| Level of spinal metastases: lumbar | 1.46 | 0.59–2.47 | 0.16 |
| Number of resected vertebrae | 1.14 | 0.82–1.58 | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yokogawa, N.; Kato, S.; Shimizu, T.; Kurokawa, Y.; Kobayashi, M.; Yamada, Y.; Nagatani, S.; Kawai, M.; Uto, T.; Murakami, H.; et al. Clinical Outcomes of Total En Bloc Spondylectomy for Previously Irradiated Spinal Metastases: A Retrospective Propensity Score-Matched Comparative Study. J. Clin. Med. 2023, 12, 4603. https://doi.org/10.3390/jcm12144603
Yokogawa N, Kato S, Shimizu T, Kurokawa Y, Kobayashi M, Yamada Y, Nagatani S, Kawai M, Uto T, Murakami H, et al. Clinical Outcomes of Total En Bloc Spondylectomy for Previously Irradiated Spinal Metastases: A Retrospective Propensity Score-Matched Comparative Study. Journal of Clinical Medicine. 2023; 12(14):4603. https://doi.org/10.3390/jcm12144603
Chicago/Turabian StyleYokogawa, Noriaki, Satoshi Kato, Takaki Shimizu, Yuki Kurokawa, Motoya Kobayashi, Yohei Yamada, Satoshi Nagatani, Masafumi Kawai, Takaaki Uto, Hideki Murakami, and et al. 2023. "Clinical Outcomes of Total En Bloc Spondylectomy for Previously Irradiated Spinal Metastases: A Retrospective Propensity Score-Matched Comparative Study" Journal of Clinical Medicine 12, no. 14: 4603. https://doi.org/10.3390/jcm12144603
APA StyleYokogawa, N., Kato, S., Shimizu, T., Kurokawa, Y., Kobayashi, M., Yamada, Y., Nagatani, S., Kawai, M., Uto, T., Murakami, H., Kawahara, N., & Demura, S. (2023). Clinical Outcomes of Total En Bloc Spondylectomy for Previously Irradiated Spinal Metastases: A Retrospective Propensity Score-Matched Comparative Study. Journal of Clinical Medicine, 12(14), 4603. https://doi.org/10.3390/jcm12144603








