Dynamics of Bone Disease Biomarkers Dickkopf-1 and Sclerostin in Patients with Multiple Myeloma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Methods
2.3. Statistical Analysis
3. Results
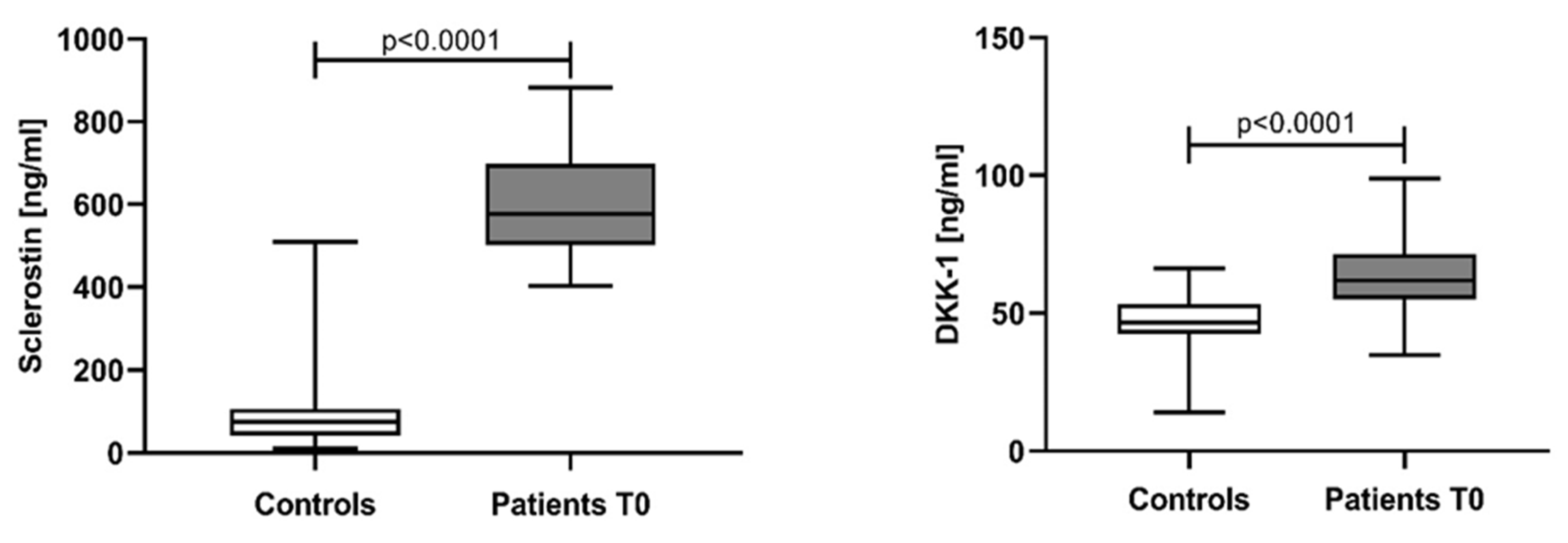
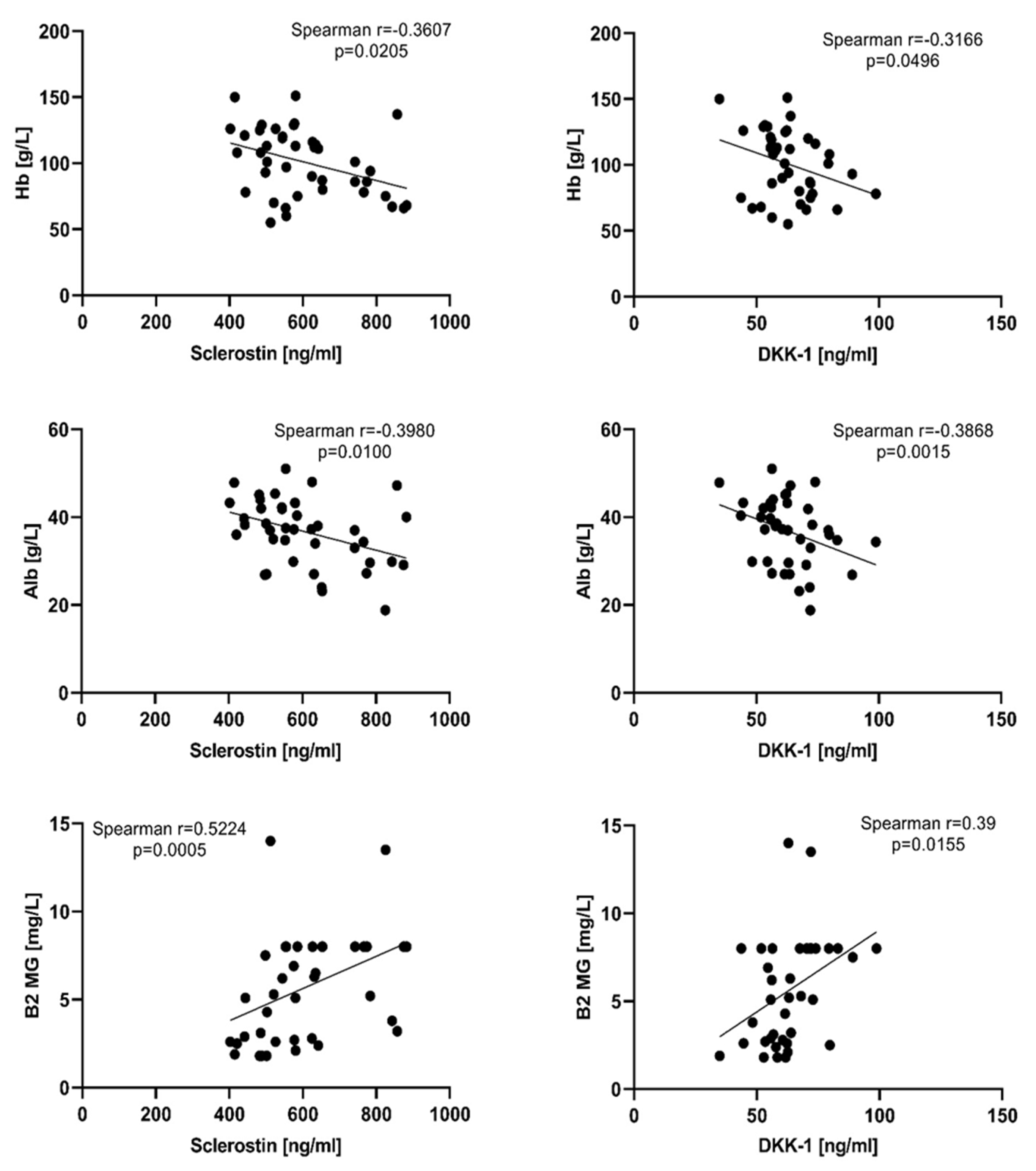
3.1. Sclerostin and Dickkopf-1 Assessed in NDMM Patients at Baseline (T0)
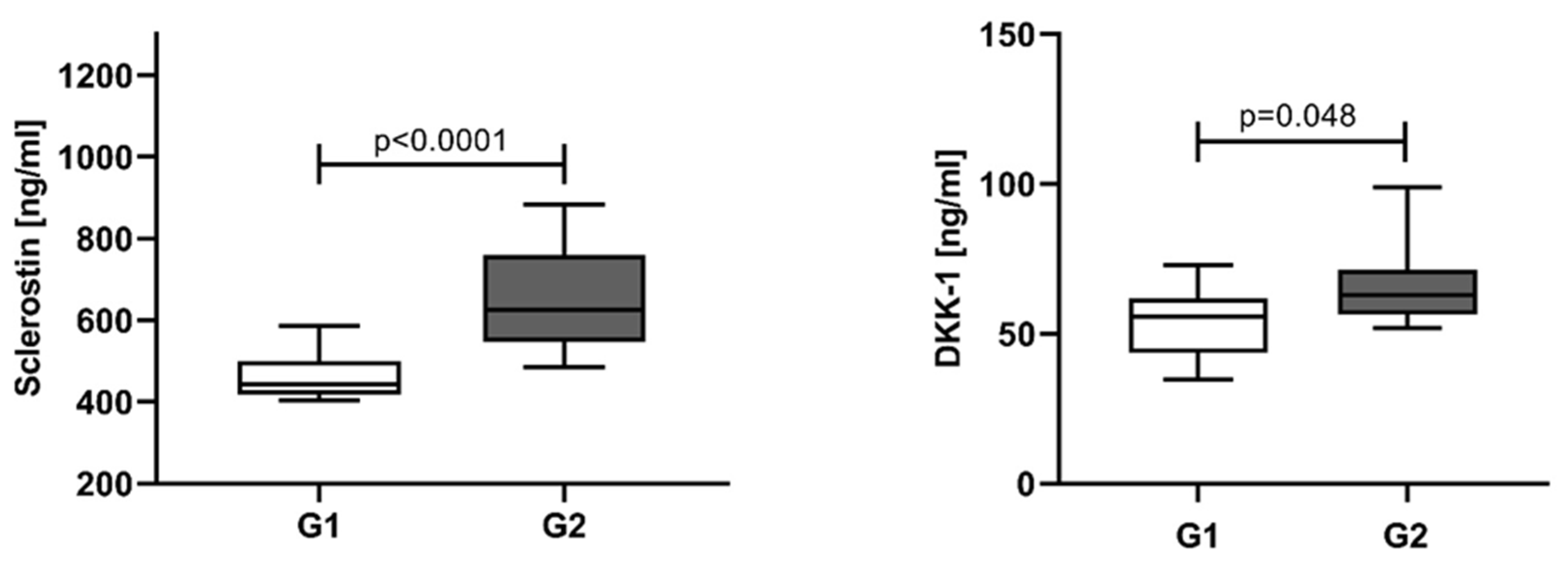
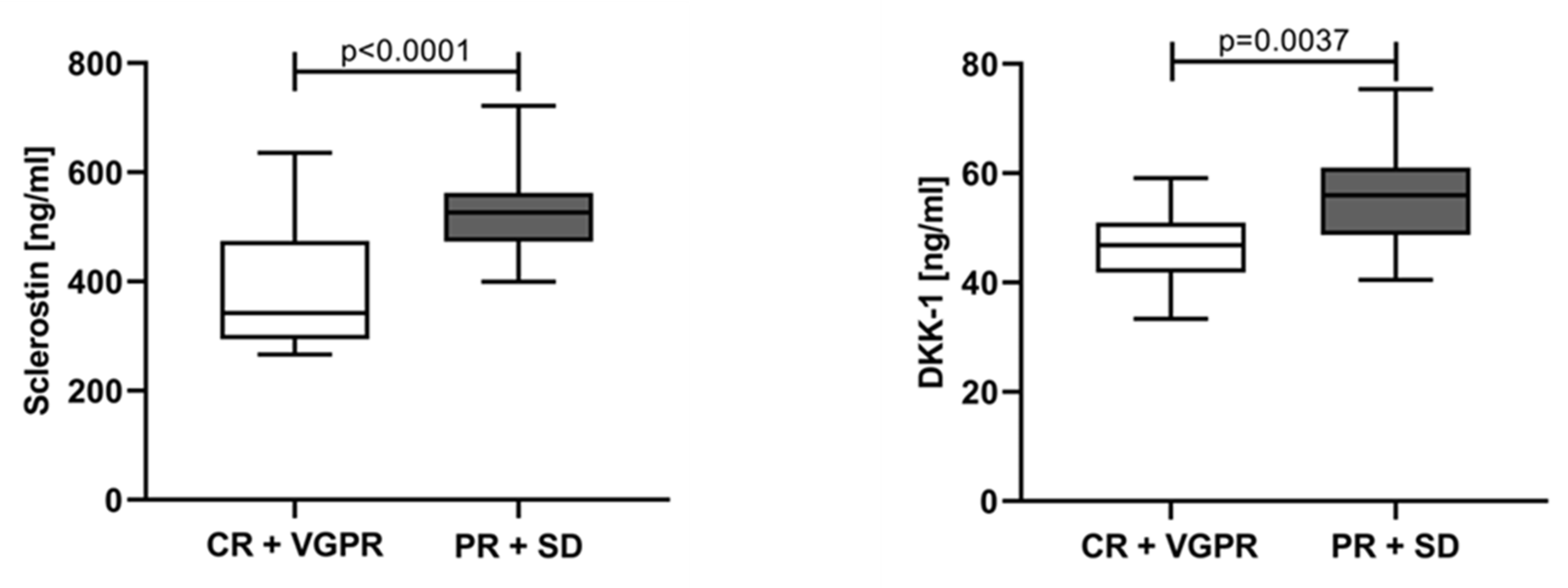
3.2. Sclerostin and Dickkopf-1 Assessed at Different Time Points and According to the Response of Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef]
- Cowan, A.J.; Allen, C.; Barac, A.; Basaleem, H.; Bensenor, I.; Curado, M.P.; Foreman, K.; Gupta, R.; Harvey, J.; Hosgood, H.D.; et al. Global Burden of Multiple Myeloma: A Systematic Analysis for the Global Burden of Disease Study 2016. JAMA Oncol. 2018, 4, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Gavriatopoulou, M.; Dimopoulos, M.A. Pathogenesis of bone disease in multiple myeloma: From bench to bedside. Blood Cancer J. 2018, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Panaroni, C.; Yee, A.J.; Raje, N.S. Myeloma and Bone Disease. Curr. Osteoporos. Rep. 2017, 15, 483–498. [Google Scholar] [CrossRef]
- Mukkamalla, S.K.R.; Malipeddi, D. Myeloma Bone Disease: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 6208. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-catenin signalling: Function, biological mechanisms, and therapeutic opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef]
- Yu, F.; Yu, C.; Li, F.; Zuo, Y.; Wang, Y.; Yao, L.; Wu, C.; Wang, C.; Ye, L. Wnt/β-catenin signaling in cancers and targeted therapies. Signal Transduct. Target. Ther. 2021, 6, 307. [Google Scholar] [CrossRef]
- Gau, Y.C.; Yeh, T.J.; Hsu, C.M.; Hsiao, S.Y.; Hsiao, H.H. Pathogenesis and Treatment of Myeloma-Related Bone Disease. Int. J. Mol. Sci. 2022, 23, 3112. [Google Scholar] [CrossRef]
- Zhang, F.; Zhuang, J. Pathophysiology and therapeutic advances in myeloma bone disease. Chronic Dis. Transl. Med. 2022, 8, 264–270. [Google Scholar] [CrossRef]
- Tian, E.; Zhan, F.; Walker, R.; Rasmussen, E.; Ma, Y.; Barlogie, B.; Shaughnessy, J.D., Jr. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N. Engl. J. Med. 2003, 349, 2483–2494. [Google Scholar] [CrossRef]
- Heath, D.J.; Chantry, A.D.; Buckle, C.H.; Coulton, L.; Shaughnessy, J.D., Jr.; Evans, H.R.; Snowden, J.A.; Stover, D.R.; Vanderkerken, K.; Croucher, P.I. Inhibiting Dickkopf-1 (Dkk1) removes suppression of bone formation and prevents the development of osteolytic bone disease in multiple myeloma. J. Bone Miner. Res. 2009, 24, 425–436. [Google Scholar] [CrossRef]
- Simic, M.K.; Mohanty, S.T.; Xiao, Y.; Cheng, T.L.; Taylor, V.E.; Charlat, O.; Croucher, P.I.; McDonald, M.M. Multi-Targeting DKK1 and LRP6 Prevents Bone Loss and Improves Fracture Resistance in Multiple Myeloma. J. Bone Miner. Res. 2023, 38, 814–828. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Y.W.; Chen, Y.; Stephens, O.; Brown, N.; Chen, B.; Epstein, J.; Barlogie, B.; Shaughnessy, J.D., Jr. Myeloma-derived Dickkopf-1 disrupts Wnt-regulated osteoprotegerin and RANKL production by osteoblasts: A potential mechanism underlying osteolytic bone lesions in multiple myeloma. Blood 2008, 112, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Koide, M.; Kobayashi, Y.; Yamashita, T.; Uehara, S.; Nakamura, M.; Hiraoka, B.Y.; Ozaki, Y.; Iimura, T.; Yasuda, H.; Takahashi, N.; et al. Bone Formation Is Coupled to Resorption via Suppression of Sclerostin Expression by Osteoclasts. J. Bone Miner. Res. 2017, 32, 2074–2086. [Google Scholar] [CrossRef]
- McDonald, M.M.; Reagan, M.R.; Youlten, S.E.; Mohanty, S.T.; Seckinger, A.; Terry, R.L.; Pettitt, J.A.; Simic, M.K.; Cheng, T.L.; Morse, A.; et al. Inhibiting the osteocyte-specific protein sclerostin increases bone mass and fracture resistance in multiple myeloma. Blood 2017, 129, 3452–3464. [Google Scholar] [CrossRef]
- Correns, A.; Zimmermann, L.A.; Baldock, C.; Sengle, G. BMP antagonists in tissue development and disease. Matrix Biol. Plus 2021, 11, 100071. [Google Scholar] [CrossRef]
- Terpos, E.; Christoulas, D.; Katodritou, E.; Bratengeier, C.; Gkotzamanidou, M.; Michalis, E.; Delimpasi, S.; Pouli, A.; Meletis, J.; Kastritis, E.; et al. Elevated circulating sclerostin correlates with advanced disease features and abnormal bone remodeling in symptomatic myeloma: Reduction post-bortezomib monotherapy. Int. J. Cancer 2012, 131, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Lee, S.H.; Koh, J.M. Potential Biomarkers to Improve the Prediction of Osteoporotic Fractures. Endocrinol. Metab. 2020, 35, 55–63. [Google Scholar] [CrossRef]
- Delgado-Calle, J.; Anderson, J.; Cregor, M.D.; Condon, K.W.; Kuhstoss, S.A.; Plotkin, L.I.; Bellido, T.; Roodman, G.D. Genetic deletion of Sost or pharmacological inhibition of sclerostin prevent multiple myeloma-induced bone disease without affecting tumor growth. Leukemia 2017, 31, 2686–2694. [Google Scholar] [CrossRef]
- Poutoglidou, F.; Samoladas, E.; Raikos, N.; Kouvelas, D. Efficacy and safety of anti-sclerostin antibodies in the treatment of osteoporosis: A meta-analysis and systematic review. J. Clin. Densitom. 2022, 25, 401–415. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Updated Diagnostic Criteria and Staging System for Multiple Myeloma. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, e418–e423. [Google Scholar] [CrossRef]
- Greipp, P.R.; San Miguel, J.; Durie, B.G.; Crowley, J.J.; Barlogie, B.; Bladé, J.; Boccadoro, M.; Child, J.A.; Avet-Loiseau, H.; Kyle, R.A.; et al. International staging system for multiple myeloma. J. Clin. Oncol. 2005, 23, 3412–3420. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef] [PubMed]
- Al Saleh, A.S.; Parmar, H.V.; Visram, A.; Muchtar, E.; Buadi, F.K.; Go, R.S.; Dispenzieri, A.; Kapoor, P.; Warsame, R.; Lacy, M.Q.; et al. Increased Bone Marrow Plasma-Cell Percentage Predicts Outcomes in Newly Diagnosed Multiple Myeloma Patients. Clin. Lymphoma Myeloma Leuk. 2020, 20, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Politou, M.C.; Heath, D.J.; Rahemtulla, A.; Szydlo, R.; Anagnostopoulos, A.; Dimopoulos, M.A.; Croucher, P.I.; Terpos, E. Serum concentrations of Dickkopf-1 protein are increased in patients with multiple myeloma and reduced after autologous stem cell transplantation. Int. J. Cancer 2006, 119, 1728–1731. [Google Scholar] [CrossRef]
- Kaiser, M.; Mieth, M.; Liebisch, P.; Oberländer, R.; Rademacher, J.; Jakob, C.; Kleeberg, L.; Fleissner, C.; Braendle, E.; Peters, M.; et al. Serum concentrations of DKK-1 correlate with the extent of bone disease in patients with multiple myeloma. Eur. J. Haematol. 2008, 80, 490–494. [Google Scholar] [CrossRef]
- Terpos, E.; Christoulas, D.; Gkotzamanidou, M.; Bratengeier, C.; Gavriatopoulou, M.; Woloszczuk, W.; Kastritis, E.; Dimopoulos, M. Dickkopf-1 and sclerostin in different phases of multiple myeloma; The effect of lenalidomide and dexamethasone treatment with or without bortezomib. Bone 2011, 48 (Suppl. 2), S254. [Google Scholar] [CrossRef]
- Heiland, G.R.; Zwerina, K.; Baum, W.; Kireva, T.; Distler, J.H.; Grisanti, M.; Asuncion, F.; Li, X.; Ominsky, M.; Richards, W.; et al. Neutralisation of Dkk-1 protects from systemic bone loss during inflammation and reduces sclerostin expression. Ann. Rheum. Dis. 2010, 69, 2152–2159. [Google Scholar] [CrossRef]
- Eda, H.; Santo, L.; Wein, M.N.; Hu, D.Z.; Cirstea, D.D.; Nemani, N.; Tai, Y.T.; Raines, S.E.; Kuhstoss, S.A.; Munshi, N.C.; et al. Regulation of Sclerostin Expression in Multiple Myeloma by Dkk-1: A Potential Therapeutic Strategy for Myeloma Bone Disease. J. Bone Miner. Res. 2016, 31, 1225–1234. [Google Scholar] [CrossRef]
- Brunetti, G.; Oranger, A.; Mori, G.; Specchia, G.; Rinaldi, E.; Curci, P.; Zallone, A.; Rizzi, R.; Grano, M.; Colucci, S. Sclerostin is overexpressed by plasma cells from multiple myeloma patients. Ann. N. Y. Acad. Sci. 2011, 1237, 19–23. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Wei, X.; Zhang, Q. Correlations of DKK1 with pathogenesis and prognosis of human multiple myeloma. Cancer Biomark. 2019, 24, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.T.; He, Y.C.; Zhou, S.Y.; Jiang, J.Z.; Huang, Y.M.; Liang, Y.Z.; Lai, Y.R. Bone marrow plasma macrophage inflammatory protein protein-1 alpha (MIP-1 alpha) and sclerostin in multiple myeloma: Relationship with bone disease and clinical characteristics. Leuk. Res. 2014, 38, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Mabille, C.; Ruyssen-Witrand, A.; Degboe, Y.; Gennero, I.; Loiseau, H.A.; Roussel, M.; Hebraud, B.; Nigon, D.; Attal, M.; Laroche, M. DKK1 and sclerostin are early markers of relapse in multiple myeloma. Bone 2018, 113, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Y.W.; Heuck, C.J.; Shaughnessy, J.D., Jr.; Barlogie, B.; Epstein, J. Proteasome inhibitors and bone disease. Semin. Hematol. 2012, 49, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Heider, U.; Kaiser, M.; Mieth, M.; Lamottke, B.; Rademacher, J.; Jakob, C.; Braendle, E.; Stover, D.; Sezer, O. Serum concentrations of DKK-1 decrease in patients with multiple myeloma responding to anti-myeloma treatment. Eur. J. Haematol. 2009, 82, 31–38. [Google Scholar] [CrossRef]
- Terpos, E.; Christoulas, D.; Kastritis, E.; Eleutherakis-Papaiakovou, E.; Gavriatopoulou, M.; Ziogas, D.; Migkou, M.; Panagiotidis, I.; Kalapanida, D.; Fotiou, D.; et al. Sclerostin Remains Elevated Even in the Plateau Phase of Myeloma Patients: Implications into the Pathogenesis of Osteoblast Dysfunction of Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2015, 15 (Suppl. 3), e229. [Google Scholar] [CrossRef]
- Lemaire, O.; Attal, M.; Bourin, P.; Laroche, M. DKK1 correlates with response and predicts rapid relapse after autologous stem cell transplantation in multiple myeloma. Eur. J. Haematol. 2010, 84, 276–277. [Google Scholar] [CrossRef]
- Terpos, E.; Kastritis, E.; Ntanasis-Stathopoulos, I.; Christoulas, D.; Papatheodorou, A.; Eleutherakis-Papaiakovou, E.; Kanellias, N.; Fotiou, D.; Ziogas, D.C.; Migkou, M.; et al. Consolidation therapy with the combination of bortezomib and lenalidomide (VR) without dexamethasone in multiple myeloma patients after transplant: Effects on survival and bone outcomes in the absence of bisphosphonates. Am. J. Hematol. 2019, 94, 400–407. [Google Scholar] [CrossRef]
- Lu, H.; Pundole, X.; Lee, H.C. The role of bone-modifying agents in myeloma bone disease. JBMR Plus 2021, 15, e10518. [Google Scholar] [CrossRef]



| Characteristic | n (%) |
|---|---|
| Patients | 41 (100%) |
| Controls | 33 (100%) |
| Age (years) | |
| Patients | 64.02 ± 12.14 * |
| Controls | 60.64 ± 7.56 * |
| Gender **: | |
| Patients (male/female) | 20/21 (48.78%/51.22%) |
| Controls (male/female) | 18/15 (54.54%/45.46%) |
| ISS stages: | |
| I | 16 (39.02%) |
| II | 6 (14.63%) |
| III | 19 (46.34%) |
| Bone disease status: | |
| G1 (≤3 osteolytic lesions) | 9 (21.95%) |
| G2 (>3 osteolytic lesions + bone fractures) | 32 (78.05%) |
| M-protein type: | |
| IgG | 23 (56.10%) |
| IgA | 6 (14.63%) |
| FLC (κ + λ) | 12 (29.27%) |
| MPCs infiltration in bone marrow: | |
| <60% | 17 (41.46%) |
| >60% | 24 (58.54%) |
| Parameters | Mean ± SD, (Range) |
|---|---|
| Hb (g/L) | 100.78 ± 25.36 (55–151) |
| WBC (×109/L) | 6.40 ± 2.95 (2.03–14.58) |
| Plt (×109/L) | 208.41 ± 103.31 (32–461) |
| Creatinine (µmol/L) | 126.05 ± 91.53 (53–449) |
| LDH (IU/L) | 425.76 ± 370.34 (200–2571) |
| Total protein (g/L) | 92.70 ± 20.99 (55–133) |
| Albumin (g/L) | 36.71 ± 7.58 (19–51) |
| Treatment Response | T1 (n = 22) | T2 (n = 11) | TA (n = 10) |
|---|---|---|---|
| CR, n (%) | 6 (27.3%) | - | 9 (90%) |
| VGPR, n (%) | 4 (18.2%) | 3 (27.3%) | 1 (10%) |
| PR, n (%) | 8 (36.4%) | 5 (45.5%) | - |
| SD, n (%) | 3 (13.6%) | 3 (27.2%) | - |
| PD, n (%) | 1 (4.5%) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerov, V.; Gerova, D.; Micheva, I.; Nikolova, M.; Mihaylova, G.; Galunska, B. Dynamics of Bone Disease Biomarkers Dickkopf-1 and Sclerostin in Patients with Multiple Myeloma. J. Clin. Med. 2023, 12, 4440. https://doi.org/10.3390/jcm12134440
Gerov V, Gerova D, Micheva I, Nikolova M, Mihaylova G, Galunska B. Dynamics of Bone Disease Biomarkers Dickkopf-1 and Sclerostin in Patients with Multiple Myeloma. Journal of Clinical Medicine. 2023; 12(13):4440. https://doi.org/10.3390/jcm12134440
Chicago/Turabian StyleGerov, Vladimir, Daniela Gerova, Ilina Micheva, Miglena Nikolova, Galya Mihaylova, and Bistra Galunska. 2023. "Dynamics of Bone Disease Biomarkers Dickkopf-1 and Sclerostin in Patients with Multiple Myeloma" Journal of Clinical Medicine 12, no. 13: 4440. https://doi.org/10.3390/jcm12134440
APA StyleGerov, V., Gerova, D., Micheva, I., Nikolova, M., Mihaylova, G., & Galunska, B. (2023). Dynamics of Bone Disease Biomarkers Dickkopf-1 and Sclerostin in Patients with Multiple Myeloma. Journal of Clinical Medicine, 12(13), 4440. https://doi.org/10.3390/jcm12134440






