High Prevalence of Non-Responders Based on Quadriceps Force after Pulmonary Rehabilitation in COPD
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Pulmonary Rehabilitation Program
2.3. Clinical Features
2.4. Quadriceps Maximal Voluntary Force
2.5. Statistical Analysis
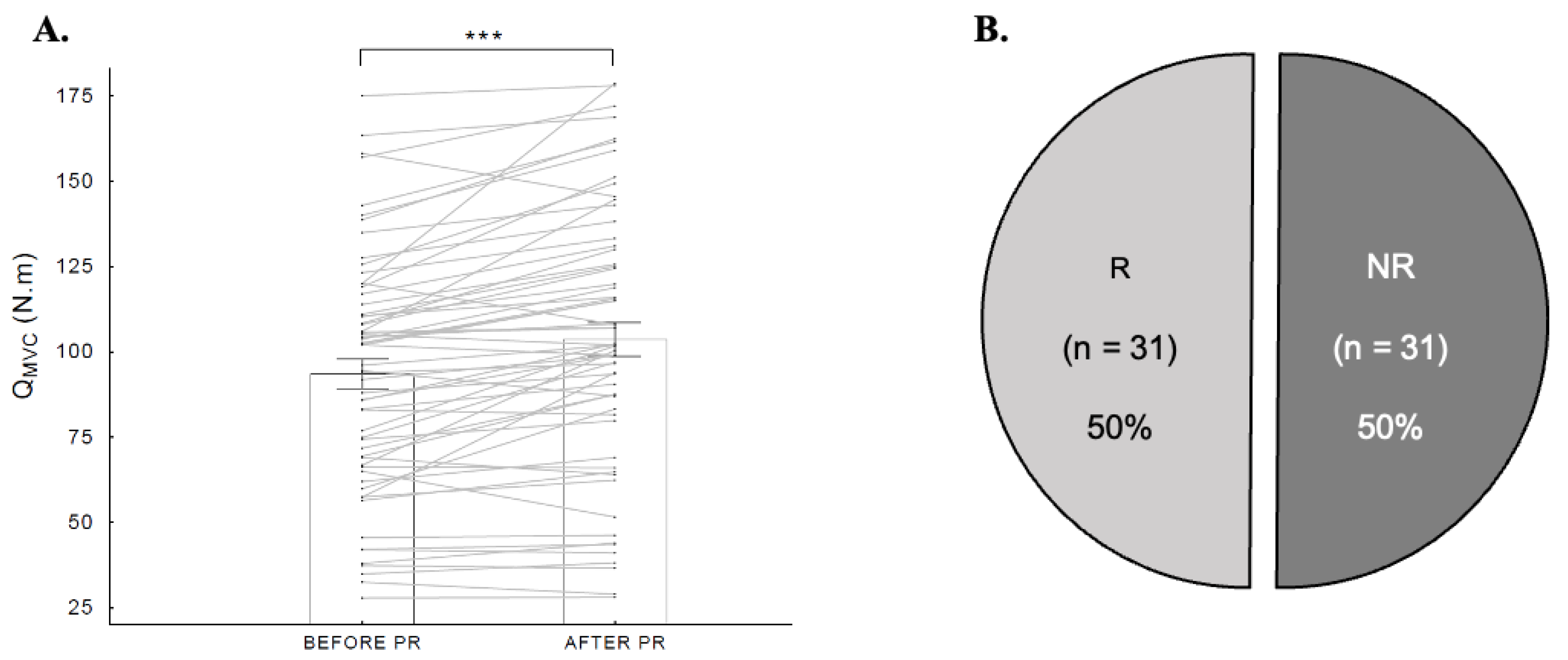
3. Results
3.1. Patient Characteristics
3.2. Prevalence and Characteristics of NRs
3.3. Predictors of Non-Response
4. Discussion
5. Conclusions
6. Methodological Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCarthy, B.; Casey, D.; Devane, D.; Murphy, K.; Murphy, E.; Lacasse, Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2015, 2, CD003793. [Google Scholar] [CrossRef]
- Spruit, M.A.; Singh, S.J.; Garvey, C.; ZuWallack, R.; Nici, L.; Rochester, C.; Hill, K.; Holland, A.E.; Lareau, S.C.; Man, W.D.-C.; et al. An official American Thoracic Society/European Respiratory Society statement: Key concepts and advances in pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2013, 188, e13–e64. [Google Scholar] [CrossRef]
- Gordon, C.S.; Waller, J.W.; Cook, R.M.; Cavalera, S.L.; Lim, W.T.; Osadnik, C.R. Effect of Pulmonary Rehabilitation on Symptoms of Anxiety and Depression in COPD: A Systematic Review and Meta-Analysis. Chest 2019, 156, 80–91. [Google Scholar] [CrossRef]
- Garrod, R.; Marshall, J.; Barley, E.; Jones, P.W. Predictors of success and failure in pulmonary rehabilitation. Eur. Respir. J. 2006, 27, 788–794. [Google Scholar] [CrossRef] [PubMed]
- Troosters, T.; Gosselink, R.; Decramer, M. Exercise training in COPD: How to distinguish responders from nonresponders. J. Cardpulm. Rehabil. 2001, 21, 10–17. [Google Scholar] [CrossRef]
- Scott, A.S.; Baltzan, M.A.; Fox, J.; Wolkove, N. Success in pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. Can. Respir. J. 2010, 17, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Spruit, M.A.; Augustin, I.M.L.; Vanfleteren, L.E.; Janssen, D.J.A.; Gaffron, S.; Pennings, H.-J.; Smeenk, F.; Pieters, W.; van den Bergh, J.J.A.M.; Michels, A.-J.; et al. Differential response to pulmonary rehabilitation in COPD: Multidimensional profiling. Eur. Respir. J. 2015, 46, 1625–1635. [Google Scholar] [CrossRef]
- Stoilkova-Hartmann, A.; Janssen, D.J.A.; Franssen, F.M.E.; Wouters, E.F.M. Differences in change in coping styles between good responders, moderate responders and non-responders to pulmonary rehabilitation. Respir. Med. 2015, 109, 1540–1545. [Google Scholar] [CrossRef]
- Simonÿ, C.; Højfeld, C.R.; Clausen, B.; Birkelund, R.; Bodtger, U. Experiences in responders and non-responders to pulmonary rehabilitation among people with chronic obstructive pulmonary disease: A clinical study with convergent mixed analysis. Disabil. Rehabil. 2022, 44, 4389–4397. [Google Scholar] [CrossRef] [PubMed]
- Seymour, J.M.; Spruit, M.A.; Hopkinson, N.S.; Natanek, S.A.; Man, W.D.-C.; Jackson, A.; Gosker, H.R.; Schols, A.M.W.J.; Moxham, J.; Polkey, M.I.; et al. The prevalence of quadriceps weakness in COPD and the relationship with disease severity. Eur. Respir. J. 2010, 36, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Gosselink, R.; Troosters, T.; Decramer, M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am. J. Respir. Crit. Care Med. 1996, 153, 976–980. [Google Scholar] [CrossRef] [PubMed]
- Locke, E.; Thielke, S.; Diehr, P.; Wilsdon, A.G.; Barr, G.; Hansel, N.; Kapur, V.K.; Krishnan, J.; Enright, P.; Heckbert, S.R.; et al. Effects of respiratory and non-respiratory factors on disability among older adults with airway obstruction: The Cardiovascular Health Study. COPD 2013, 10, 588–596. [Google Scholar] [CrossRef]
- Swallow, E.B.; Reyes, D.; Hopkinson, N.S.; Man, W.D.-C.; Porcher, R.; Cetti, E.J.; Moore, A.J.; Moxham, J.; Polkey, M.I. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax 2007, 62, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Holland, A.E.; Cox, N.S.; Houchen-Wolloff, L.; Rochester, C.L.; Garvey, C.; ZuWallack, R.; Nici, L.; Limberg, T.; Lareau, S.C.; Yawn, B.P.; et al. Defining Modern Pulmonary Rehabilitation. An Official American Thoracic Society Workshop Report. Ann. Am. Thorac. Soc. 2021, 18, e12–e29. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, J.; Wang, Y.; Xia, J.; Liu, X. Effects of Exercise Intervention on Peripheral Skeletal Muscle in Stable Patients With COPD: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 766841. [Google Scholar] [CrossRef] [PubMed]
- Garvey, C.; Bayles, M.P.; Hamm, L.F.; Hill, K.; Holland, A.; Limberg, T.M.; Spruit, M.A. Pulmonary Rehabilitation Exercise Prescription in Chronic Obstructive Pulmonary Disease: Review of Selected Guidelines: An Official Statement from the American Association of Cardiovascular and Pulmonary Rehabilitation. J. Cardiopulm. Rehabil. Prev. 2016, 36, 75–83. [Google Scholar] [CrossRef]
- Bove, A.M.; Lynch, A.D.; DePaul, S.M.; Terhorst, L.; Irrgang, J.J.; Fitzgerald, G.K. Test-Retest Reliability of Rating of Perceived Exertion and Agreement With 1-Repetition Maximum in Adults. J. Orthop. Sport. Phys. Ther. 2016, 46, 768–774. [Google Scholar] [CrossRef]
- Brzycki, M. Strength Testing—Predicting a One-Rep Max from Reps-to-Fatigue. J. Phys. Educ. Recreat. Dance 1993, 64, 88–90. [Google Scholar] [CrossRef]
- Abdul-Hameed, U.; Rangra, P.; Shareef, M.Y.; Hussain, M.E. Reliability of 1-Repetition Maximum Estimation for Upper and Lower Body Muscular Strength Measurement in Untrained Middle Aged Type 2 Diabetic Patients. Asian J. Sport. Med. 2012, 3, 267–273. [Google Scholar] [CrossRef]
- Holland, A.E.; Spruit, M.A.; Troosters, T.; Puhan, M.A.; Pepin, V.; Saey, D.; McCormack, M.C.; Carlin, B.W.; Sciurba, F.C.; Pitta, F.; et al. An official European Respiratory Society/American Thoracic Society technical standard: Field walking tests in chronic respiratory disease. Eur. Respir. J. 2014, 44, 1428–1446. [Google Scholar] [CrossRef]
- Ninot, G.; Soyez, F.; Fiocco, S.; Nassih, K.; Morin, A.J.S.; Prefaut, C. The VQ11, a short health-related quality of life questionnaire for routine practice in COPD patients. Rev. Mal. Respir. 2010, 27, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Sillen, M.J.H.; Franssen, F.M.E.; Delbressine, J.M.L.; Vaes, A.W.; Wouters, E.F.M.; Spruit, M.A. Efficacy of lower-limb muscle training modalities in severely dyspnoeic individuals with COPD and quadriceps muscle weakness: Results from the DICES trial. Thorax 2014, 69, 525–531. [Google Scholar] [CrossRef]
- Vaidya, T.; Beaumont, M.; de Bisschop, C.; Bazerque, L.; Le Blanc, C.; Vincent, A.; Ouksel, H.; Chambellan, A. Determining the minimally important difference in quadriceps strength in individuals with COPD using a fixed dynamometer. Int. J. Chron. Obstruct. Pulmon. Dis. 2018, 13, 2685–2693. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.Z.I.; Turin, T.C. Variable selection strategies and its importance in clinical prediction modelling. Fam. Med. Community Health 2020, 8, e000262. [Google Scholar] [CrossRef] [PubMed]
- Nici, L.; Donner, C.; Wouters, E.; Zuwallack, R.; Ambrosino, N.; Bourbeau, J.; Carone, M.; Celli, B.; Engelen, M.; Fahy, B.; et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am. J. Respir. Crit. Care Med. 2006, 173, 1390–1413. [Google Scholar] [CrossRef]
- Nyberg, A.; Carvalho, J.; Bui, K.-L.; Saey, D.; Maltais, F. Adaptations in limb muscle function following pulmonary rehabilitation in patients with COPD—A review. Rev. Port. Pneumol. Engl. Ed. 2016, 22, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Menon, M.K.; Houchen, L.; Harrison, S.; Singh, S.J.; Morgan, M.D.; Steiner, M.C. Ultrasound assessment of lower limb muscle mass in response to resistance training in COPD. Respir. Res. 2012, 13, 119. [Google Scholar] [CrossRef]
- Gibbons, J.D.; Chakraborti, S. Nonparametric Statistical Inference, 5th ed.; Chapman and Hall/CRC: New York, NY, USA, 2010; ISBN 978-0-429-11188-4. [Google Scholar]
- Leys, C.; Ley, C.; Klein, O.; Bernard, P.; Licata, L. Detecting outliers: Do not use standard deviation around the mean, use absolute deviation around the median. J. Exp. Soc. Psychol. 2013, 49, 764–766. [Google Scholar] [CrossRef]
- Jaitovich, A.; Angulo, M.; Lecuona, E.; Dada, L.A.; Welch, L.C.; Cheng, Y.; Gusarova, G.; Ceco, E.; Liu, C.; Shigemura, M.; et al. High CO2 Levels Cause Skeletal Muscle Atrophy via AMP-activated Kinase (AMPK), FoxO3a Protein, and Muscle-specific Ring Finger Protein 1 (MuRF1). J. Biol. Chem. 2015, 290, 9183–9194. [Google Scholar] [CrossRef]
- Korponay, T.C.; Balnis, J.; Vincent, C.E.; Singer, D.V.; Chopra, A.; Adam, A.P.; Ginnan, R.; Singer, H.A.; Jaitovich, A. High CO2 Downregulates Skeletal Muscle Protein Anabolism via AMP-activated Protein Kinase α2–mediated Depressed Ribosomal Biogenesis. Am. J. Respir. Cell Mol. Biol. 2020, 62, 74–86. [Google Scholar] [CrossRef]
- Balnis, J.; Lee, C.G.; Elias, J.A.; Jaitovich, A. Hypercapnia-Driven Skeletal Muscle Dysfunction in an Animal Model of Pulmonary Emphysema Suggests a Complex Phenotype. Front. Physiol. 2020, 11, 600290. [Google Scholar] [CrossRef] [PubMed]
- Brugarolas, J.; Lei, K.; Hurley, R.L.; Manning, B.D.; Reiling, J.H.; Hafen, E.; Witters, L.A.; Ellisen, L.W.; Kaelin, W.G. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004, 18, 2893–2904. [Google Scholar] [CrossRef] [PubMed]
- Favier, F.B.; Costes, F.; Defour, A.; Bonnefoy, R.; Lefai, E.; Baugé, S.; Peinnequin, A.; Benoit, H.; Freyssenet, D. Downregulation of Akt/mammalian target of rapamycin pathway in skeletal muscle is associated with increased REDD1 expression in response to chronic hypoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1659–R1666. [Google Scholar] [CrossRef] [PubMed]
- DeYoung, M.P.; Horak, P.; Sofer, A.; Sgroi, D.; Ellisen, L.W. Hypoxia regulates TSC1/2-mTOR signaling and tumor suppression through REDD1-mediated 14-3-3 shuttling. Genes Dev. 2008, 22, 239–251. [Google Scholar] [CrossRef]
- Brunelle, J.K.; Chandel, N.S. Oxygen deprivation induced cell death: An update. Apoptosis 2002, 7, 475–482. [Google Scholar] [CrossRef]
- Jensen, K.S.; Binderup, T.; Jensen, K.T.; Therkelsen, I.; Borup, R.; Nilsson, E.; Multhaupt, H.; Bouchard, C.; Quistorff, B.; Kjær, A.; et al. FoxO3A promotes metabolic adaptation to hypoxia by antagonizing Myc function. EMBO J. 2011, 30, 4554–4570. [Google Scholar] [CrossRef]
- Bakker, W.J.; Harris, I.S.; Mak, T.W. FOXO3a is activated in response to hypoxic stress and inhibits HIF1-induced apoptosis via regulation of CITED2. Mol. Cell 2007, 28, 941–953. [Google Scholar] [CrossRef]
- Zappe, A.C.; Uludağ, K.; Oeltermann, A.; Uğurbil, K.; Logothetis, N.K. The influence of moderate hypercapnia on neural activity in the anesthetized nonhuman primate. Cereb. Cortex 2008, 18, 2666–2673. [Google Scholar] [CrossRef]
- Thesen, T.; Leontiev, O.; Song, T.; Dehghani, N.; Hagler, D.; Huang, M.-X.; Buxton, R.; Halgren, E. Depression of Cortical Activity in Humans by Mild Hypercapnia. Hum. Brain Mapp. 2012, 33, 715–726. [Google Scholar] [CrossRef]
- Chaouat, A.; Weitzenblum, E.; Kessler, R.; Charpentier, C.; Enrhart, M.; Schott, R.; Levi-Valensi, P.; Zielinski, J.; Delaunois, L.; Cornudella, R.; et al. A randomized trial of nocturnal oxygen therapy in chronic obstructive pulmonary disease patients. Eur. Respir. J. 1999, 14, 1002–1008. [Google Scholar] [CrossRef]
- Alexandre, F.; Heraud, N.; Sanchez, A.M.J.; Tremey, E.; Oliver, N.; Guerin, P.; Varray, A. Brain Damage and Motor Cortex Impairment in Chronic Obstructive Pulmonary Disease: Implication of Nonrapid Eye Movement Sleep Desaturation. Sleep 2016, 39, 327–335. [Google Scholar] [CrossRef]
- Maltais, F.; Decramer, M.; Casaburi, R.; Barreiro, E.; Burelle, Y.; Debigaré, R.; Dekhuijzen, P.N.R.; Franssen, F.; Gayan-Ramirez, G.; Gea, J.; et al. An Official American Thoracic Society/European Respiratory Society Statement: Update on Limb Muscle Dysfunction in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2014, 189, e15–e62. [Google Scholar] [CrossRef]
- Serres, I.; Hayot, M.; Préfaut, C.; Mercier, J. Skeletal muscle abnormalities in patients with COPD: Contribution to exercise intolerance. Med. Sci. Sport. Exerc. 1998, 30, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, R.A.; Vilaró, J. Structural and functional changes of peripheral muscles in chronic obstructive pulmonary disease patients. Curr. Opin. Pulm. Med. 2010, 16, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Man, W.D.-C.; Kemp, P.; Moxham, J.; Polkey, M.I. Exercise and muscle dysfunction in COPD: Implications for pulmonary rehabilitation. Clin. Sci. 2009, 117, 281–291. [Google Scholar] [CrossRef]
- Alexandre, F.; Heraud, N.; Oliver, N.; Varray, A. Cortical Implication in Lower Voluntary Muscle Force Production in Non-Hypoxemic COPD Patients. PLoS ONE 2014, 9, e100961. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, F.; Héraud, N.; Tremey, E.; Oliver, N.; Bourgouin, D.; Varray, A. Specific motor cortex hypoexcitability and hypoactivation in COPD patients with peripheral muscle weakness. BMC Pulm. Med. 2020, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Nuzzo, J.L.; Barry, B.K.; Jones, M.D.; Gandevia, S.C.; Taylor, J.L. Effects of Four Weeks of Strength Training on the Corticomotoneuronal Pathway. Med. Sci. Sport. Exerc. 2017, 49, 2286–2296. [Google Scholar] [CrossRef] [PubMed]
- Eklund, D.; Pulverenti, T.; Bankers, S.; Avela, J.; Newton, R.; Schumann, M.; Häkkinen, K. Neuromuscular adaptations to different modes of combined strength and endurance training. Int. J. Sport. Med. 2015, 36, 120–129. [Google Scholar] [CrossRef]
- Schoenfeld, B.J.; Grgic, J.; Ogborn, D.; Krieger, J.W. Strength and Hypertrophy Adaptations Between Low- vs. High-Load Resistance Training: A Systematic Review and Meta-analysis. J. Strength Cond. Res. 2017, 31, 3508–3523. [Google Scholar] [CrossRef]
- MacMillan, N.J.; Kapchinsky, S.; Konokhova, Y.; Gouspillou, G.; de Sousa Sena, R.; Jagoe, R.T.; Baril, J.; Carver, T.E.; Andersen, R.E.; Richard, R.; et al. Eccentric Ergometer Training Promotes Locomotor Muscle Strength but Not Mitochondrial Adaptation in Patients with Severe Chronic Obstructive Pulmonary Disease. Front. Physiol. 2017, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Perrey, S. Brain activation associated with eccentric movement: A narrative review of the literature. Eur. J. Sport Sci. 2018, 18, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Gondin, J.; Guette, M.; Ballay, Y.; Martin, A. Electromyostimulation training effects on neural drive and muscle architecture. Med. Sci. Sport. Exerc. 2005, 37, 1291–1299. [Google Scholar] [CrossRef]
- Carson, R.G.; Buick, A.R. Neuromuscular electrical stimulation-promoted plasticity of the human brain. J. Physiol. 2021, 599, 2375–2399. [Google Scholar] [CrossRef]
- Mador, M.J.; Kufel, T.J.; Pineda, L.A.; Steinwald, A.; Aggarwal, A.; Upadhyay, A.M.; Khan, M.A. Effect of pulmonary rehabilitation on quadriceps fatiguability during exercise. Am. J. Respir. Crit. Care Med. 2001, 163, 930–935. [Google Scholar] [CrossRef]
- Vivodtzev, I.; Flore, P.; Lévy, P.; Wuyam, B. Voluntary activation during knee extensions in severely deconditioned patients with chronic obstructive pulmonary disease: Benefit of endurance training. Muscle Nerve 2008, 37, 27–35. [Google Scholar] [CrossRef]
- Vivodtzev, I.; Minet, C.; Wuyam, B.; Borel, J.-C.; Vottero, G.; Monneret, D.; Baguet, J.-P.; Lévy, P.; Pépin, J.-L. Significant improvement in arterial stiffness after endurance training in patients with COPD. Chest 2010, 137, 585–592. [Google Scholar] [CrossRef]
- Kongsgaard, M.; Backer, V.; Jørgensen, K.; Kjaer, M.; Beyer, N. Heavy resistance training increases muscle size, strength and physical function in elderly male COPD-patients—A pilot study. Respir. Med. 2004, 98, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, A.; Lindström, B.; Rickenlund, A.; Wadell, K. Low-load/high-repetition elastic band resistance training in patients with COPD: A randomized, controlled, multicenter trial. Clin. Respir. J. 2015, 9, 278–288. [Google Scholar] [CrossRef]
- Menon, M.K.; Houchen, L.; Singh, S.J.; Morgan, M.D.; Bradding, P.; Steiner, M.C. Inflammatory and satellite cells in the quadriceps of patients with COPD and response to resistance training. Chest 2012, 142, 1134–1142. [Google Scholar] [CrossRef]
- Houchen, L.; Deacon, S.; Sandland, C.; Collier, R.; Steiner, M.; Morgan, M.; Singh, S. Preservation of lower limb strength after a short course of pulmonary rehabilitation with no maintenance: A 6-month follow-up study. Physiotherapy 2011, 97, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Portilla-Cueto, K.; Medina-Pérez, C.; Romero-Pérez, E.M.; Hernández-Murúa, J.A.; Vila-Chã, C.; de Paz, J.A. Reliability of Isometric Muscle Strength Measurement and Its Accuracy Prediction of Maximal Dynamic Force in People with Multiple Sclerosis. Med. Kaunas Lith. 2022, 58, 948. [Google Scholar] [CrossRef] [PubMed]
- Marklund, S.; Bui, K.-L.; Nyberg, A. Measuring and monitoring skeletal muscle function in COPD: Current perspectives. Int. J. Chron. Obstruct. Pulmon. Dis. 2019, 14, 1825–1838. [Google Scholar] [CrossRef] [PubMed]
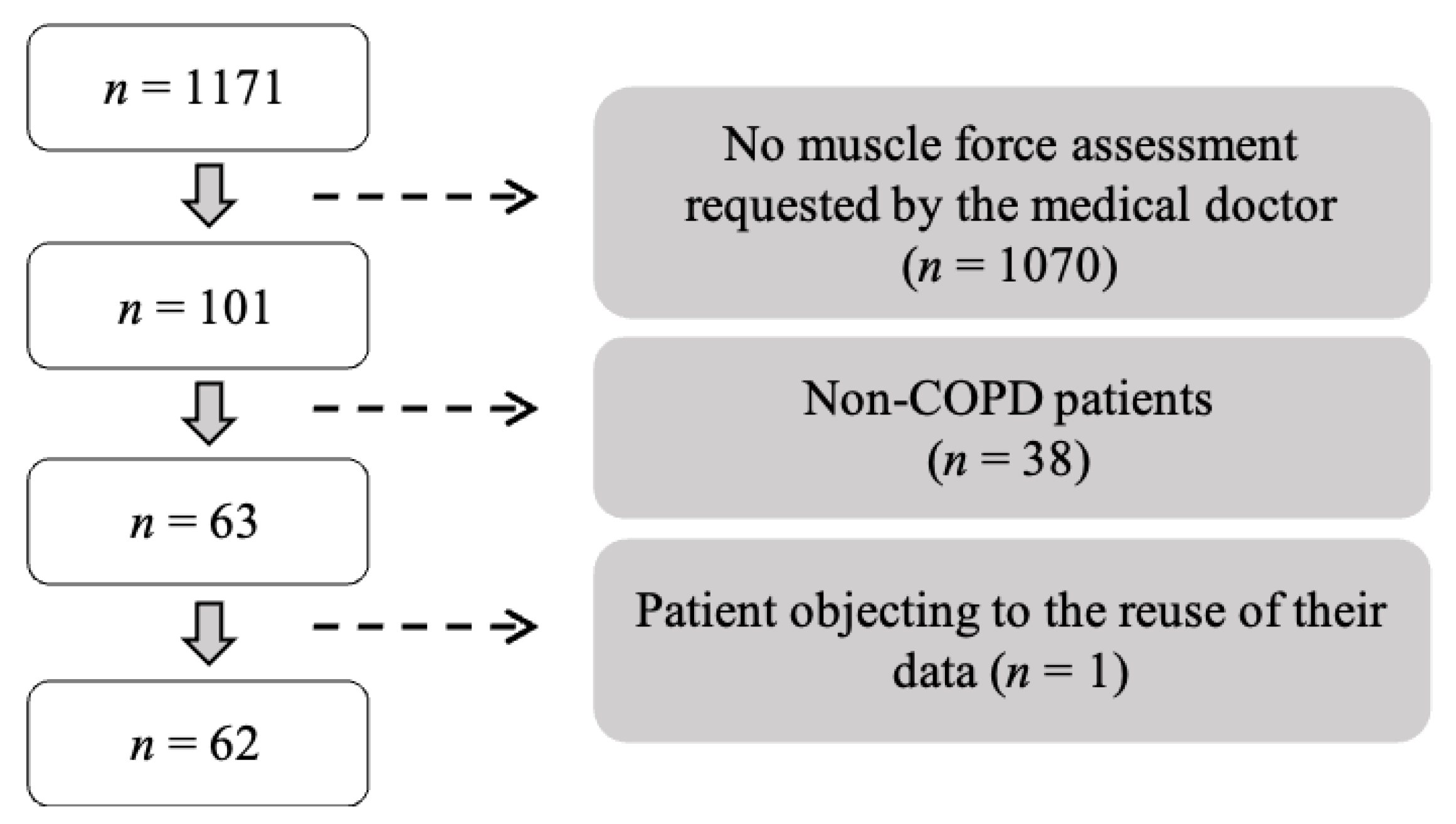
| n | 62 |
| Male/female | 41/21 |
| Age (years) | 64.1 ± 7.9 |
| Body mass (kg) | 72.5 ± 19.3 |
| BMI (kg/m2) | 25.8 ± 6.2 |
| FEV1 (% predicted) | 52.6 ± 21.2 |
| FEV1 (L) | 1.4 ± 0.6 |
| 6 MWD (m) | 448.5 ± 91.5 |
| QMVC (N·m) | 93.4 ± 34.6 |
| QMVC (% predicted) | 73.4 ± 17.9 |
| Non- Responders (n = 31) | Responders (n = 31) | Mean between-Group Difference | 95% CI | |
|---|---|---|---|---|
| Anthropometric parameters | ||||
| Sex (M/F) | 19/12 | 22/9 | / | / |
| Age (years) | 63.9 ± 8.0 | 64.3 ± 7.9 | 0.35 | [−3.71; 4.42] |
| Body mass (kg) | 65.9 ± 20.7 | 79.1 ± 15.5 | 13.16 | [3.86; 22.47] |
| Height (cm) | 165.2 ± 7.1 | 169.2 ± 7.6 | 4 | [0.25; 7.75] |
| BMI (kg/m2) | 23.9 ± 6.4 | 27.7 ± 5.5 | 3.81 | [0.78; 6.84] |
| FFMI (kg/m2) | 16.8 ± 2.5 | 17.7 ± 1.9 | 0.86 | [−0.28; 2.02] |
| Pulmonary parameters | ||||
| FEV1 (L) | 1.25 ± 0.63 | 1.56 ± 0.11 | 0.31 | [−0.00; 0.62] |
| FEV1 (% predicted) | 49.1 ± 22.8 | 56.1 ± 19.2 | 7.03 | [−3.70; 17.76] |
| FEV1/FVC (%) | 47.9 ± 11.3 | 52.9 ± 10.6 | 4.95 | [−0.62; 10.53] |
| LTOT patients (%) | 58 | 19 | / | / |
| Resting blood gases (room air) | ||||
| PaO2 (mmHg) | 60.6 ± 13.2 | 66.5 ± 8.9 | 5.90 | [0.13; 11.64] |
| PaCO2 (mmHg) | 40.2 ± 11.5 | 37.6 ± 4.4 | −2.60 | [−7.03; 1.82] |
| Exercise capacity and QoL | ||||
| 6 MWD (m) | 430 ± 17 | 466 ± 15 | 36.16 | [−9.75; 82.07] |
| VQ11 score | 32 ± 9 | 29 ± 11 | −2.7 | [−9.20; 3.79] |
| Quadriceps force | ||||
| QMVC (N·m) | 83.1 ± 38.4 | 103.8 ± 27.2 | 20.81 | [3.89; 37.73] |
| QMVC (% predicted) | 71.0 ± 19.4 | 75.8 ± 16.2 | 4.77 | [−4.32; 13.87] |
| ΔQMVC (N·m) | 0.5 ± 5.7 | 19.7 ± 10.8 | 19.22 | [14.81; 23.64] |
| Prevalence of muscle weakness (%) | 77 | 71 | / | / |
| Odds Ratio | 95% IC | VIF | |
|---|---|---|---|
| PaCO2 | 1.07 | [1.0–1.15] | 1.16 |
| Sex | 0.97 | [0.50–9.4] | 1.60 |
| QMVC | 0.97 | [0.94–0.99] | 1.79 |
| Predicted | ||||
|---|---|---|---|---|
| Responders | Non-Responders | % Correct | ||
| Observed | Responders | 25 | 6 | 80.6 |
| Non-responders | 8 | 23 | 74.2 | |
| Overall % correct | 77.4 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desachy, M.; Alexandre, F.; Varray, A.; Molinier, V.; Four, E.; Charbonnel, L.; Héraud, N. High Prevalence of Non-Responders Based on Quadriceps Force after Pulmonary Rehabilitation in COPD. J. Clin. Med. 2023, 12, 4353. https://doi.org/10.3390/jcm12134353
Desachy M, Alexandre F, Varray A, Molinier V, Four E, Charbonnel L, Héraud N. High Prevalence of Non-Responders Based on Quadriceps Force after Pulmonary Rehabilitation in COPD. Journal of Clinical Medicine. 2023; 12(13):4353. https://doi.org/10.3390/jcm12134353
Chicago/Turabian StyleDesachy, Marion, François Alexandre, Alain Varray, Virginie Molinier, Elodie Four, Laurène Charbonnel, and Nelly Héraud. 2023. "High Prevalence of Non-Responders Based on Quadriceps Force after Pulmonary Rehabilitation in COPD" Journal of Clinical Medicine 12, no. 13: 4353. https://doi.org/10.3390/jcm12134353
APA StyleDesachy, M., Alexandre, F., Varray, A., Molinier, V., Four, E., Charbonnel, L., & Héraud, N. (2023). High Prevalence of Non-Responders Based on Quadriceps Force after Pulmonary Rehabilitation in COPD. Journal of Clinical Medicine, 12(13), 4353. https://doi.org/10.3390/jcm12134353







