Circulating Profiles of Serum Proguanylin, S100A12 Protein and Pentraxin 3 as Diagnostic Markers of Ulcerative Colitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Assessing the Serum Proguanylin Concentration
2.3. Assessing the Serum S100A12 Concentration
2.4. Assessing the Serum Pentraxin 3 Concentration
2.5. Statistical Analysis
3. Results
3.1. Research Data
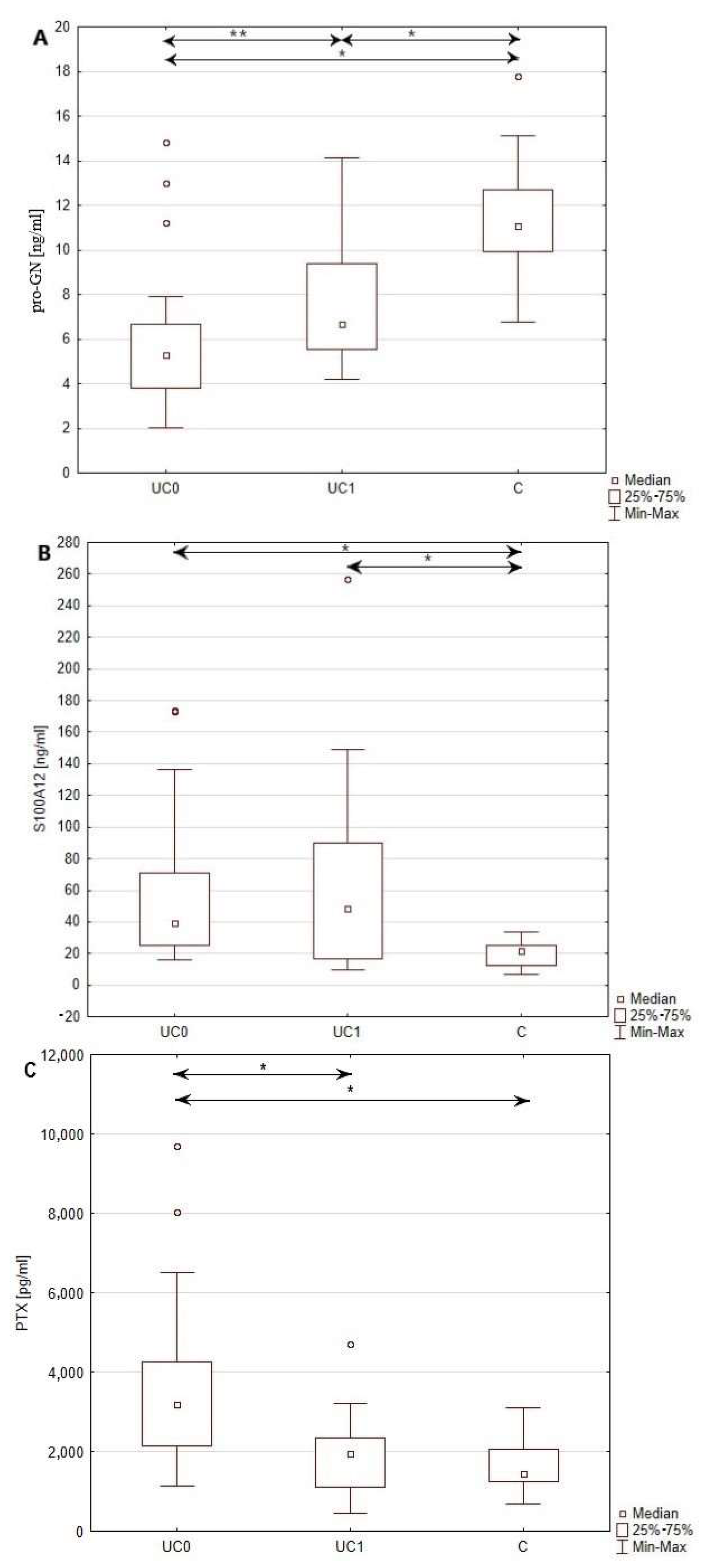
3.2. Serum Level of Pro-GN, S100A12 and PTX3 in Patients with Ulcerative Colitis and Healthy Individuals
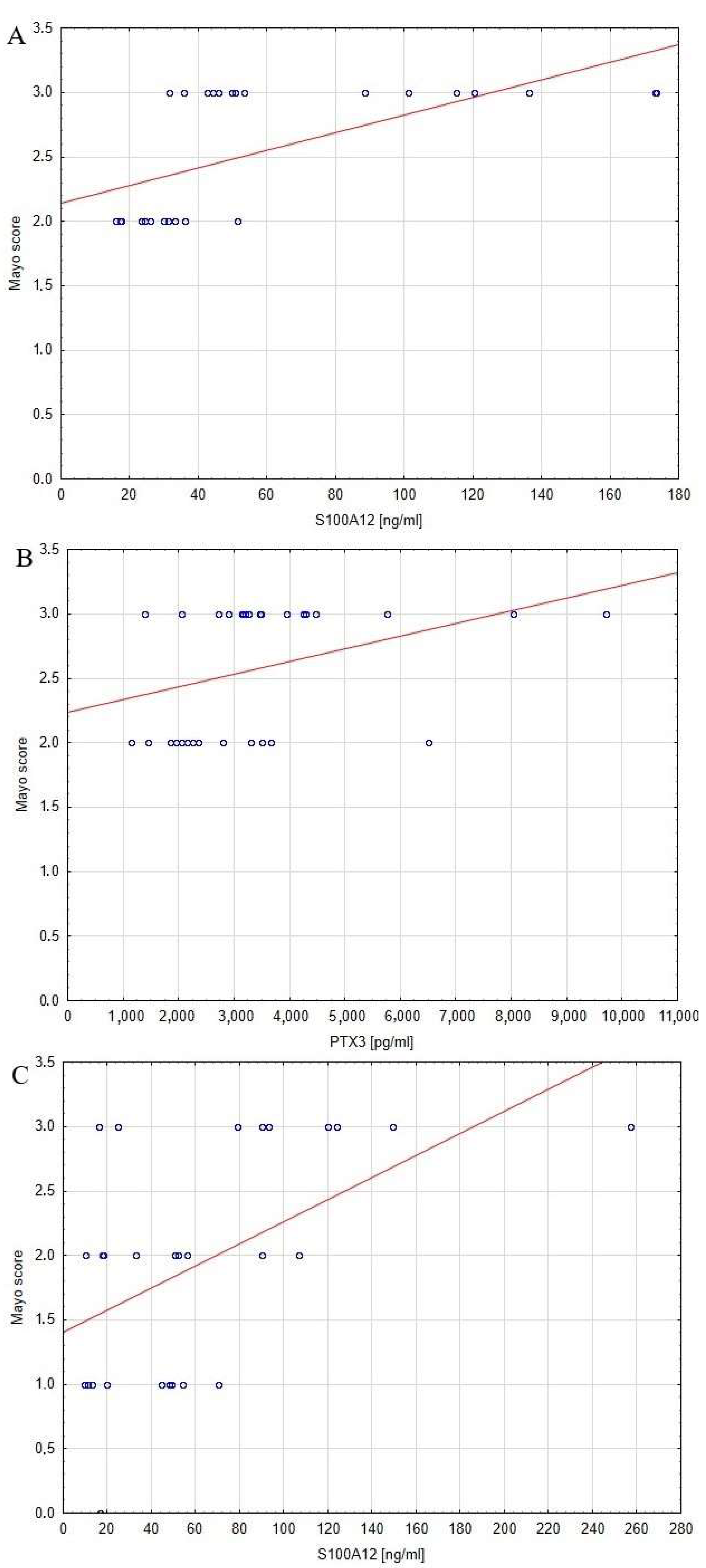
3.3. The Relationship between Serum Pro-GN, S100A12, PTX3 and Inflammatory Process and Disease Activity
3.4. The Influence of One Year of Biological Treatment with Adalimumab on the Serum Profile of Pro-GN, S100A12 and PTX3
4. Discussion
4.1. Serum Profile of the Pro-GN, S100A12 and PTX3 in Patients Diagnosed with UC and Healthy Individuals
4.2. The Relationship between Disease Activity and Serum Profile of Pro-GN, S100A12 and PTX3 in Patients with UC
4.3. The Influence of Biological Treament with Adalimumab on the Circulating Profile of Pro-GN, S100A12 and PTX3
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Porter, R.J.; Kalla, R.; Ho, G.T. Ulcerative colitis: Recent advances in the understanding of disease pathogenesis. F100 Res. 2020, 9, 294. [Google Scholar] [CrossRef]
- Leone, S.; Samhan-Arias, A.; Ben-Shachar, I.; Derieppe, M.; Dinc, F.; Grosu, I.; Guinea, C.; Lignell, J.; Smailys, G.; Sturludóttir, S.; et al. ECCO EFCCA Patient Guidelines on Ulcerative Colitis Ulcerosa; European Chron’s and Colitis Organisation: Barcelona, Spain, 2017. [Google Scholar]
- Soubières, A.A.; Poullis, A. Emerging role of novel biomarkers in the diagnosis of inflammatory bowel disease. World J. Gastrointest. Pharmacol. Ther. 2016, 7, 41–50. [Google Scholar] [CrossRef]
- Khaki-Khatibi, F.; Qujeq, D.; Kashifard, M.; Moein, S.; Maniati, M.; Vaghari-Tabari, M. Calprotectin in inflammatory bowel disease. Clin. Chim. Acta 2020, 510, 556–565. [Google Scholar] [CrossRef]
- Uranga, J.A.; Castro, M.; Abalo, R. Guanylate Cyclase C: A Current Hot Target, from Physiology to Pathology. Curr. Med. Chem. 2018, 25, 1879–1908. [Google Scholar] [CrossRef] [PubMed]
- Lan, D.; Wen, Y.; Dong, X.; Yang, Q.; Liu, Y.; Wang, K.; Li, H.; Miao, Y. The endogenous ligand for guanylate cyclase-C activation reliefs intestinal inflammation in the DSS colitis model. Acta Biochim. Pol. 2020, 67, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.E.; Li, P.; Snook, A.E.; Schulz, S.; Dasgupta, A.; Hyslop, T.M.; Gibbons, A.V.; Marszlowicz, G.; Pitari, G.M.; Waldman, S.A. The hormone receptor GUCY2C suppresses intestinal tumor formation by inhibiting AKT signaling. Gastroenterology 2010, 138, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.E.; Snook, A.E.; Li, P.; Stoecker, B.A.; Kim, G.W.; Magee, M.S.; Garcia, A.V.; Valentino, M.A.; Hyslop, T.; Schulz, S.; et al. GUCY2C opposes systemic genotoxic tumorigenesis by regulating AKT-dependent intestinal barrier integrity. PLoS ONE 2012, 7, e31686. [Google Scholar] [CrossRef] [PubMed]
- Oesterle, A.; Hofmann-Bowman, M.A. S100A12 and the S100/Calgranulins—Emerging biomarkers for atherosclerosis and possibly therapeutic targets. Arterioscler. Thromb. Vasc. Biol. 2015, 25, 2496–2507. [Google Scholar] [CrossRef]
- Donato, R.; Cannon, B.R.; Sorci, G.; Riuzzi, F.; Hsu, K.; Weber, D.J.; Geczy, C.L. Functions of S100 Proteins. Curr. Mol. Med. 2013, 13, 24–57. [Google Scholar] [CrossRef]
- Bierhaus, A.; Humpert, P.M.; Morcos, M.; Wendt, T.; Chavakis, T.; Arnold, B.; Stern, D.M.; Nawroth, P.P. Understanding RAGE, the receptor for advanced glycation end products. J. Mol. Med. 2005, 83, 876–886. [Google Scholar] [CrossRef]
- Kaur, A.; Goggolidou, P. Ulcerative colitis: Understanding its cellular pathology could provide insight into novel therapies. J. Inflamm. 2020, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Savchenko, A.S.; Inoue, A.; Ohashi, R.; Jiang, S.; Hasegawa, G.; Tanaka, T.; Hamakubo, T.; Kodama, T.; Aoyagi, Y.; Ushiki, T.; et al. Long pentraxin 3 (PTX3) expression and release by neutrophils in vitro and in ulcerative colitis. Pathol. Int. 2011, 61, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Cao, F.; Tao, J.; Li, X.; Zheng, S.G.; Pan, H.F. Pentraxin 3: A promising therapeutic target for autoimmune diseases. Autoimmun. Rev. 2020, 19, 102584. [Google Scholar] [CrossRef]
- Porte, R.; Davoudian, S.; Asgari, F.; Parente, R.; Mantovani, A.; Garlanda, C.; Bottazzi, B. The Long Pentraxin PTX3 as a Humoral Innate Immunity Functional Player and Biomarker of Infections and Sepsis. Front. Immunol. 2019, 10, 794. [Google Scholar] [CrossRef] [PubMed]
- Bennike, T.B.; Carlsen, T.G.; Ellingsen, T.; Bonderup, O.K.; Glerup, H.; Bøgsted, M.; Christiansen, G.; Birkelund, S.; Stensballe, A.; Andersen, V. Neutrophil Extracellular Traps in Ulcerative Colitis: A Proteome Analysis of Intestinal Biopsies. Inflamm. Bowel Dis. 2015, 21, 2052–2067. [Google Scholar] [CrossRef]
- Kato, S.; Ochiai, M.; Sakurada, T.; Ohno, S.; Miyamoto, K.; Sagara, M.; Ito, M.; Takeuchi, K.; Imaki, J.; Itoh, K.; et al. Increased Expression of Long Pentraxin PTX3 in Inflammatory Bowel Diseases. Dig. Dis. Sci. 2008, 53, 1910–1916. [Google Scholar] [CrossRef]
- Doni, A.; Stravalaci, M.; Inforzato, A.; Magrini, E.; Mantovani, A.; Garlanda, C.; Bottazzi, B. The Long Pentraxin PTX3 as a Link Between Innate Immunity, Tissue Remodeling, and Cancer. Front. Immunol. 2019, 10, 712. [Google Scholar] [CrossRef]
- Komosinska-Vassev, K.; Kałużna, K.; Jura-Półtorak, A.; Derkacz, A.; Olczyk, K. Circulating Profile of ECM-Related Proteins as Diagnostic Markers in Inflammatory Bowel Diseases. J. Clin. Med. 2022, 11, 5618. [Google Scholar] [CrossRef]
- Lan, D.; Niu, J.; Miao, J.; Dong, X.; Wang, H.; Yang, G.; Wang, K.; Miao, Y. Expression of guanylate cyclase-C, guanylin, and uroguanylin is downregulated proportionally to the ulcerative colitis disease activity index. Sci. Rep. 2016, 6, 25034. [Google Scholar] [CrossRef]
- Johansson, M.E.V.; Sjövall, H.; Hansson, G.C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenetrol. Hepatol. 2013, 10, 352–361. [Google Scholar] [CrossRef]
- Kmieć, Z.; Cyman, M.; Ślebioda, T.J. Cells of the innate and adaptive immunity and their interactions in inflammatory bowel disease. Adv. Med. Sci. 2017, 62, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Martini, E.; Krug, S.M.; Siegmund, B.; Neurath, M.F.; Becker, C. Mend your fence. The epithelial barrier and its relationship with mucosal immunity in inflammatory bowel disease. Cell. Mol. Gastroenterol. Hepatol. 2017, 4, 33–46. [Google Scholar] [CrossRef]
- Dinallo, V.; Marafini, I.; Di Fusco, D.; Laudisi, F.; Franze, E.; Di Grazia, A.; Figliuzzi, M.M.; Caprioli, F.; Stolfi, C.; Monteleone, I.; et al. Neutrophil extracellular traps sustain inflammatory signals in ulcerative colitis. J. Crohn’s Colitis 2019, 13, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Foell, D.; Kucharzik, T.; Kraft, M.; Vogl, T.; Sorg, C.; Domschke, W.; Roth, J. Neutrophil derived human S100A12 (EN-RAGE) is strongly expressed during chronic active inflammatory bowel disease. Gut 2003, 52, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, T.; Langhorst, J.; Wittkowski, H.; Becker, K.; Friedrich, A.W.; Rueffer, A.; Dobos, G.J.; Roth, J.; Foell, D. Fecal S100A12 as a non-invasive marker distinguishing inflammatory bowel disease from irritable bowel syndrome. Gut 2007, 56, 1706–1713. [Google Scholar] [CrossRef]
- Ashour, D.S.; Othman, A.A.; Shareef, M.M.; Gaballah, H.H.; Mayah, W.W. Interactions between Trichinella spiralis infection and induced colitis in mice. J. Helminthol. 2014, 88, 210–218. [Google Scholar] [CrossRef]
- Foell, D.; Wittkowski, H.; Kessel, C.; Lüken, A.; Weinhage, T.; Varga, G.; Vogl, T.; Wirth, T.; Viemann, D.; Björk, P.; et al. Proinflammatory S100A12 can activate human monocytes via Toll-like receptor 4. Am. J. Respir. Crit. Care Med. 2013, 187, 1324–1334. [Google Scholar] [CrossRef]
- Brenna, Ø.; Bruland, T.; Furnes, M.W.; Granlund, A.; Drozdov, I.; Emgård, J.; Brønstad, G.; Kidd, M.; Sandvik, A.K.; Gustafsson, B.I. The guanylate cyclase-C signaling pathway is down-regulated in inflammatory bowel disease. Scand. J. Gastroenterol. 2015, 50, 1241–1252. [Google Scholar] [CrossRef]
- Harmel-Laws, E.; Mann, E.A.; Cohen, M.B.; Steinbrecher, K.A. Guanylate cyclase C deficiency causes severe inflammation in a murine model of spontaneous colitis. PLoS ONE 2013, 8, e79180. [Google Scholar] [CrossRef]
- Boschetti, G.; Garnero, P.; Moussata, D.; Cuerq, C.; Préaudat, C.; Duclaux-Loras, R.; Mialon, A.; Drai, J.; Flourié, B.; Nancey, S. Accuracies of serum and fecal S100 proteins (calprotectin and calgranulin C) to predict the response to TNF antagonists in patients with Crohn’s disease. Inflamm. Bowel Dis. 2015, 21, 331–336. [Google Scholar] [CrossRef]
- Hasegawa, T.; Kosaki, A.; Kimura, T.; Matsubara, H.; Mori, Y.; Okigaki, M.; Masaki, H.; Toyoda, N.; Inoue-Shibata, M.; Kimura, Y.; et al. The regulation of EN-RAGE (S100A12) gene expression in human THP-1 macrophages. Atherosclerosis 2003, 171, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Goyette, J.; Geczy, C.L. Inflammation-associated S100 proteins: New mechanisms that regulate function. Amino Acids 2011, 41, 821–842. [Google Scholar] [CrossRef] [PubMed]
| Parameter | UC0 | UC1 | p |
|---|---|---|---|
| Mayo score | 3 (2–3) | 2 (1–3) | 0.000 |
| CRP [mg/L] | 3.4 (1.26–17.51) | 2.47 (1.51–7.68) | 0.160 |
| Glucose [mmol/L] | 4.99 ± 0.70 | 4.81 ± 0.81 | 0.293 |
| Cholesterol [mmol/L] | 4.98 ± 0.79 | 4.93 ± 0.91) | 0.724 |
| Triglycerides [mmol/L] | 1.23 (1.01–1.49) | 1.0 (0.87–1.36) | 0.017 |
| Indirect bilirubin [μmol/L] | 4.75 (1.80–7.70) | 8.3 (5.50–16.00) | 0.000 |
| Direct bilirubin [μmol/L] | 3.45 (1.90–3.80) | 5.30 (3.50–8.20) | 0.000 |
| ALT [U/L] | 15.00 (10.00–26.00) | 14.50 (10.00–23.00) | 0.591 |
| AST [U/L] | 17.92 ± 4.81 | 19.42 ± 6.18 | 0.136 |
| Creatnine [μmol/L] | 77.8 (68.50–87.90) | 74.70 (63.40–87.10) | 0.117 |
| Total protein [g/L] | 73.48 ± 5.43 | 74.73 ± 5.63 | 0.231 |
| Albumin [g/L] | 42.52 ± 4.73 | 43.34 ± 4.52 | 0.358 |
| Sodium [mmol/L] | 140 (138.00–142.00) | 140.00 (138.00–141.00) | 0.381 |
| Potassium [mmol/L] | 4.17 ± 0.40 | 3.97 ± 0.33 | 0.011 |
| Calcium [mmol/L] | 2.36 ± 0.09 | 2.33 ± 0.12 | 0.660 |
| Hemoglobin [g/dl] | 12.83 ± 2.28 | 13.49 ± 2.29 | 0.005 |
| Neutrophils [%] | 66.65 ± 11.25 | 64.52 ± 10.92 | 0.282 |
| Lymphocytes [%] | 24.27 ± 10.71 | 26.99 ± 11.74 | 0.259 |
| Basophils [%] | 0.76 ± 0.43 | 0.71 ± 0.37 | 1.000 |
| Eosinophils [%] | 2.6 (1.10–3.30) | 2.03 ± 1.43 | 0.063 |
| Monocytes [%] | 5.72 ± 2.27 | 5.75 ± 1.79 | 0.859 |
| PLT [×109/L] | 375.93 ± 108.79 | 342.04 ± 101.72 | 0.043 |
| Parameter | UC0 | UC1 | C |
|---|---|---|---|
| Pro-GN [ng/mL] | 5.27 (3.80–6.65) # | 6.68 (5.51–9.36) # | 11.35 ± 2.59 * |
| S100A12 [ng/mL] | 39.36 (25.15–70.99) # | 48.32 (16.84–89.99) # | 19.74 ± 8.07 * |
| PTX3 [pg/mL] | 3197.05 (2148.20–4248.95) # | 1946.4 (1103.61–2334.93) # | 1608.37 ± 587.05 * |
| Parameter | Mayo Score | |
| Pro-GN | UC0 | p > 0.05 |
| UC1 | p > 0.05 | |
| S100A12 | UC0 | p < 0.0005 |
| UC1 | p < 0.005 | |
| PTX3 | UC0 | p < 0.05 |
| UC1 | p > 0.05 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kałużna, A.; Jura-Półtorak, A.; Derkacz, A.; Jaruszowiec, J.; Olczyk, K.; Komosinska-Vassev, K. Circulating Profiles of Serum Proguanylin, S100A12 Protein and Pentraxin 3 as Diagnostic Markers of Ulcerative Colitis. J. Clin. Med. 2023, 12, 4339. https://doi.org/10.3390/jcm12134339
Kałużna A, Jura-Półtorak A, Derkacz A, Jaruszowiec J, Olczyk K, Komosinska-Vassev K. Circulating Profiles of Serum Proguanylin, S100A12 Protein and Pentraxin 3 as Diagnostic Markers of Ulcerative Colitis. Journal of Clinical Medicine. 2023; 12(13):4339. https://doi.org/10.3390/jcm12134339
Chicago/Turabian StyleKałużna, Aleksandra, Agnieszka Jura-Półtorak, Alicja Derkacz, Julia Jaruszowiec, Krystyna Olczyk, and Katarzyna Komosinska-Vassev. 2023. "Circulating Profiles of Serum Proguanylin, S100A12 Protein and Pentraxin 3 as Diagnostic Markers of Ulcerative Colitis" Journal of Clinical Medicine 12, no. 13: 4339. https://doi.org/10.3390/jcm12134339
APA StyleKałużna, A., Jura-Półtorak, A., Derkacz, A., Jaruszowiec, J., Olczyk, K., & Komosinska-Vassev, K. (2023). Circulating Profiles of Serum Proguanylin, S100A12 Protein and Pentraxin 3 as Diagnostic Markers of Ulcerative Colitis. Journal of Clinical Medicine, 12(13), 4339. https://doi.org/10.3390/jcm12134339







