1. Introduction
The World Health Organization (WHO) declared the end of the pandemic emergency on 5 May 2023, but COVID-19 is far from being defeated, especially when it comes to vulnerable populations such as solid organ transplant recipients. Based on information from the Centers for Disease Control and Prevention, COVID-19 commonly presents with symptoms such as fever, cough, shortness of breath, muscle aches and, in some cases, loss of taste or smell. Gastrointestinal symptoms may also occur, including nausea, vomiting, diarrhea and abnormal liver function. Less common manifestations include headache, dizziness, conjunctivitis, eye irritation and a rash characterized by redness of the skin [
1]. In recent years, several retrospective studies have focused on analyzing the outcomes, clinical features and management of COVID-19 in vulnerable patient populations, including those who have undergone allogeneic stem cell transplantation (allo SCT) and lung transplantation (LT) [
2,
3]. These studies provide valuable insights into the management and care of these specific patient populations. A study conducted in German and Austrian transplant centers aimed to analyze the outcomes of COVID-19 in allo SCT recipients. The cohort included 130 patients who had previously undergone allo SCT who then contracted SARS-CoV-2 between February 2020 and July 2021. The median age at COVID-19 diagnosis was 59 years, and the median age at allo SCT was 55 years. Common underlying diseases among patients included acute myeloid leukemia, Hodgkin’s and non-Hodgkin’s lymphoma, acute lymphoblastic leukemia and myelodysplastic syndrome. Many patients were in incomplete remission at the time of their COVID-19 diagnosis and had active graft versus host disease (GVHD) requiring systemic immunosuppressive treatment. COVID-19 detection methods varied, with nasopharyngeal swab Polymerase Chain Reaction (PCR) being the most common. Transmission was mainly within the family and home environment. Symptoms such as fever, cough, dyspnea and fatigue were common, and a significant proportion of patients developed pneumonia. Around 19% of patients required intensive care, and some patients received specific treatments such as corticosteroids, convalescent plasma, remdesivir and bamlanivimab. This study aimed to identify risk factors for severe disease and mortality in allo SCT recipients with COVID-19, highlighting the need for a comprehensive understanding of the impact of COVID-19 on this patient population to guide appropriate management strategies and to optimize patient care. Similarly, a retrospective, multicenter cohort study conducted in French lung transplant centers had investigated the clinical characteristics and outcomes of LT recipients with COVID-19 [
4]. The study included 35 LT recipients with confirmed or highly suspected SARS-CoV-2 infection. Double lung transplants were the most common, with a median time to LT of 38.2 months. Immunosuppressive therapies, including calcineurin inhibitors and corticosteroids, were commonly used in these patients.
Clinical presentation was variable, with fever being the predominant symptom. Chest CT scans typically showed ground-glass opacities. The study reported cases of hospital-acquired, healthcare-associated and community-acquired infections. Management strategies included the adjustment of immunosuppressive therapy and the administration of specific antiviral treatments such as hydroxychloroquine and remdesivir. The results of the study showed an overall survival rate of 85.7% after a median follow-up of 50 days. However, five patients died due to multi-organ failure and acute respiratory distress syndrome (ARDS). Thrombotic events and pulmonary superinfections were observed in some patients. Overweight status was identified as a potential risk factor for death in LT recipients with COVID-19, while no other significant risk factors for severe disease or mortality were found. In addition, ongoing research and studies are being conducted to assess the effectiveness and safety of COVID-19 vaccination in lung transplant recipients, providing valuable insights and guidance for their healthcare management. COVID-19 vaccination has been a crucial tool in fighting the global pandemic. With the development and distribution of effective vaccines, it offers hope for a safer and healthier future. Vaccines help stimulate the immune system to recognize and fight off the virus, reducing the severity of symptoms and preventing hospitalizations and deaths. However, for individuals who have undergone lung transplants, the importance of vaccination is even more significant. Transplant recipients typically have a weakened immune system, making them more vulnerable to infections. Getting vaccinated against COVID-19 can provide an additional layer of protection and minimize the risk of severe illness or complications in this vulnerable population. It is essential for transplant recipients to consult their healthcare providers for specific vaccination guidelines and recommendations. These studies contribute to our understanding of the clinical characteristics and outcomes of COVID-19 in allo SCT and LT recipients and highlight the importance of tailored management strategies for this vulnerable population. Further research is needed to gain a comprehensive understanding of the risk factors and optimal management approaches for COVID-19 in these patient groups.
4. Discussion
COVID-19 was a global challenge for the entire scientific community. However, over time, improved knowledge of the physiopathology of the virus has led to more successful treatments. Currently, we need to be aware of the potential threat that COVID-19 poses to frail patients. Solid-organ recipients are highly susceptible to developing severe forms of COVID-19 due to their chronic immunosuppression. This risk is even higher in lung transplant recipients, who have a higher degree of immunosuppression, a high incidence of allograft rejection and exposure of the graft to the external environment [
5,
6]. Although the majority of lung transplants performed in our center in the last four years were for pulmonary hypertension, the cases presented in this paper are related not only to vascular lung pathology but also to parenchymal disease, such as emphysema. This is to highlight the different initial conditions that led the two patients to these unusual scenarios. The most interesting finding, in our opinion, is the completely different evolution of the disease, in terms of radiological and clinical findings, as well as the timing of their progression. The second important point are the different treatments adopted: in the first case, medical treatment was modified, whereas in the second case, we initially opted for endoscopic bronchial dilatation or surgical resection (which was not eventually performed given the futility of the procedure) and subsequently for listing for retransplantation.
The last evidence resulting from our cases regards the identification of possible risk factors: immunosuppressive therapy, CLAD and bronchial stenosis.
As described in non-immunocompromised hosts, the clinical presentation of COVID-19 in transplant patients is mostly characterized by fever and cough, especially after the introduction of anti-COVID-19 vaccines [
4]. Our cohort of patients confirms this trend: out of 60 lung transplant patients followed by our unit, 30 developed COVID-19 infection, but only two patients developed a severe form of the infection. However, it is important to note that this series of patients has not been published.
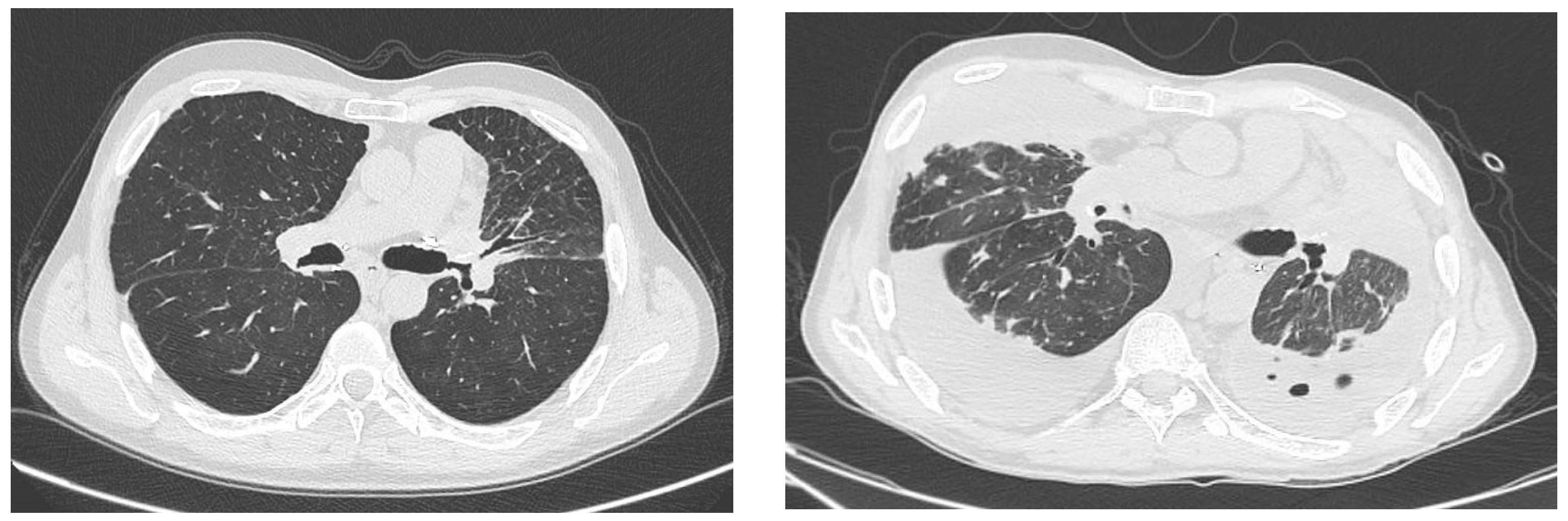
The radiological findings are characterized by the presence of ground-glass opacities on CT, as described in the general population [
4]. Ground-glass opacities are also the main radiological findings of COVID-19 in lung transplant recipients, but radiological presentation may vary among individuals and can be influenced by factors such as the timing of the scan, disease severity, and the presence of pre-existing lung conditions or anatomical changes related to the transplant. The two reported cases have unique features. In particular, Case #1, interestingly, developed a clinically relevant form of COVID-19 one month after testing positive for the virus. Despite treatment with molnupiravir, the patient’s respiratory status deteriorated dramatically 30 days after infection. The recent kidney transplant probably played a critical role in further weakening the patient’s immune system and making him more susceptible to COVID-19. According to Raja et al., lung transplant recipients, and solid-organ recipients in general, show longer viral clearance of SARS-CoV-2 than the general population—almost 3–5 weeks. T-cell immunity, which is pharmacologically inhibited after transplantation, is thought to be the first actor in the antiviral response [
7]. On the other hand, Opsomer et al. reviewed 77 articles and did not find any association between the type of immunosuppression and mortality [
8]. They also found a less relevant immune response after the first two doses of vaccination. These findings are corroborated by Altneu et al., who also support the need for a fourth dose in recipients, especially those infected with the Omicron variant [
9]. In Case #2, the patient was already suffering from significant bilateral bronchial stenosis, and COVID-19 contributed to accelerating the process of respiratory deterioration. In our opinion, Case #1 has a rather peculiar aspect, as the late onset of COVID-19-related pneumonia in the immunosuppressed patient has never been described in lung transplant recipients. Therefore, we believe that these two case reports represent opposite forms of COVID-19 presentation in lung transplant recipients. In the first case, we saw a late-onset ARDS due to parenchymal involvement. In the second case, the pathophysiological mechanism is more related to the involvement of large bronchial structures. This highlights some important aspects.
The issue that is more discussed in literature is the extreme frailty of these patients, not only in the acute phase but also one month after infection.
In COVID-19 infection, the treatment strategy includes the withdrawal of antimetabolites and an increase in corticosteroids, with or without withdrawal of calcineurin inhibitors. Treatment of the infection consists of specific antiviral drugs (remdesivir or lopinavir–ritonavir) [
4]. The treatment for Case #1 was exclusively based on medical therapy. There are no specific guidelines for the timing for antimetabolite withdrawal, but we could hypnotize that a late modification of therapy may be the reason for the severe pneumonia onset. Modification of immunosuppressive therapy probably played an important role in the progression of the parenchymal involvement in Case #1. The French Transplant Society has suggested that the treatment strategy should be based on the severity of the disease [
4]. In this latter study, the most common change in immunosuppressive therapy was also the withdrawal of antimetabolites, followed by an increase in the corticosteroid dose [
4]. However, no definitive indication can be given, as each transplant center has adopted a strategy tailored to the clinical characteristics of the patient.
On the other hand, in Case #2, we observed the worsening of bronchial stenosis. In this specific case, the medical treatment was unsuccessful, and we resorted to surgical solutions without the possibility of success. There were no similar cases in the literature to compare with.
These cases highlight potential prognostic factors in lung transplant recipients who develop COVID-19-related disease. The mortality associated with COVID-19 in lung transplant recipients reported in the literature ranges from 10% to 46%. In our cohort of lung transplant recipients, 30 out of 60 patients developed COVID-19, reporting a mortality of 6.6% (2/30 patients). This cohort of patients has never been published. We propose two causes to explain these data. First, in Italy, we observed a high level of compliance with the COVID-19 restrictions (isolation, use of masks, etc.), thus limiting the rate of infection, even in our transplant population as a whole, during the first phase of the pandemic, when no vaccines were available. Second, the majority of COVID-19 infections in our population were observed during the second wave, when the population was already widely vaccinated, and the clinical manifestations of infection were therefore milder.
Regarding long-term follow-up after COVID-19 infection, it has been shown that the FEV1 remained stable, whereas the TLC and DLCO decreased significantly due to the interstitial involvement and restrictive pattern resulting from the infection [
4]. Kamp et al. showed that the burden of comorbidities, as assessed by the Charlson Comorbidity Index, was a predictor of poor outcome in this group of patients [
5]. In addition, Messika et al. reported that overweight status (body mass index >25 and <30 kg/m
2) was associated with an increased risk of death [
4].
In CLAD, COVID-19 may play a crucial role in determining the clinical course in terms of death (43% of cases) or a further decline in the FEV1 (43% of cases), and it should be noted that none of these patients received any vaccination prior to infection. Messika et al. showed a lower mortality (14.3%) but, as reported by Kamp et al., their series had a lower rate of CLAD, a lower median age and BMI and a shorter observation period [
4,
5], while Permpalung et al. reported no significant association between COVID-19 and the possible worsening of pre-existing CLAD [
10]. These cases show that we must be aware of the possible long-term ineffectiveness of preventive measures and acute treatments such as vaccines, social distancing and antiviral therapy. Indeed, all preventive measures may be ineffective when combined with the frailty of lung transplant recipients.
Finally, the interaction between viral injury and post-transplant anatomical changes, such as bronchial stenosis, must never be underestimated. There is little evidence of bronchial changes after SARS-CoV-2 infection as in Case #1. However, Kanne et al. reported that bronchial thickening is a common complication of long COVID-19 [
11]. Visconti et al., analyzing a Brazilian cohort of pulmonary COVID-19, found many late complications of infection. They reported an 18% rate of bronchial thickening two months after discharge as measured by CT scans [
12].
In conclusion, the first case highlights the challenges faced by transplant recipients with COVID-19, particularly those with complex medical histories and immunosuppressive regimens.
The patient’s experience highlights the need for continuous monitoring, individualized treatment approaches and further research to improve the management and outcomes of COVID-19 in this vulnerable population. We also believe, based on the second case report, that in susceptible patients, SARS-CoV-2 may involve the bronchus and exacerbate stenosis or bronchiectasis. We also do not yet know the full spectrum of viral effects on lung anatomy, but it is reasonable to assume that COVID-19 infection played a primary role in the final occlusion of the bronchial tree in Case Report #2.