Prokineticin 2 and Cytokine Content in the Synovial Fluid of Knee Osteoarthritis and Traumatic Meniscal Tear Patients: Preliminary Results
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Clinical and Radiological Follow-Up after 5 Years for Meniscal Tears
2.3. Synovial Fluid Collection and Storage
2.4. PK2 and Cytokine Analysis
2.5. Statistics
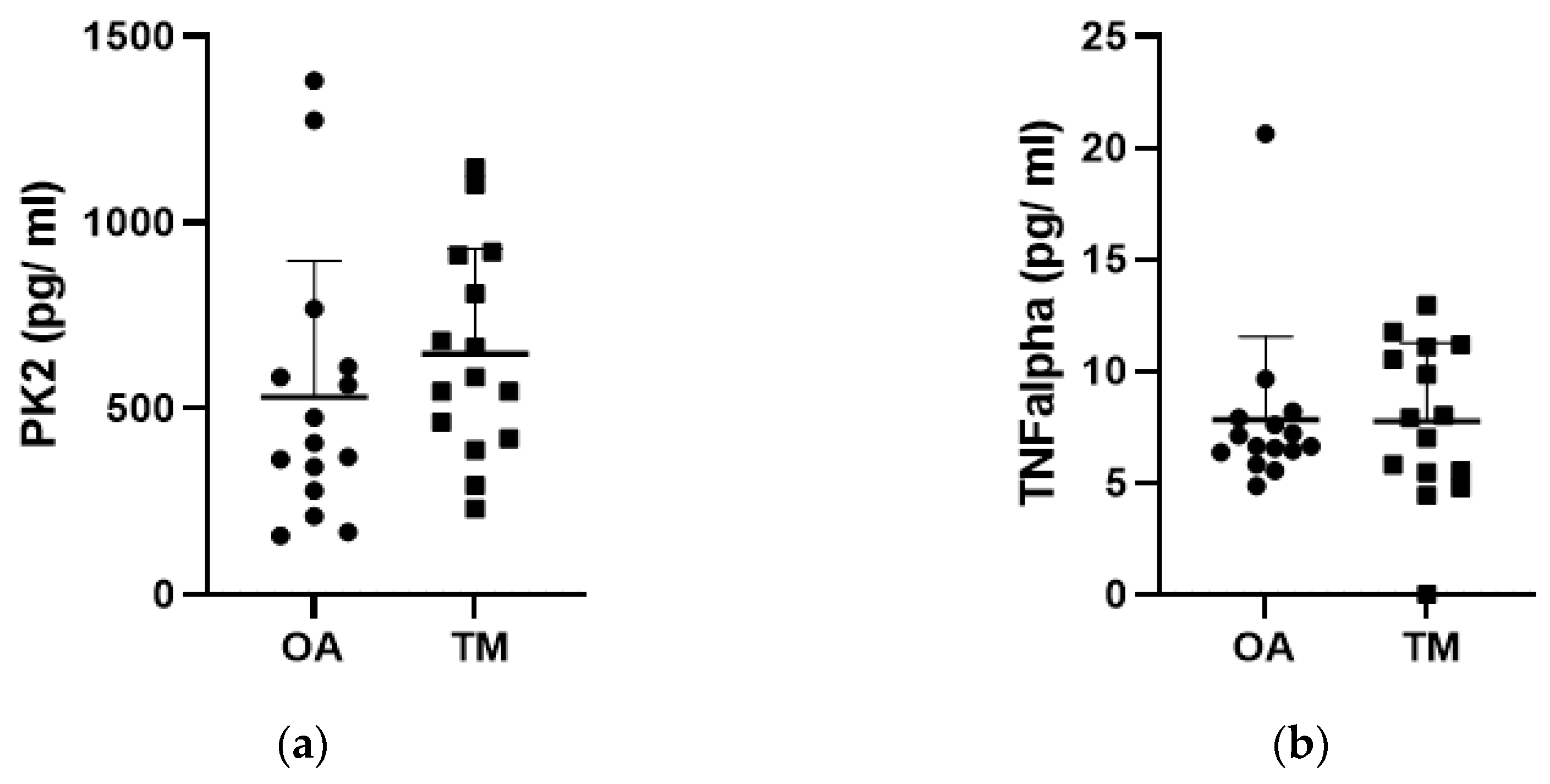
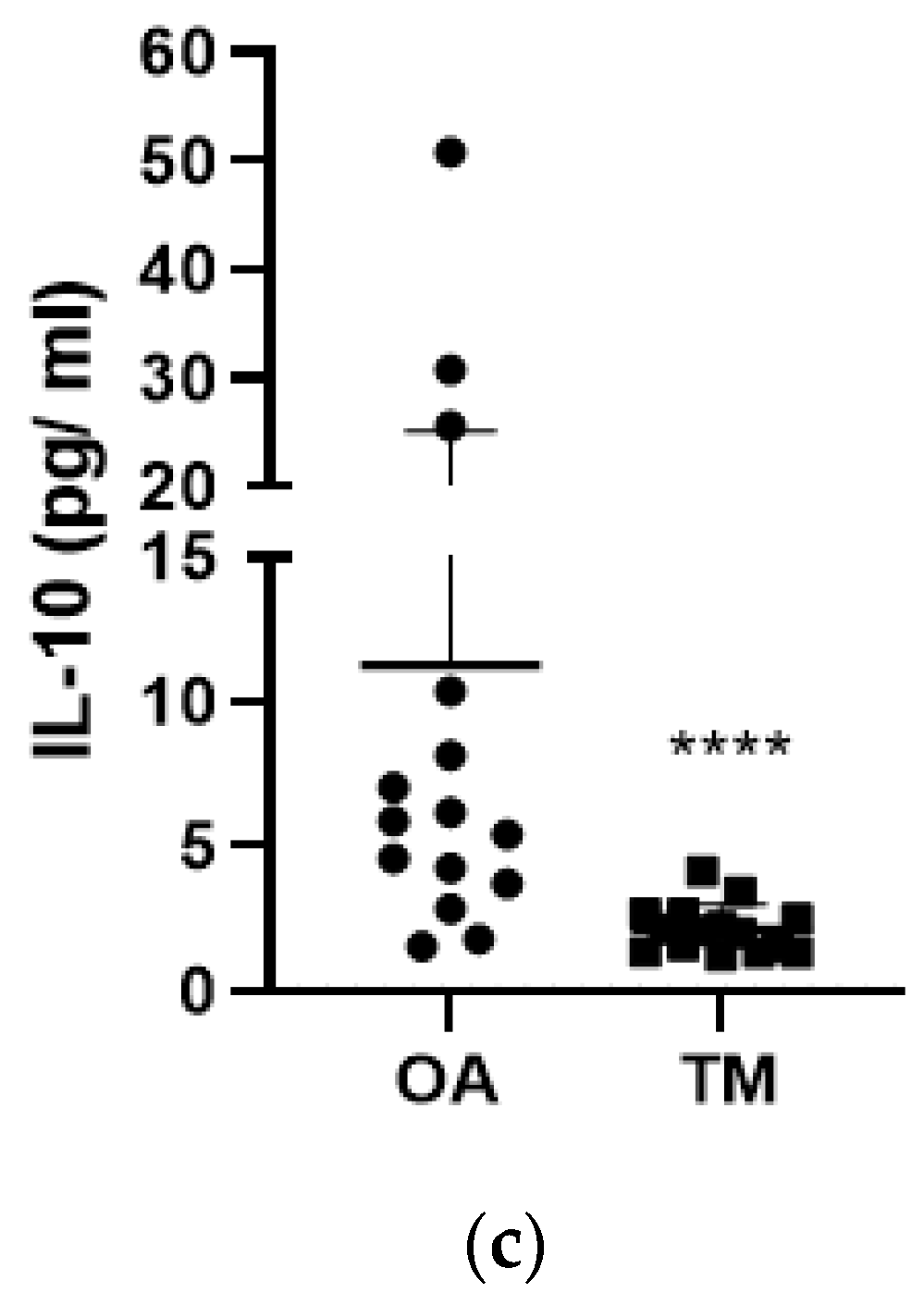
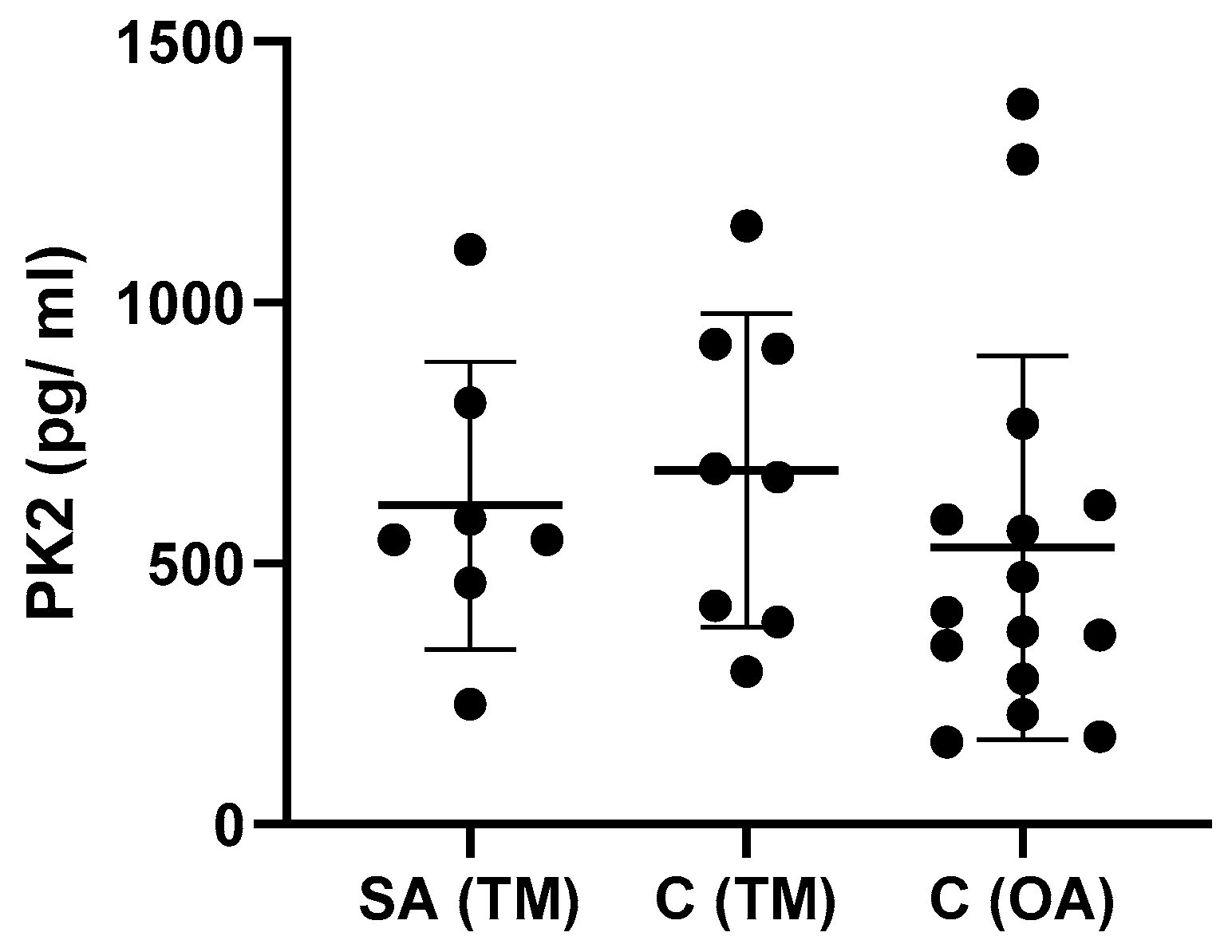
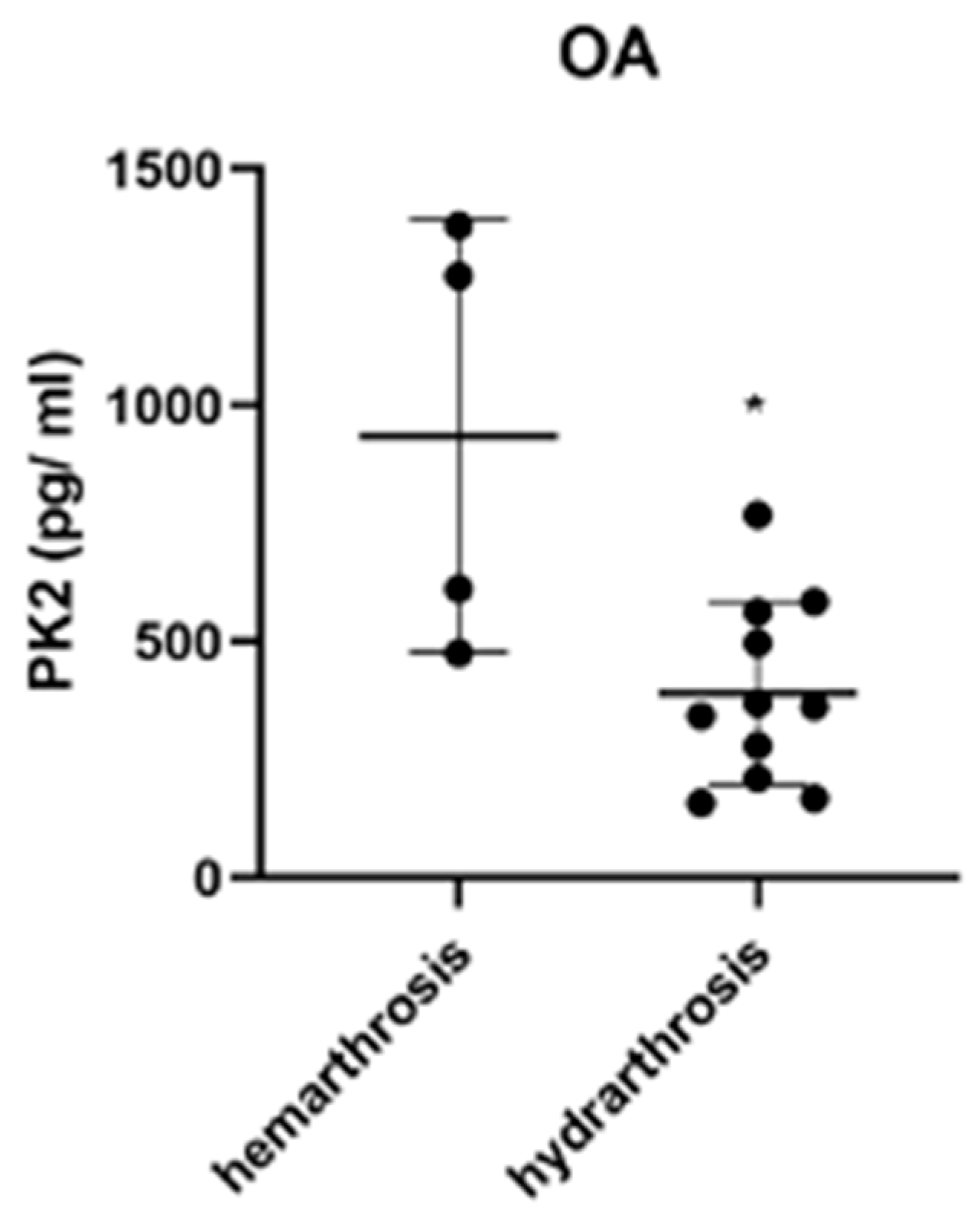
3. Results
3.1. Effect of the Type of Injury on PK2 and Cytokine Levels
3.2. Effect of Time between Injury and Synovial Fluid Sampling on PK2 and Cytokine Levels
3.3. Effect of Type of Samples on PK2 TNF-α and IL-10 Levels
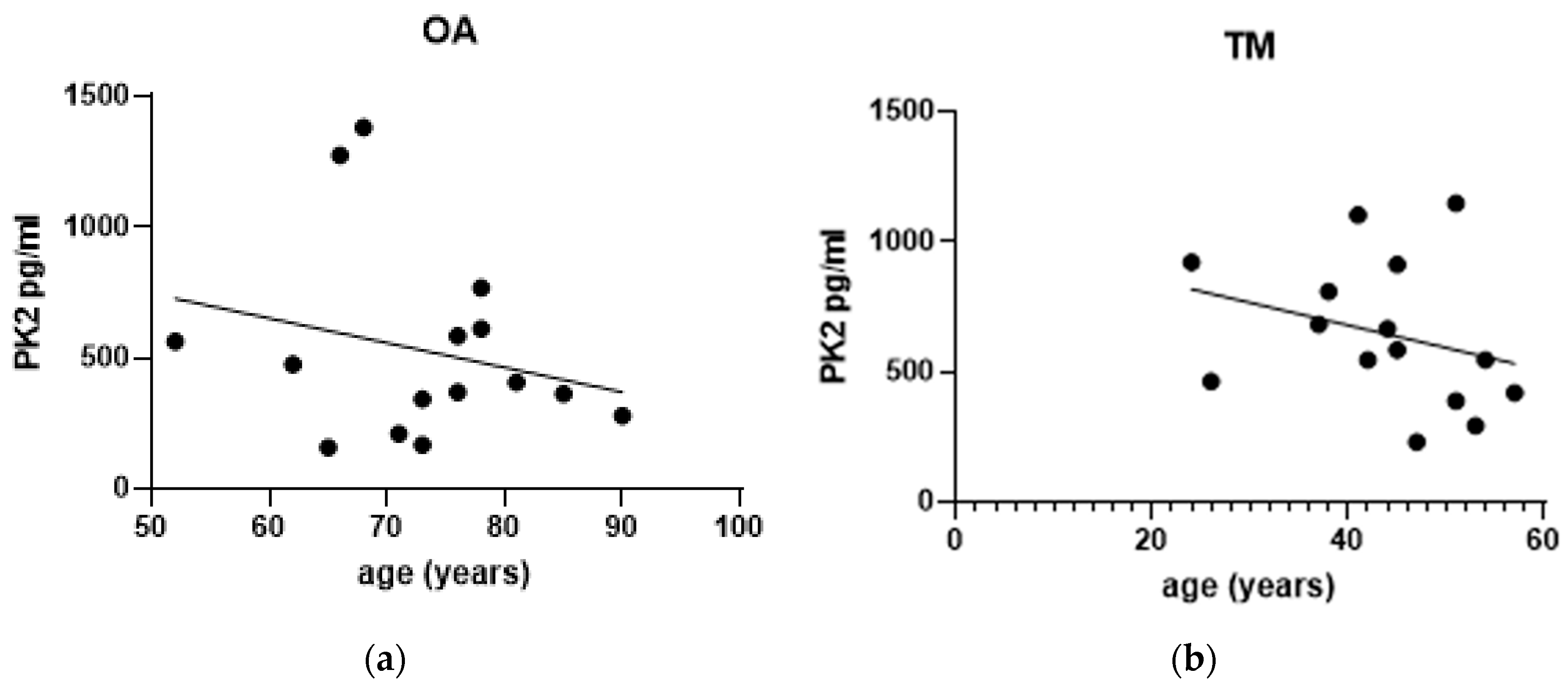
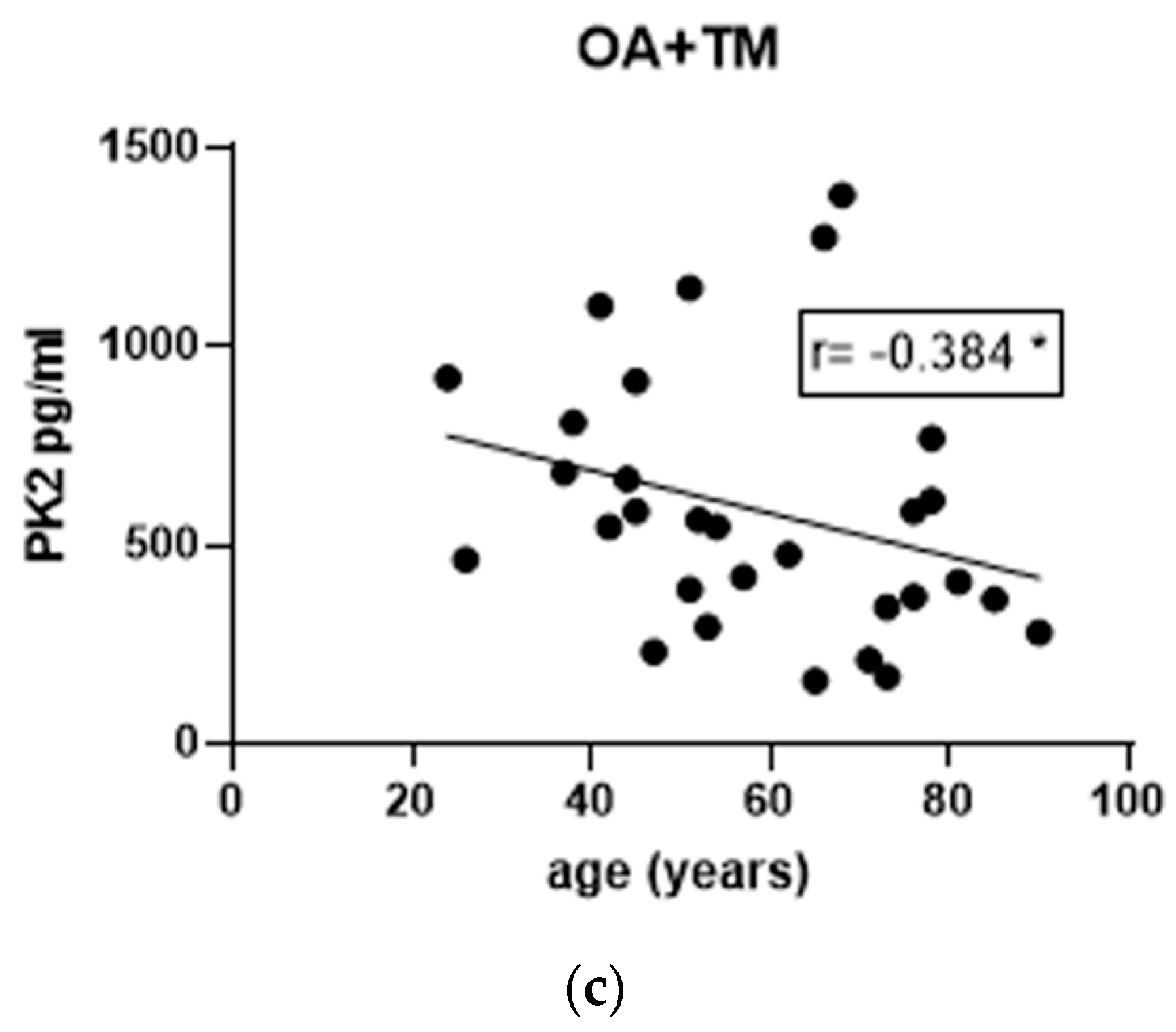
3.4. Effect of Gender and Age on PK2 Levels
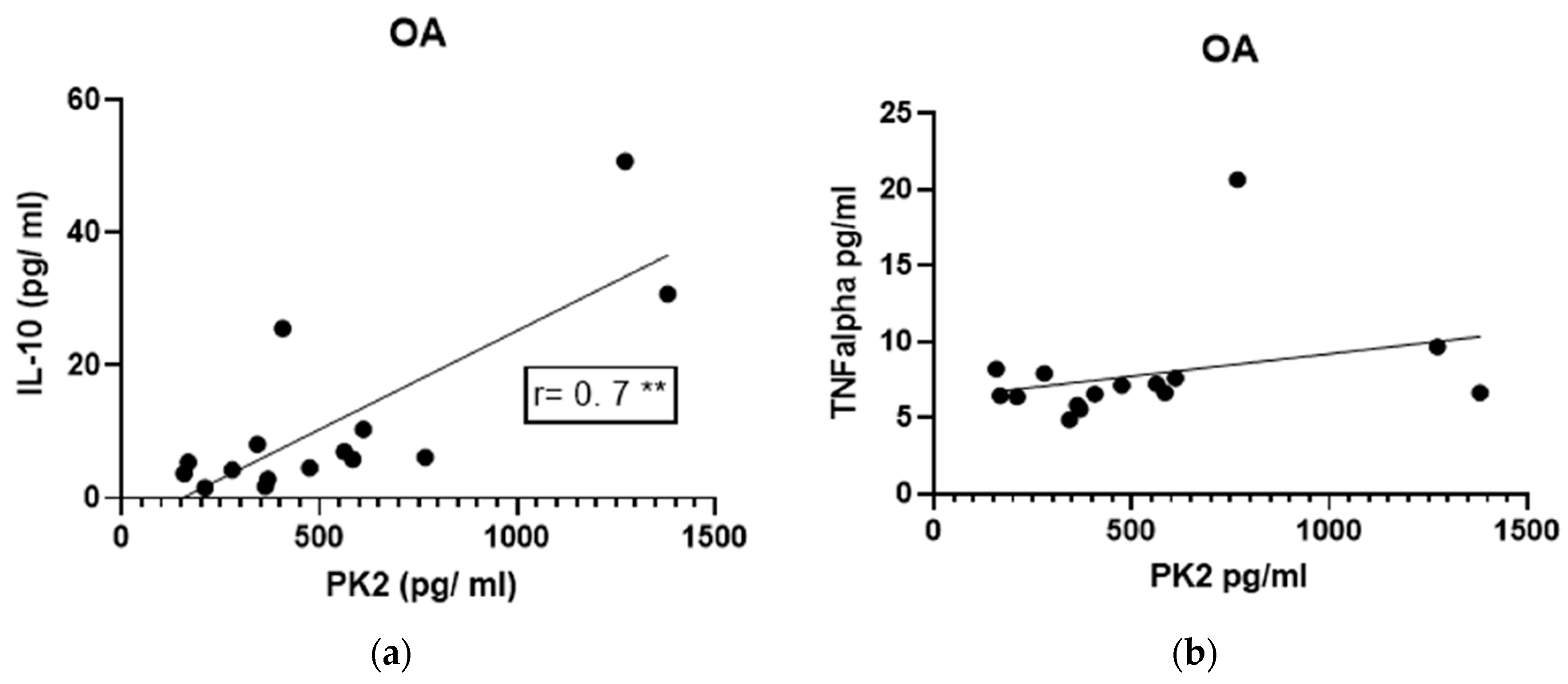
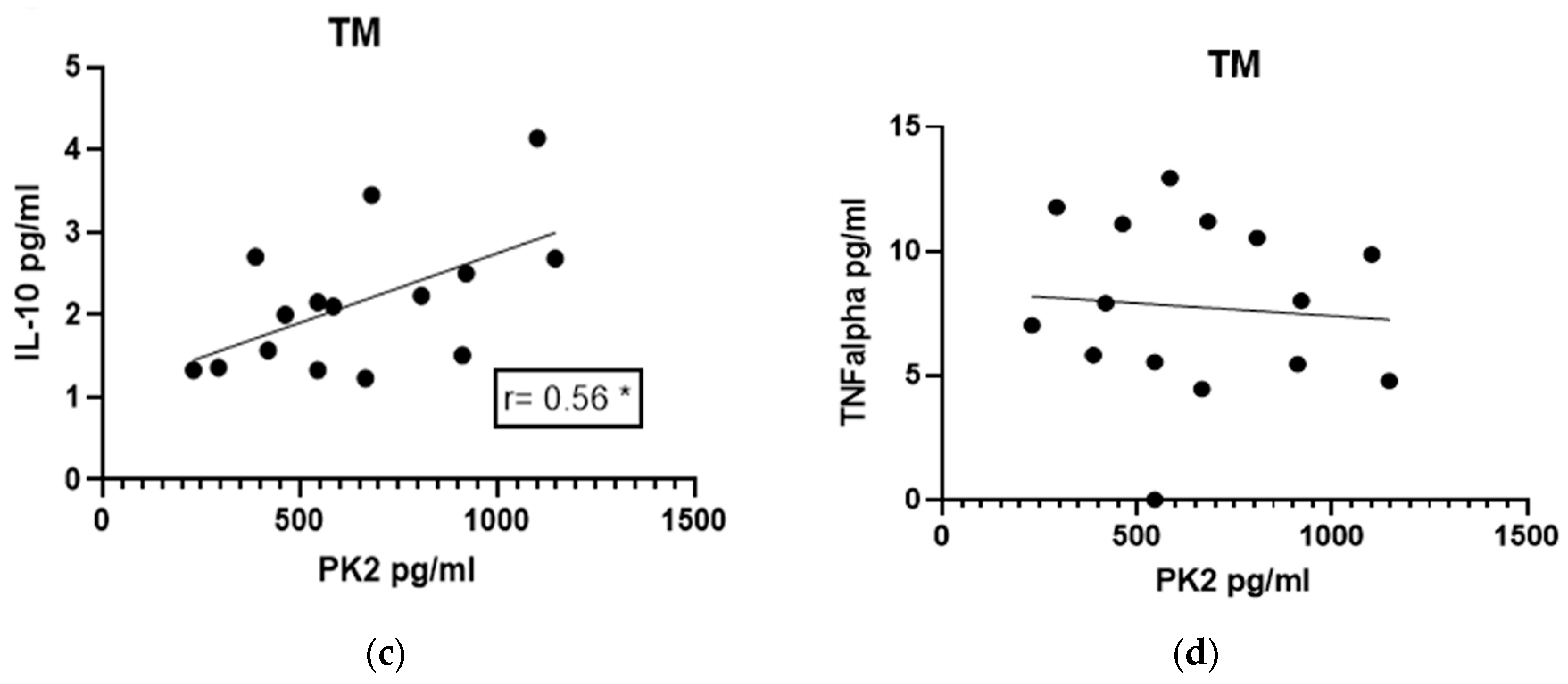
3.5. Correlations of IL-10 and TNF-α Levels with PK2
3.6. Correlations between Cytokine Levels and PROMS and X-ray
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Michael, J.W.-P.; Schlüter-Brust, K.U.; Eysel, P. The Epidemiology, Etiology, Diagnosis, and Treatment of Osteoarthritis of the Knee. Dtsch. Arztebl. Int. 2010, 107, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T. The Epidemiology of Knee Osteoarthritis: Results from the Framingham Osteoarthritis Study. Semin. Arthritis Rheum. 1990, 20, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Wallace, I.J.; Worthington, S.; Felson, D.T.; Jurmain, R.D.; Wren, K.T.; Maijanen, H.; Woods, R.J.; Lieberman, D.E. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc. Natl. Acad. Sci. USA 2017, 114, 9332–9336. [Google Scholar] [CrossRef] [PubMed]
- Bigoni, M.; Zanchi, N.; Turati, M. Healing potential and surgical treatment of anterior cruciate ligament rupture in pediatric population. Sport Sci. Health 2017, 13, 645–646. [Google Scholar] [CrossRef]
- Turati, M.; Boerci, L.; Piatti, M.; Zanchi, N.; Zatti, G.; Accadbled, F.; Bigoni, M. Updates on etiopathogenesis of musculoskeletal injuries in adolescent athletes. Minerva Pediatr. 2023, 75, 133–135. [Google Scholar] [CrossRef]
- Rai, M.F.; Brophy, R.H.; Sandell, L.J. Osteoarthritis following meniscus and ligament injury: Insights from translational studies and animal models. Curr. Opin. Rheumatol. 2019, 31, 70–79. [Google Scholar] [CrossRef]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.-P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Cameron, M.L.; Fu, F.H.; Paessler, H.H.; Schneider, M.; Evans, C.H. Synovial problems Synovial fluid cytokine concentrations as possible prognostic indicators in the ACL-deflcient knee. Knee Surg. Sport. Traumatol. Arthrosc. 1994, 2, 38–44. [Google Scholar] [CrossRef]
- Mabey, T.; Honsawek, S.; Tanavalee, A.; Yuktanandana, P.; Wilairatana, V.; Poovorawan, Y. Plasma and synovial fluid inflammatory cytokine profiles in primary knee osteoarthritis. Biomarkers 2016, 21, 639–644. [Google Scholar] [CrossRef]
- Franchi, S.; Sacerdote, P.; Panerai, A. The prokineticin system: An interface between neural inflammation and pain. Neurol. Sci. 2017, 38, 27–30. [Google Scholar] [CrossRef]
- Negri, L.; Ferrara, N. The Prokineticins: Neuromodulators and Mediators of Inflammation and Myeloid Cell-Dependent Angiogenesis. Physiol. Rev. 2018, 98, 1055–1082. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, R.; Miele, R. Versatile Role of Prokineticins and Prokineticin Receptors in Neuroinflammation. Biomedicines 2021, 9, 1648. [Google Scholar] [CrossRef] [PubMed]
- Abou-Hamdan, M.; Costanza, M.; Fontana, E.; Di Dario, M.; Musio, S.; Congiu, C.; Onnis, V.; Lattanzi, R.; Radaelli, M.; Martinelli, V.; et al. Critical role for prokineticin 2 in CNS autoimmunity. Neurol.-Neuroimmunol. Neuroinflamm. 2015, 2, e95. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Shen, C.; Lu, Q.; Li, J.; Wei, Y.; He, L.; Bai, R.; Zheng, J.; Luan, N.; Zhang, Z.; et al. Prokineticin 2 Plays a Pivotal Role in Psoriasis. Ebiomedicine 2016, 13, 248–261. [Google Scholar] [CrossRef]
- Noda, K.; Dufner, B.; Ito, H.; Yoshida, K.; Balboni, G.; Straub, R.H. Differential inflammation-mediated function of prokineticin 2 in the synovial fibroblasts of patients with rheumatoid arthritis compared with osteoarthritis. Sci. Rep. 2021, 11, 18399. [Google Scholar] [CrossRef]
- Ito, H.; Noda, K.; Yoshida, K.; Otani, K.; Yoshiga, M.; Oto, Y.; Saito, S.; Kurosaka, D. Prokineticin 2 antagonist, PKRA7 suppresses arthritis in mice with collagen-induced arthritis. BMC Musculoskelet. Disord. 2016, 17, 387. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Maftei, D.; Severini, C.; Miele, R.; Balboni, G.; Siracusa, R.; Cordaro, M.; Di Paola, R.; Cuzzocrea, S.; Lattanzi, R. Blocking prokineticin receptors attenuates synovitis and joint destruction in collagen-induced arthritis. J. Mol. Med. 2023, 101, 569–580. [Google Scholar] [CrossRef]
- Bigoni, M.; Zanchi, N.; Omeljaniuk, R.J.; Zatti, G.; Locatelli, V.; Torsello, A.; Turati, M. Role of interleukin-10 in the synovial fluid of the anterior cruciate ligament injured knee. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 932–940. [Google Scholar]
- Kellgren, J.H.; Lawrence, J.S. Radiological Assessment of Osteo-Arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef]
- E Outerbridge, R.; Dunlop, J.A.Y. The Problem of Chondromalacia Patellae. Clin. Orthop. Relat. Res. 1961, 110, 177–196. [Google Scholar] [CrossRef]
- Lysholm, J.; Gillquist, J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am. J. Sport. Med. 1982, 10, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Tegner, Y.; Lysholm, J. Rating systems in the evaluation of knee ligament injuries. Clin. Orthop. Relat. Res. 1985, 198, 43–49. [Google Scholar] [CrossRef]
- Irrgang, J.J.; Anderson, A.F.; Boland, A.L.; Harner, C.D.; Kurosaka, M.; Neyret, P.; Richmond, J.C.; Shelborne, K.D. Development and Validation of the International Knee Documentation Committee Subjective Knee Form. Am. J. Sport. Med. 2001, 29, 600–613. [Google Scholar] [CrossRef] [PubMed]
- Marot, V.; Justo, A.; Alshanquiti, A.; Reina, N.; Accadbled, F.; Berard, E.; Cavaignac, E. Simple Knee Value: A simple evaluation correlated to existing knee PROMs. Knee Surg. Sport. Traumatol. Arthrosc. 2021, 29, 1952–1959. [Google Scholar] [CrossRef]
- Rosenberg, T.D.; E Paulos, L.; Parker, R.D.; Coward, D.B.; Scott, S.M. The forty-five-degree posteroanterior flexion weight-bearing radiograph of the knee. J. Bone Jt. Surg. 1988, 70, 1479–1483. [Google Scholar] [CrossRef]
- Wright, R.W.; Benjamin, C. The MARS Group Osteoarthritis Classification Scales: Interobserver Reliability and Arthroscopic Correlation. J. Bone Jt. Surg. 2014, 96, 1145–1151. [Google Scholar] [CrossRef]
- Bigoni, M.; Turati, M.; Zatti, G.; Gandolla, M.; Sacerdote, P.; Piatti, M.; Castelnuovo, A.; Rigamonti, L.; Munegato, D.; Franchi, S.; et al. Intra-Articular Cytokine Levels in Adolescent Patients after Anterior Cruciate Ligament Tear. Mediat. Inflamm. 2018, 2018, 4210593. [Google Scholar] [CrossRef]
- Kingery, M.T.; Anil, U.; Berlinberg, E.J.; Clair, A.J.; Kenny, L.; Strauss, E.J. Changes in the Synovial Fluid Cytokine Profile of the Knee Between an Acute Anterior Cruciate Ligament Injury and Surgical Reconstruction. Am. J. Sport. Med. 2022, 50, 451–460. [Google Scholar] [CrossRef]
- Turati, M.; Franchi, S.; Leone, G.; Piatti, M.; Zanchi, N.; Gandolla, M.; Rigamonti, L.; Sacerdote, P.; Rizzi, L.; Pedrocchi, A.; et al. Resolvin E1 and Cytokines Environment in Skeletally Immature and Adult ACL Tears. Front. Med. 2021, 8, 610866. [Google Scholar] [CrossRef]
- Turati, M.; Maggioni, D.; Zanchi, N.; Gandolla, M.; Gorla, M.; Sacerdote, P.; Franchi, S.; Rizzi, L.; Pedrocchi, A.; Omeljaniuk, R.J.; et al. Characterization of Synovial Cytokine Patterns in Bucket-Handle and Posterior Horn Meniscal Tears. Mediat. Inflamm. 2020, 2020, 5071934. [Google Scholar] [CrossRef]
- Barker, T.; Rogers, V.E.; Henriksen, V.T.; Trawick, R.H.; Momberger, N.G.; Rasmussen, G.L. Circulating IL-10 is compromised in patients predisposed to developing and in patients with severe knee osteoarthritis. Sci. Rep. 2021, 11, 1812. [Google Scholar] [CrossRef] [PubMed]
- Bigoni, M.; Turati, M.; Gandolla, M.; Sacerdote, P.; Piatti, M.; Castelnuovo, A.; Franchi, S.; Gorla, M.; Munegato, D.; Gaddi, D.; et al. Effects of ACL Reconstructive Surgery on Temporal Variations of Cytokine Levels in Synovial Fluid. Mediat. Inflamm. 2016, 2016, 8243601. [Google Scholar] [CrossRef] [PubMed]
- Bigoni, M.; Turati, M.; Sacerdote, P.; Gaddi, D.; Piatti, M.; Castelnuovo, A.; Franchi, S.; Gandolla, M.; Pedrocchi, A.; Omeljaniuk, R.J.; et al. Characterization of synovial fluid cytokine profiles in chronic meniscal tear of the knee. J. Orthop. Res. 2017, 35, 340–346. [Google Scholar] [CrossRef]
- Lotz, M. Cytokines in Cartilage Injury and Repair. Clin. Orthop. Relat. Res. 2001, 391, S108–S115. [Google Scholar] [CrossRef]
- Wassilew, G.I.; Lehnigk, U.; Duda, G.N.; Taylor, W.R.; Matziolis, G.; Dynybil, C. The Expression of Proinflammatory Cytokines and Matrix Metalloproteinases in the Synovial Membranes of Patients with Osteoarthritis Compared with Traumatic Knee Disorders. Arthrosc. J. Arthrosc. Relat. Surg. 2010, 26, 1096–1104. [Google Scholar] [CrossRef]
- Bigoni, M.; Sacerdote, P.; Turati, M.; Franchi, S.; Gandolla, M.; Gaddi, D.; Moretti, S.; Munegato, D.; Augusti, C.A.; Bresciani, E.; et al. Acute and late changes in intraarticular cytokine levels following anterior cruciate ligament injury. J. Orthop. Res. 2012, 31, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Wojdasiewicz, P.; Poniatowski, L.A.; Szukiewicz, D. The Role of Inflammatory and Anti-Inflammatory Cytokines in the Pathogenesis of Osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Wang, Y.; Crawford, R.; Xiao, Y. Synovial macrophages in cartilage destruction and regeneration—Lessons learnt from osteoarthritis and synovial chondromatosis. Biomed. Mater. 2021, 17, 012001. [Google Scholar] [CrossRef]
- Zhang, J.; Rong, Y.; Luo, C.; Cui, W. Bone marrow mesenchymal stem cell-derived exosomes prevent osteoarthritis by regulating synovial macrophage polarization. Aging 2020, 12, 25138–25152. [Google Scholar] [CrossRef]
- Jansen, N.W.D.; Roosendaal, G.; Hooiveld, M.J.J.; Bijlsma, J.W.J.; van Roon, J.A.G.; Theobald, M.; Lafeber, F.P.J.G. Interleukin-10 protects against blood-induced joint damage. Br. J. Haematol. 2008, 142, 953–961. [Google Scholar] [CrossRef]
- Bigoni, M.; Zanchi, N.; Turati, M.; Pirovano, G.; Zatti, G.; Munegato, D. Short-term differences in anterior knee pain and clinical outcomes between rotating and fixed platform posterior stabilized total knee arthroplasty with a new femoral component design. World J. Orthop. 2019, 10, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Jomha, N.M.; Borton, D.C.; Clingeleffer, A.J.; Pinczewski, L. Long Term Osteoarthritic Changes in Anterior Cruciate Ligament Reconstructed Knees. Clin. Orthop. Relat. Res. 1999, 358, 188–193. [Google Scholar] [CrossRef]
- Seon, J.K.; Song, E.K.; Park, S.J. Osteoarthritis after anterior cruciate ligament reconstruction using a patellar tendon autograft. Int. Orthop. 2006, 30, 94–98. [Google Scholar] [CrossRef] [PubMed]
| OA Group | TM Group | Total | |
|---|---|---|---|
| Gender | |||
| Male | 7 (46.67%) | 15 (100%) | 22 (73.33%) |
| Female | 8 (53.33%) | 0 (0%) | 8 (26.67%) |
| Age | |||
| Mean Age (Years) | 72.9 ± 9.5 | 43.7 ± 9.6 | 58.3 ± 17.59 |
| Timing | |||
| Subacute (SA) | / | 7 (46.67%) | 7 (23.33%) |
| Chronic (C) | 15 (100%) | 8 (53.33%) | 23 (76.67%) |
| Synovial Fluid | |||
| Hydrarthrosis | 11 (73.33%) | 15 (100%) | 26 (86.67%) |
| Hemarthrosis | 4 (26.67%) | 0 (0%) | 4 (13.33%) |
| Laterality | |||
| Medial Meniscus | / | 12 (80%) | 12 (80%) |
| Lateral Meniscus | / | 3 (20%) | 3 (20%) |
| Score | |
|---|---|
| PROMs | |
| Tegner—pre-injury | 5.25 ± 2.71 |
| Tegner—after injury | 4.50 ± 2.00 |
| Lysholm | 80.63 ± 10.35 |
| IKDC | 69.55 ± 16.42 |
| SKV | 71.25 ± 13.30 |
| RX | |
| Kellgren–Lawrence | 0 = 0% |
| 1 = 50% | |
| 2 = 50% | |
| 3 = 0% | |
| 4 = 0% |
| PK2 | IL-10 | TNF-α | |
|---|---|---|---|
| Gonarthrosis | 530.3 ± 367.1 | 11.26 ± 13.81 | 7.813 ± 3.733 |
| Gender | |||
| Male | 588.6 ± 377.8 | 11.85 ± 11.65 | 6.689 ± 0.576 |
| Female | 479.3 ± 375.2 | 10.74 ± 16.26 | 8.798 ± 5.022 |
| Synovial Fluid | |||
| Hemarthrosis | 935.6 ± 458.0 | 24.12 ± 21.02 | 7.755 ± 1.329 |
| Hydrarthrosis | 391.1 ± 193.2 | 6.584 ± 6.643 | 7.835 ± 4.357 |
| PK2 | IL-10 | TNF-α | |
|---|---|---|---|
| Meniscal tear | 647.2 ± 281.0 | 2.15 ± 0.843 | 8.314 ± 2.885 |
| Timing | |||
| SA | 611.4 ± 275.6 | 2.182 ± 0.94 | 9.502 ± 2.733 |
| C | 678.6 ± 300.7 | 2.12 ± 0.81 | 7.423 ± 2.828 |
| Laterality | |||
| Medial Meniscus | 626.4 ± 296.3 | 2.13 ± 0.94 | 7.888 ± 3.057 |
| Lateral Meniscus | 730.8 ± 238.4 | 2.24 ± 0.25 | 9.873 ± 1.646 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turati, M.; Franchi, S.; Crippa, M.; Rizzi, L.; Rigamonti, L.; Sacerdote, P.; Gatti, S.D.; Piatti, M.; Galimberti, G.; Munegato, D.; et al. Prokineticin 2 and Cytokine Content in the Synovial Fluid of Knee Osteoarthritis and Traumatic Meniscal Tear Patients: Preliminary Results. J. Clin. Med. 2023, 12, 4330. https://doi.org/10.3390/jcm12134330
Turati M, Franchi S, Crippa M, Rizzi L, Rigamonti L, Sacerdote P, Gatti SD, Piatti M, Galimberti G, Munegato D, et al. Prokineticin 2 and Cytokine Content in the Synovial Fluid of Knee Osteoarthritis and Traumatic Meniscal Tear Patients: Preliminary Results. Journal of Clinical Medicine. 2023; 12(13):4330. https://doi.org/10.3390/jcm12134330
Chicago/Turabian StyleTurati, Marco, Silvia Franchi, Marco Crippa, Laura Rizzi, Luca Rigamonti, Paola Sacerdote, Simone Daniel Gatti, Massimiliano Piatti, Giulia Galimberti, Daniele Munegato, and et al. 2023. "Prokineticin 2 and Cytokine Content in the Synovial Fluid of Knee Osteoarthritis and Traumatic Meniscal Tear Patients: Preliminary Results" Journal of Clinical Medicine 12, no. 13: 4330. https://doi.org/10.3390/jcm12134330
APA StyleTurati, M., Franchi, S., Crippa, M., Rizzi, L., Rigamonti, L., Sacerdote, P., Gatti, S. D., Piatti, M., Galimberti, G., Munegato, D., Amodeo, G., Omeljaniuk, R. J., Zatti, G., Torsello, A., & Bigoni, M. (2023). Prokineticin 2 and Cytokine Content in the Synovial Fluid of Knee Osteoarthritis and Traumatic Meniscal Tear Patients: Preliminary Results. Journal of Clinical Medicine, 12(13), 4330. https://doi.org/10.3390/jcm12134330








