Influence of Periodontal Status and Prosthetic Treatment on Survival and Success Rates in Implant Therapy: A 5-Year Retrospective Follow-Up Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Setting
2.3. Participants
2.4. Variables, Data Sources, and Measurement
- Minor: easily handled chairside (loss of retention or de-bonding of implant crown(s), fracture of porcelain on implant crown(s), loss of screw hole sealing, or abutment screw loosening).
- Medium: manageable but at a greater cost and chair time required (fracture of abutment screw or abutment screw loosening).
- Major: new construction required or major repairs with even greater costs and a substantial amount of chair time (fracture of porcelain on implant crown(s), implant fracture, abutment screw loosening, or fracture of prosthesis).
- Survival/success of implants was divided into two categories:
- Survival: the implant exists in the mouth.
- Success: an implant without biological and/or technical complications.
2.5. Statistical Analyses
3. Results
3.1. Patient Demographics
3.2. Implant and Surgical Site Characteristics
3.3. Implant Survival and Success Rate
3.3.1. Survival Rate
3.3.2. Success Rate
3.4. Marginal Bone Level
4. Discussion
- Periodontal diagnosis at baseline had a significant impact on the level of marginal bone loss prediction. Regardless of treatment, Stage 3 and 4 periodontitis patients experienced a higher level of marginal bone loss in comparison to Stages 1 and 2. A comprehensive review of articles published over 42 years concluded that “There is an increased risk of peri-implantitis in smokers compared with non-smokers (reported odds ratios from 3.6 to 4.6). The combination of a history of treated periodontitis and smoking increases the risk of implant failure and peri-implant bone loss” [55].
- For the implants researched in our study, data show that the cylindrical implant with an internal connection of a 45° medium taper (Bego Semados S implant system BEGO Implant Systems GmbH & Co. KG, Bremen, Germany) experienced a higher rate of bone resorption compared to the tapered implant with the 5° internal connection (MegaGen Any Ridge implant, MegaGen group, Daegu, Korea). Both implant systems have a rough surface and a machined polished neck. Although the studies in the literature are inconclusive regarding the survival/success rate of different implant types (tapered vs. cylindrical), the connection type seems to have an impact on long-term stability, especially the morse-taper connection [56]. The primary stability of tapered implants has been demonstrated to be higher than that of cylindrical ones [57], which can have a direct impact on the marginal bone level if loaded immediately [58]. Even though our findings are similar to other studies, we cannot neglect the risk of bias due to the large difference in implant numbers on the two used systems and the observational nature of our study.
- The type of prosthetic work showed a significant prediction of marginal bone loss. Screw-retained restorations and over dentures showed a statistical decrease in the marginal bone level height when compared to the cemented restorations. In the literature, there is still a debate regarding cemented vs. screw-retained implant restorations. With the use of stock abutments in the past, a risk for developing peri-implantitis due to improper excess removal after cementation was demonstrated [59,60]. As a mechanical complication, screw loosening happened more often for the screw-retained ones than for the cemented abutments [61]. A recent systematic review and meta-analysis demonstrated that “multiple abutments disconnections significantly affected marginal bone loss changes in partially edentulous patients” [62]. With the use of standardized titanium-based implant connections and customized implant abutments, we can nowadays make cemented prosthetic restorations that overcome the drawbacks from the past and best preserve the marginal bone level. The cemented restorations used in this study all followed these principles in accordance with other research [63].
- Even though other studies reported the lack of evidence for the association between recall visits and the rate of complications in dental implant treatment [2], data from our study suggest that not only is there a powerful association between these two variables, but a lack of professional maintenance predicts a higher bone level loss during the 5-year functional period.
5. Conclusions
5.1. Limitations and Strengths
5.2. Implications for Practice
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adell, R.; Lekholm, U.; Rockler, B.; Brånemark, P.I. A 15-Year Study of Osseointegrated Implants in the Treatment of the Edentulous Jaw. Int. J. Oral Surg. 1981, 10, 387–416. [Google Scholar] [CrossRef]
- Adler, L.; Buhlin, K.; Jansson, L. Survival and Complications: A 9- to 15-Year Retrospective Follow-up of Dental Implant Therapy. J. Oral Rehabil. 2020, 47, 67–77. [Google Scholar] [CrossRef]
- Ali, Z.; Baker, S.R.; Shahrbaf, S.; Martin, N.; Vettore, M.V. Oral Health-Related Quality of Life after Prosthodontic Treatment for Patients with Partial Edentulism: A Systematic Review and Meta-Analysis. J. Prosthet. Dent. 2019, 121, 59–68.e3. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Thoma, D.; Jung, R.; Zwahlen, M.; Zembic, A. A Systematic Review of the Survival and Complication Rates of Implant-Supported Fixed Dental Prostheses (FDPs) after a Mean Observation Period of at Least 5 Years. Clin. Oral Implant. Res. 2012, 23 (Suppl. S6), 22–38. [Google Scholar] [CrossRef]
- van Velzen, F.J.J.; Ofec, R.; Schulten, E.A.J.M.; Ten Bruggenkate, C.M. 10-Year Survival Rate and the Incidence of Peri-Implant Disease of 374 Titanium Dental Implants with a SLA Surface: A Prospective Cohort Study in 177 Fully and Partially Edentulous Patients. Clin. Oral Implant. Res. 2015, 26, 1121–1128. [Google Scholar] [CrossRef]
- Moraschini, V.; Poubel, L.A.d.C.; Ferreira, V.F.; Barboza, E.d.S.P. Evaluation of Survival and Success Rates of Dental Implants Reported in Longitudinal Studies with a Follow-up Period of at Least 10 Years: A Systematic Review. Int. J. Oral Maxillofac. Surg. 2015, 44, 377–388. [Google Scholar] [CrossRef]
- Simonis, P.; Dufour, T.; Tenenbaum, H. Long-Term Implant Survival and Success: A 10-16-Year Follow-up of Non-Submerged Dental Implants. Clin. Oral Implant. Res. 2010, 21, 772–777. [Google Scholar] [CrossRef]
- Charyeva, O.; Altynbekov, K.; Zhartybaev, R.; Sabdanaliev, A. Long-Term Dental Implant Success and Survival—A Clinical Study after an Observation Period up to 6 Years. Swed. Dent. J. 2012, 36, 1–6. [Google Scholar] [PubMed]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A.R. The Long-Term Efficacy of Currently Used Dental Implants: A Review and Proposed Criteria of Success. Int. J. Oral Maxillofac. Implant. 1986, 1, 11–25. [Google Scholar]
- Smith, D.E.; Zarb, G.A. Criteria for Success of Osseointegrated Endosseous Implants. J. Prosthet. Dent. 1989, 62, 567–572. [Google Scholar] [CrossRef]
- Schwarz, F.; Ramanauskaite, A. It Is All about Peri-Implant Tissue Health. Periodontol. 2000 2022, 88, 9–12. [Google Scholar] [CrossRef]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A New Classification Scheme for Periodontal and Peri-Implant Diseases and Conditions-Introduction and Key Changes from the 1999 Classification. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S1–S8. [Google Scholar] [CrossRef]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-Implant Diseases and Conditions: Consensus Report of Workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S286–S291. [Google Scholar] [CrossRef]
- Araujo, M.G.; Lindhe, J. Peri-Implant Health. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S230–S236. [Google Scholar] [CrossRef] [PubMed]
- French, D.; Grandin, H.M.; Ofec, R. Retrospective Cohort Study of 4591 Dental Implants: Analysis of Risk Indicators for Bone Loss and Prevalence of Peri-Implant Mucositis and Peri-Implantitis. J. Periodontol. 2019, 90, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Diaz, P.; Gonzalo, E.; Villagra, L.J.G.; Miegimolle, B.; Suarez, M.J. What is the prevalence of peri-implantitis? A systematic review and meta-analysis. BMC Oral Health 2022, 22, 449. [Google Scholar] [CrossRef]
- Romandini, M.; Lima, C.; Pedrinaci, I.; Araoz, A.; Soldini, M.C.; Sanz, M. Prevalence and risk/protective indicators of peri-implant diseases: A university-representative cross-sectional study. Clin. Oral Implant. Res. 2021, 32, 112–122. [Google Scholar] [CrossRef]
- Sailer, I.; Karasan, D.; Todorovic, A.; Ligoutsikou, M.; Pjetursson, B.E. Prosthetic Failures in Dental Implant Therapy. Periodontol. 2000 2022, 88, 130–144. [Google Scholar] [CrossRef]
- Lang, N.P.; Wilson, T.G.; Corbet, E.F. Biological Complications with Dental Implants: Their Prevention, Diagnosis and Treatment. Clin. Oral Implant. Res. 2000, 11 (Suppl. S1), 146–155. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Heimisdottir, K. Dental Implants-Are They Better than Natural Teeth? Eur. J. Oral Sci. 2018, 126 (Suppl. S1), 81–87. [Google Scholar] [CrossRef] [PubMed]
- Sinjari, B.; D’Addazio, G.; Santilli, M.; D’Avanzo, B.; Rexhepi, I.; Scarano, A.; Traini, T.; Piattelli, M.; Caputi, S. A 4 Year Human, Randomized, Radiographic Study of Scalloped versus Non-Scalloped Cemented Implants. Materials 2020, 13, 2190. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Brägger, U. Mechanical and Technical Risks in Implant Therapy. Int. J. Oral Maxillofac. Implant. 2009, 24 (Suppl. S3), 69–85. [Google Scholar]
- Francisco, H.; Marques, D.; Pinto, C.; Aiquel, L.; Caramês, J. Is the Timing of Implant Placement and Loading Influencing Esthetic Outcomes in Single-Tooth Implants?-A Systematic Review. Clin. Oral Implant. Res. 2021, 32 (Suppl. S21), 28–55. [Google Scholar] [CrossRef]
- Fürhauser, R.; Florescu, D.; Benesch, T.; Haas, R.; Mailath, G.; Watzek, G. Evaluation of Soft Tissue around Single-Tooth Implant Crowns: The Pink Esthetic Score. Clin. Oral Implant. Res. 2005, 16, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Genetti, L.; Ercoli, C.; Kotsailidi, E.A.; Feng, C.; Tsigarida, A.; Russo, L.L.; Chochlidakis, K. Clinical Evaluation of Pink Esthetic Score of Immediately Impressed Posterior Dental Implants. J. Prosthodont. 2022, 31, 496–501. [Google Scholar] [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S. EFP Workshop Participants and Methodological Consultants. Treatment of stage I–III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 (Suppl. S22), 4–60, Erratum in: J. Clin. Periodontol. 2021, 48, 163. [Google Scholar] [CrossRef]
- Serroni, M.; Paolantonio, M.; Romano, L.; Santamaria, P.; Rexhepi, I.; Sinjari, B.; Paolantonio, G.; Secondi, L.; De Ninis, P.; Femminella, B. Added benefit of L-PRF to autogenous bone grafts in the treatment of degree II furcation involvement in mandibular molars. J. Periodontol. 2022, 93, 1486–1499. [Google Scholar] [CrossRef]
- Rexhepi, I.; Paolantonio, M.; Romano, L.; Serroni, M.; Santamaria, P.; Secondi, L.; Paolantonio, G.; Sinjari, B.; De Ninis, P.; Femminella, B. Efficacy of inorganic bovine bone combined with leukocyte and platelet-rich fibrin or collagen membranes for treating unfavorable periodontal infrabony defects: Randomized non-inferiority trial. J. Periodontol. 2021, 92, 1576–1587. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), S173–S182. [Google Scholar] [CrossRef]
- Buser, D.; Mericske-Stern, R.; Bernard, J.P.; Behneke, A.; Behneke, N.; Hirt, H.P.; Belser, U.C.; Lang, N.P. Long-Term Evaluation of Non-Submerged ITI Implants. Part 1: 8-Year Life Table Analysis of a Prospective Multi-Center Study with 2359 Implants. Clin. Oral Implant. Res. 1997, 8, 161–172. [Google Scholar] [CrossRef]
- Duong, H.-Y.; Roccuzzo, A.; Stähli, A.; Salvi, G.E.; Lang, N.P.; Sculean, A. Oral Health-Related Quality of Life of Patients Rehabilitated with Fixed and Removable Implant-Supported Dental Prostheses. Periodontol. 2000 2022, 88, 201–237. [Google Scholar] [CrossRef] [PubMed]
- Calvert, M.; Kyte, D.; Mercieca-Bebber, R.; Slade, A.; Chan, A.-W.; King, M.T.; the SPIRIT-PRO Group; Hunn, A.; Bottomley, A.; Regnault, A.; et al. Guidelines for Inclusion of Patient-Reported Outcomes in Clinical Trial Protocols: The SPIRIT-PRO Extension. JAMA 2018, 319, 483–494. [Google Scholar] [CrossRef] [PubMed]
- De Bruyn, H.; Raes, S.; Matthys, C.; Cosyn, J. The Current Use of Patient-Centered/Reported Outcomes in Implant Dentistry: A Systematic Review. Clin. Oral Implant. Res. 2015, 26 (Suppl. S11), 45–56. [Google Scholar] [CrossRef] [PubMed]
- Gurgel, B.C.d.V.; Montenegro, S.C.L.; Dantas, P.M.C.; Pascoal, A.L.d.B.; Lima, K.C.; Calderon, P.D.S. Frequency of Peri-Implant Diseases and Associated Factors. Clin. Oral Implant. Res. 2017, 28, 1211–1217. [Google Scholar] [CrossRef]
- Berglundh, T.; Persson, L.; Klinge, B. A Systematic Review of the Incidence of Biological and Technical Complications in Implant Dentistry Reported in Prospective Longitudinal Studies of at Least 5 Years. J. Clin. Periodontol. 2002, 29 (Suppl. S3), 197–212, discussion 232–233. [Google Scholar] [CrossRef]
- Jung, R.E.; Zembic, A.; Pjetursson, B.E.; Zwahlen, M.; Thoma, D.S. Systematic Review of the Survival Rate and the Incidence of Biological, Technical, and Aesthetic Complications of Single Crowns on Implants Reported in Longitudinal Studies with a Mean Follow-up of 5 Years. Clin. Oral Implant. Res. 2012, 23 (Suppl. S6), 2–21. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Brägger, U.; Lang, N.P.; Zwahlen, M. Comparison of Survival and Complication Rates of Tooth-Supported Fixed Dental Prostheses (FDPs) and Implant-Supported FDPs and Single Crowns (SCs). Clin. Oral Implant. Res. 2007, 18 (Suppl. S3), 97–113. [Google Scholar] [CrossRef]
- Menini, M.; Pesce, P.; Delucchi, F.; Ambrogio, G.; Canepa, C.; Carossa, M.; Pera, F. One-stage versus two-stage technique using two splinted extra-short implants: A multicentric split-mouth study with a one-year follow-up. Clin. Implant Dent. Relat. Res. 2022, 24, 602–610. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Asgeirsson, A.G.; Zwahlen, M.; Sailer, I. Improvements in Implant Dentistry over the Last Decade: Comparison of Survival and Complication Rates in Older and Newer Publications. Int. J. Oral Maxillofac. Implant. 2014, 29, 308–324. [Google Scholar] [CrossRef]
- Brägger, U.; Karoussis, I.; Persson, R.; Pjetursson, B.; Salvi, G.; Lang, N. Technical and Biological Complications/Failures with Single Crowns and Fixed Partial Dentures on Implants: A 10-Year Prospective Cohort Study. Clin. Oral Implant. Res. 2005, 16, 326–334. [Google Scholar] [CrossRef]
- Chappuis, V.; Buser, R.; Brägger, U.; Bornstein, M.M.; Salvi, G.E.; Buser, D. Long-Term Outcomes of Dental Implants with a Titanium Plasma-Sprayed Surface: A 20-Year Prospective Case Series Study in Partially Edentulous Patients. Clin. Implant. Dent. Relat. Res. 2013, 15, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Daubert, D.M.; Weinstein, B.F.; Bordin, S.; Leroux, B.G.; Flemming, T.F. Prevalence and Predictive Factors for Peri-Implant Disease and Implant Failure: A Cross-Sectional Analysis. J. Periodontol. 2015, 86, 337–347. [Google Scholar] [CrossRef]
- Derks, J.; Schaller, D.; Håkansson, J.; Wennström, J.L.; Tomasi, C.; Berglundh, T. Effectiveness of Implant Therapy Analyzed in a Swedish Population: Prevalence of Peri-Implantitis. J. Dent. Res. 2016, 95, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Sgolastra, F.; Petrucci, A.; Severino, M.; Gatto, R.; Monaco, A. Smoking and the Risk of Peri-Implantitis. A Systematic Review and Meta-Analysis. Clin. Oral Implant. Res. 2015, 26, e62–e67. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, A.D.; Salame, Z.; Chrcanovic, B.R. Smoking and Dental Implants: A Systematic Review and Meta-Analysis. Medicina 2021, 58, 39. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.O.; Takenaka-Martinez, S.; Cota, L.O.M.; Ferreira, S.D.; Silva, G.L.M.; Costa, J.E. Peri-Implant Disease in Subjects with and without Preventive Maintenance: A 5-Year Follow-Up. J. Clin. Periodontol. 2012, 39, 173–181. [Google Scholar] [CrossRef]
- de Siqueira, R.A.C.; Savaget Gonçalves Junior, R.; Dos Santos, P.G.F.; de Mattias Sartori, I.A.; Wang, H.-L.; Fontão, F.N.G.K. Effect of Different Implant Placement Depths on Crestal Bone Levels and Soft Tissue Behavior: A 5-Year Randomized Clinical Trial. Clin. Oral Implant. Res. 2020, 31, 282–293. [Google Scholar] [CrossRef]
- Fu, J.-H.; Lee, A.; Wang, H.-L. Influence of Tissue Biotype on Implant Esthetics. Int. J. Oral Maxillofac. Implant. 2011, 26, 499–508. [Google Scholar]
- Tarnow, D.P.; Cho, S.C.; Wallace, S.S. The Effect of Inter-Implant Distance on the Height of Inter-Implant Bone Crest. J. Periodontol. 2000, 71, 546–549. [Google Scholar] [CrossRef]
- Vervaeke, S.; Matthys, C.; Nassar, R.; Christiaens, V.; Cosyn, J.; De Bruyn, H. Adapting the Vertical Position of Implants with a Conical Connection in Relation to Soft Tissue Thickness Prevents Early Implant Surface Exposure: A 2-Year Prospective Intra-Subject Comparison. J. Clin. Periodontol. 2018, 45, 605–612. [Google Scholar] [CrossRef]
- Perussolo, J.; Souza, A.B.; Matarazzo, F.; Oliveira, R.P.; Araújo, M.G. Influence of the Keratinized Mucosa on the Stability of Peri-Implant Tissues and Brushing Discomfort: A 4-Year Follow-up Study. Clin. Oral Implant. Res. 2018, 29, 1177–1185. [Google Scholar] [CrossRef]
- Linkevicius, T.; Linkevicius, R.; Alkimavicius, J.; Linkeviciene, L.; Andrijauskas, P.; Puisys, A. Influence of Titanium Base, Lithium Disilicate Restoration and Vertical Soft Tissue Thickness on Bone Stability around Triangular-Shaped Implants: A Prospective Clinical Trial. Clin. Oral Implant. Res. 2018, 29, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Spinato, S.; Stacchi, C.; Lombardi, T.; Bernardello, F.; Messina, M.; Zaffe, D. Biological Width Establishment around Dental Implants Is Influenced by Abutment Height Irrespective of Vertical Mucosal Thickness: A Cluster Randomized Controlled Trial. Clin. Oral Implant. Res. 2019, 30, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Nóvoa, L.; Batalla, P.; Caneiro, L.; Pico, A.; Liñares, A.; Blanco, J. Influence of Abutment Height on Maintenance of Peri-Implant Crestal Bone at Bone-Level Implants: A 3-Year Follow-up Study. Int. J. Periodontics Restor. Dent. 2017, 37, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Heitz-Mayfield, L.J.A.; Huynh-Ba, G. History of Treated Periodontitis and Smoking as Risks for Implant Therapy. Int. J. Oral Maxillofac. Implant. 2009, 24, 39–68. [Google Scholar]
- Caricasulo, R.; Malchiodi, L.; Ghensi, P.; Fantozzi, G.; Cucchi, A. The Influence of Implant-Abutment Connection to Peri-Implant Bone Loss: A Systematic Review and Meta-Analysis. Clin. Implant. Dent. Relat. Res. 2018, 20, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Nandini, N.; Kunusoth, R.; Alwala, A.M.; Prakash, R.; Sampreethi, S.; Katkuri, S. Cylindrical Implant Versus Tapered Implant: A Comparative Study. Cureus 2022, 14, e29675. [Google Scholar] [CrossRef]
- Alshehri, M.; Alshehri, F. Influence of Implant Shape (Tapered vs. Cylindrical) on the Survival of Dental Implants Placed in the Posterior Maxilla: A Systematic Review. Implant. Dent. 2016, 25, 855–860. [Google Scholar] [CrossRef]
- Wittneben, J.-G.; Joda, T.; Weber, H.-P.; Brägger, U. Screw Retained vs. Cement Retained Implant-Supported Fixed Dental Prosthesis. Periodontol. 2000 2017, 73, 141–151. [Google Scholar] [CrossRef]
- De la Rosa, M.; Rodríguez, A.; Sierra, K.; Mendoza, G.; Chambrone, L. Predictors of Peri-Implant Bone Loss during Long-Term Maintenance of Patients Treated with 10-Mm Implants and Single Crown Restorations. Int. J. Oral Maxillofac. Implant. 2013, 28, 798–802. [Google Scholar] [CrossRef]
- Assenza, B.; Artese, L.; Scarano, A.; Rubini, C.; Perrotti, V.; Piattelli, M.; Thams, U.; San Roman, F.; Piccirilli, M.; Piattelli, A. Screw vs. Cement-Implant-Retained Restorations: An Experimental Study in the Beagle. Part 2. Immunohistochemical Evaluation of the Peri-Implant Tissues. J. Oral Implantol. 2006, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Vatėnas, I.; Linkevičius, T. One Abutment One Time vs. Repeatable Abutment Disconnections in Implants, Restored with Cemented / Screw Retained Fixed Partial Dentures: Marginal Bone Level Changes. A Systematic Review and Meta-Analysis. Stomatologija 2021, 23, 35–40. [Google Scholar] [PubMed]
- Muñoz, M.; Busoms, E.; Vilarrasa, J.; Albertini, M.; Ruíz-Magaz, V.; Nart, J. Bone-Level Changes around Implants with 1- or 3-Mm-High Abutments and Their Relation to Crestal Mucosal Thickness: A 1-Year Randomized Clinical Trial. J. Clin. Periodontol. 2021, 48, 1302–1311. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | All Patients (n = 615) | Patients with Implants Surviving ≥ 60 Months (n = 324) |
|---|---|---|
| Age, median (IQR) [range] | 45 (38–57) [17–83] | 45 (38–57) [17–79] |
| Gender (F vs. M) | 359/615 (58.37) | 185/324 (57.1) |
| Smoker, n (%) | 162/615 (26.34) | 82/324 (25.31) |
| No of cigarettes/day, median (IQR) | 0 (0–5) [0–30] | 0 (0–0) [0–30] |
| No of cigarettes/day ≥ 10, n (%) | 148/615 (24.07) | 76/324 (23.46) |
| Diabetes (total), n (%) | 14/615 (2.28) | 5/324 (1.54) |
| Diabetes, n (%) | ||
| No: | 601/615 (97.72) | 319/324 (98.46) |
| Type1: | 1/615 (0.16) | 0/324 (0) |
| Type2: | 13/615 (2.11) | 5/324 (1.54) |
| Cardiovascular disease, n (%) | 29/615 (4.72) | 21/324 (6.48) |
| Osteoporosis, n (%) | 46/615 (7.48) | 20/324 (6.17) |
| Periodontal diagnosis at baseline, n (%) | ||
| Edentulous: | 13/615 (2.11) | 8/324 (2.47) |
| Health: | 49/615 (7.97) | 29/324 (8.95) |
| Stage 1: | 109/615 (17.72) | 48/324 (14.81) |
| Stage 2: | 170/615 (27.64) | 90/324 (27.78) |
| Stage 3: | 158/615 (25.69) | 83/324 (25.62) |
| Stage 4: | 116/615 (18.86) | 66/324 (20.37) |
| Treated periodontitis, n (%) | 348/615 (56.59) | 193/324 (59.57) |
| No. of natural teeth at baseline, median (IQR) [range] | 20 (15–24) [0–31] | 19.5 (16–24.25) [0–31] |
| No. of natural teeth at recall, median (IQR) [range] | 19 (14–24) [0–31] | 19 (13–24) [0–31] |
| No. of inserted dental implants, median (IQR) [range] | 2 (1–3) [1–22] | 2 (1–3) [1–8] |
| No. of prosthetic units/implant ≤ 3, n (%) | 610/615 (99.19) | 321/324 (99.07) |
| Characteristic | All Implants (n = 1427) | Implants Surviving ≥ 60 Months (n = 792) |
|---|---|---|
| Implant, n (%) | ||
| MEGAGEN | 1237/1427 (86.69) | 716/792 (90.4) |
| BEGO | 190/1427 (13.31) | 76/792 (9.6) |
| Prosthetics, n (%) | ||
| Cemented: | 522/1427 (36.58) | 275/792 (34.72) |
| OD: | 199/1427 (13.95) | 114/792 (14.39) |
| Screw: | 706/1427 (49.47) | 403/792 (50.88) |
| Implant position, n (%) | ||
| Mandibular anterior: | 87/1427 (6.1) | 53/792 (6.69) |
| Mandibular posterior: | 559/1427 (39.17) | 295/792 (37.25) |
| Maxillary anterior: | 208/1427 (14.58) | 124/792 (15.66) |
| Maxillary posterior: | 573/1427 (40.15) | 320/792 (40.4) |
| Bone reconstruction before implant therapy, n (%) | ||
| Iliac crest: | 18/1425 (1.26) | 9/791 (1.14) |
| GBR: | 13/1425 (0.91) | 11/791 (1.39) |
| No: | 1100/1425 (77.19) | 616/791 (77.88) |
| Onlay graft: | 91/1425 (6.39) | 43/791 (5.44) |
| Alveolar grafting: | 66/1425 (4.63) | 41/791 (5.18) |
| Buccal reconstruction: | 10/1425 (0.7) | 5/791 (0.63) |
| Sinus lift: | 127/1425 (8.91) | 66/791 (8.34) |
| Bone reconstruction during implant therapy, n (%) | ||
| No: | 748/1427 (52.42) | 421/792 (53.16) |
| Onlay graft: | 2/1427 (0.14) | 0/792 (0) |
| Alveolar grafting: | 107/1427 (7.5) | 66/792 (8.33) |
| Buccal reconstruction: | 460/1427 (32.24) | 249/792 (31.44) |
| Sinus lift: | 77/1427 (5.4) | 38/792 (4.8) |
| Socket shield: | 3/1427 (0.21) | 3/792 (0.38) |
| Splitting: | 30/1427 (2.1) | 15/792 (1.89) |
| Bone reconstruction all (before and during implant therapy), n (%) | 949/1427 (66.5) | 517/792 (65.28) |
| Healing (Open vs. Closed), n (%) | 372/1427 (26.07) | 228/792 (28.79) |
| Free gingival graft, n (%) | 103/1427 (7.22) | 60/792 (7.58) |
| Connective tissue graft, n (%) | 130/1427 (9.11) | 73/792 (9.22) |
| Loading, n (%) | ||
| Conventional: | 1079/1427 (75.61) | 564/792 (71.21) |
| Early: | 161/1427 (11.28) | 109/792 (13.76) |
| Immediate: | 187/1427 (13.1) | 119/792 (15.03) |
| Recall at a specialized clinic, n (%) | 718/1427 (50.32) | 541/792 (68.31) |
| Periodontal check-up at specialized clinic, n (%) | 683/1427 (47.86) | 525/792 (66.29) |
| Implant loss (early/late), n (%) | 37/1427 (2.59) | 8/792 (1.01) |
| No. of prosthetic units/implant, median (IQR) [range] | 1 (1–1.5) [1–6] | 1 (1–1.5) [1–6] |
| Implant survival time (months), median (IQR) [range] | 60 (30–67) [0–84] | 66.5 (62.75–72) [60–84] |
| Implant Diameter (mm) | Implant Length | No of Implants (%) n = 792 |
|---|---|---|
| 3 | 11.5 ± 0.84 | 10 (1.26) |
| 3.25 | 10.75 ± 1.67 | 15 (1.89) |
| 3.5 | 10 ± 2.12 | 302 (38.13) |
| 3.75 | 11.5 ± 1.22 | 49 (6.18) |
| 4 | 10.75 ± 2.68 | 284 (35.85) |
| 4.1 | 12.25 ± 0.75 | 7 (0.88) |
| 4.5 | 10.75 ± 2.68 | 95 (11.99) |
| 5 | 10 ± 2.12 | 21 (2.65) |
| 5.5 | 10.75 ± 1.67 | 9 (1.13) |
| Characteristic | All Implants (n = 1427) | Implants Surviving ≥ 60 Months (n = 792) |
|---|---|---|
| Implant survival, n (%) | 1390/1427 (97.41) | 784/792 (98.99) |
| Implant success rate, n (%) | 1300/1427 (91.1) | 728/792 (91.92) |
| Implant Survival Rate: | Yes (n = 784) | No (n = 8) | p-Value |
|---|---|---|---|
| Sex (F vs. M), no (%) | 445 (56.76) | 2 (25) | 0.085 |
| Smoker, no (%) | 215 (27.42) | 6 (75) | 0.007 |
| No. of cigarettes/day ≥ 10, no (%) | 205 (26.15) | 6 (75) | 0.006 |
| Diabetes, no (%) | 18 (2.3) | 0 (0) | 1 |
| Osteoporosis, no (%) | 58 (7.4) | 0 (0) | 1 |
| Periodontal diagnosis at baseline, no (%) | 0.234 | ||
| Stage 1: | 82 (11.47) | 0 (0) | |
| Stage 2: | 193 (26.99) | 0 (0) | |
| Stage 3: | 210 (29.37) | 4 (66.67) | |
| Stage 4: | 230 (32.17) | 2 (33.33) | |
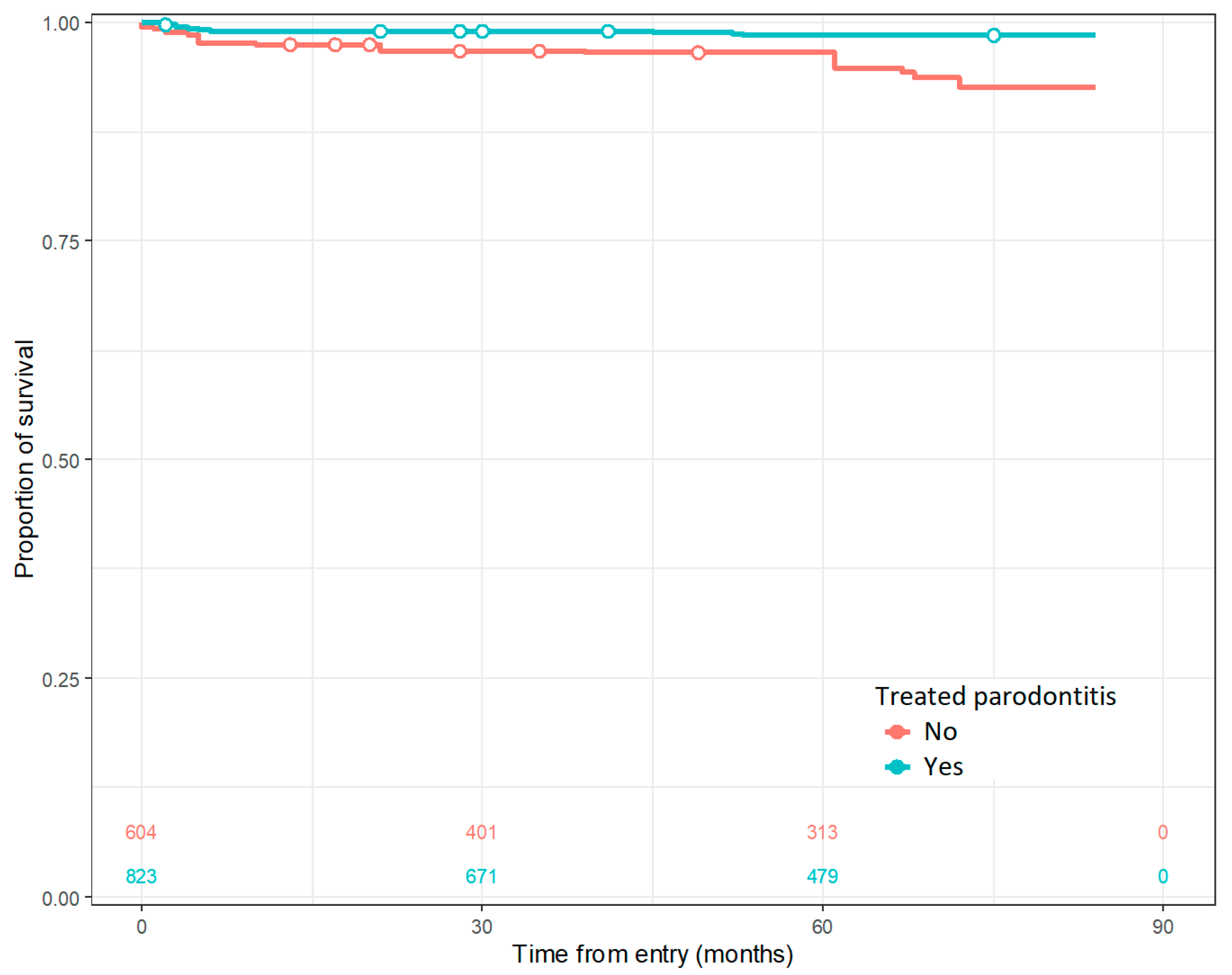
| Treated periodontitis, no (%) | 479 (61.1) | 0 (0) | <0.001 |
| Periodontal diagnosis at baseline and treatment no (%) | <0.001 | ||
| Stage 1,2 untreated: | 91 (12.73) | 0 (0) | |
| Stage 1,2 treated: | 184 (25.73) | 0 (0) | |
| Stage 3,4 untreated: | 145 (20.28) | 6 (100) | |
| Stage 3,4 treated: | 295 (41.26) | 0 (0) | |
| Implant type (BEGO vs. MEGAGEN), no (%) | 76 (9.69) | 0 (0) | 1 |
| Prosthetics, no (%) | 0.065 | ||
| Cemented: | 275 (35.08) | 0 (0) | |
| OD: | 112 (14.29) | 2 (25) | |
| Screw: | 397 (50.64) | 6 (75) | |
| Loading no (%) | <0.001 | ||
| Conventional: | 563 (71.81) | 1 (12.5) | |
| Early: | 107 (13.65) | 2 (25) | |
| Immediate: | 114 (14.54) | 5 (62.5) | |
| No of prosthetic units/implant ≤ 3, no (%) | 775 (98.85) | 8 (100) | 1 |
| Implant position, no (%) | 0.213 | ||
| Mandibular anterior: | 53 (6.76) | 0 (0) | |
| Mandibular posterior: | 294 (37.5) | 1 (12.5) | |
| Maxillary anterior: | 121 (15.43) | 3 (37.5) | |
| Maxillary posterior: | 316 (40.31) | 4 (50) | |
| Bone reconstruction before implant placement, no (%) | 0.674 | ||
| Iliac crest: | 9 (1.15) | 0 (0) | |
| GBR: | 11 (1.4) | 0 (0) | |
| No: | 609 (77.78) | 7 (87.5) | |
| Onlay graft: | 43 (5.49) | 0 (0) | |
| Alveolar grafting: | 40 (5.11) | 1 (12.5) | |
| Buccal reconstruction: | 5 (0.64) | 0 (0) | |
| Sinus lift: | 66 (8.43) | 0 (0) | |
| Bone reconstruction and implant placement, no (%) | 0.184 | ||
| No: | 418 (53.32) | 3 (37.5) | |
| Onlay graft: | 0 (0) | 0 (0) | |
| Alveolar grafting: | 66 (8.42) | 0 (0) | |
| Buccal reconstruction: | 246 (31.38) | 3 (37.5) | |
| Sinus lift: | 36 (4.59) | 2 (25) | |
| Socket shield: | 3 (0.38) | 0 (0) | |
| Splitting: | 15 (1.91) | 0 (0) | |
| Free gingival graft, no (%) | 60 (7.65) | 0 (0) | 1 |
| Connective tissue graft, no (%) | 73 (9.31) | 0 (0) | 1 |
| Healing (Open vs. close), no (%) | 220 (28.06) | 8 (100) | <0.001 |
| Recall at a specialized clinic, no (%) | 537 (68.49) | 4 (50) | 0.272 |
| Periodontal check-up at specialized clinic, no (%) | 521 (66.45) | 4 (50) | 0.453 |
| Characteristic | HR 1 | 95% CI 2 | p-Value |
|---|---|---|---|
| Loading | |||
| Early vs. conventional | 1.57 | 0.50, 4.97 | 0.4 |
| Immediate vs. conventional | 1.74 | 0.46, 6.65 | 0.4 |
| Previously treated periodontitis | 0.29 | 0.12, 0.73 | 0.008 |
| Bone reconstruction (total vs. none) | 1.11 | 0.43, 2.87 | 0.8 |
| Characteristic | All Implants (n = 1427) | Implants Surviving ≥ 60 Months (n = 792) |
|---|---|---|
| Technical complication 1 | ||
| Absent: | 1361/1427 (95.37) | 751/792 (94.82) |
| Major: | 11/1427 (0.77) | 10/792 (1.26) |
| Medium: | 6/1427 (0.42) | 4/792 (0.51) |
| Minor: | 49/1427 (3.43) | 27/792 (3.41) |
| Technical complication 2 (Minor vs. Absent) | 9/1427 (0.63) | 9/792 (1.14) |
| Technical complication 3 (Minor vs. Absent) | 2/1427 (0.14) | 2/792 (0.25) |
| Biological complications | ||
| Absent: | 1392/1427 (97.55) | 772/792 (97.47) |
| Mucositis: | 3/1427 (0.21) | 2/792 (0.25) |
| No integration: | 18/1427 (1.26) | 8/792 (1.01) |
| Periimplantitis: | 14/1427 (0.98) | 10/792 (1.26) |
| Implant Success Rate: | Yes (n = 728) | No (n = 64) | p-Value |
|---|---|---|---|
| Sex (F vs. M), no (%) | 417 (57.28) | 30 (46.88) | 0.107 |
| Smoker, no (%) | 199 (27.34) | 22 (34.38) | 0.229 |
| No. of cigarettes/day ≥ 10, no (%) | 190 (26.1) | 21 (32.81) | 0.244 |
| Diabetes, no (%) | 16 (2.2) | 2 (3.12) | 0.651 |
| Osteoporosis, no (%) | 55 (7.55) | 3 (4.69) | 0.615 |
| Periodontal diagnosis at baseline, no (%) | 0.018 | ||
| Stage 1: | 78 (11.66) | 4 (7.69) | |
| Stage 2: | 179 (26.76) | 14 (26.92) | |
| Stage 3: | 206 (30.79) | 8 (15.38) | |
| Stage 4: | 206 (30.79) | 26 (50) | |
| Periodontal treatment, no (%) | 447 (61.4) | 32 (50) | 0.074 |
| Periodontal diagnosis at baseline and treatment no (%) | 0.827 | ||
| Stage 1,2 untreated: | 84 (12.56) | 7 (13.46) | |
| Stage 1,2 treated: | 173 (25.86) | 11 (21.15) | |
| Stage 3,4 untreated: | 138 (20.63) | 13 (25) | |
| Stage 3,4 treated: | 274 (40.96) | 21 (40.38) | |
| Implant type (BEGO vs. MEGAGEN), no (%) | 68 (9.34) | 8 (12.5) | 0.411 |
| Prosthetic, no (%) | 0.116 | ||
| Cemented: | 249 (34.2) | 26 (40.62) | |
| OD: | 101 (13.87) | 13 (20.31) | |
| Screw: | 378 (51.92) | 25 (39.06) | |
| Loading, no (%) | 0.373 | ||
| Conventional: | 523 (71.84) | 41 (64.06) | |
| Early: | 99 (13.6) | 10 (15.62) | |
| Immediate: | 106 (14.56) | 13 (20.31) | |
| No of prosthetic units/implant ≤ 3, no (%) | 722 (99.18) | 61 (95.31) | 0.03 |
| Implant position, no (%) | 0.424 | ||
| Mandibular anterior: | 49 (6.73) | 4 (6.25) | |
| Mandibular posterior: | 270 (37.09) | 25 (39.06) | |
| Maxillary anterior: | 110 (15.11) | 14 (21.88) | |
| Maxillary posterior: | 299 (41.07) | 21 (32.81) | |
| Bone reconstruction before implant placement, no (%) | 0.896 | ||
| Iliac crest: | 9 (1.24) | 0 (0) | |
| GBR: | 11 (1.51) | 0 (0) | |
| No: | 562 (77.3) | 54 (84.38) | |
| Onlay graft: | 39 (5.36) | 4 (6.25) | |
| Alveolar grafting: | 38 (5.23) | 3 (4.69) | |
| Buccal reconstruction: | 5 (0.69) | 0 (0) | |
| Sinus lift: | 63 (8.67) | 3 (4.69) | |
| Bone reconstruction and implant placement, no (%) | 0.758 | ||
| No: | 389 (53.43) | 32 (50) | |
| Onlay graft: | 0 (0) | 0 (0) | |
| Alveolar grafting: | 59 (8.1) | 7 (10.94) | |
| Buccal reconstruction: | 226 (31.04) | 23 (35.94) | |
| Sinus lift: | 36 (4.95) | 2 (3.12) | |
| Socket shield: | 3 (0.41) | 0 (0) | |
| Splitting: | 15 (2.06) | 0 (0) | |
| Free gingival graft, no (%) | 53 (7.28) | 7 (10.94) | 0.319 |
| Connective tissue graft, no (%) | 67 (9.2) | 6 (9.38) | 0.964 |
| Healing (Open vs. Close), no (%) | 205 (28.16) | 23 (35.94) | 0.188 |
| Recall at a specialized clinic, no (%) | 493 (67.72) | 48 (75) | 0.23 |
| Periodontal check-up at specialized clinic, no (%) | 478 (65.66) | 47 (73.44) | 0.207 |
| Implant Success: | Yes (n = 1300) | No (n = 127) | p-Value |
|---|---|---|---|
| Gender (F vs. M), nr (%) | 768 (59.08) | 60 (47.24) | 0.01 |
| Smoker, nr (%) | 347 (26.69) | 53 (41.73) | <0.001 |
| Number of cigarettes per day ≥ 10, nr (%) | 325 (25) | 52 (40.94) | <0.001 |
| Baseline periodontal diagnostic, nr (%) | <0.001 | ||
| Stage 1: | 173 (14.46) | 16 (14.41) | |
| Stage 2: | 329 (27.51) | 23 (20.72) | |
| Stage 3: | 364 (30.43) | 16 (14.41) | |
| Stage 4: | 330 (27.59) | 56 (50.45) | |
| Loading, nr (%) | <0.001 | ||
| Conventional: | 1001 (77) | 78 (61.42) | |
| Early: | 136 (10.46) | 25 (19.69) | |
| Immediate: | 163 (12.54) | 24 (18.9) | |
| Healing (open vs. close), nr (%) | 320 (24.62) | 52 (40.94) | <0.001 |
| Recall at a specialized clinic, no (%) | 631 (48.54) | 87 (68.5) | <0.001 |
| Periodontal check-up at specialized clinic, no (%) | 600 (46.15) | 83 (65.35) | <0.001 |
| Implant Success: | Yes (n = 550) | No (n = 65) | p-Value |
|---|---|---|---|
| Baseline periodontal diagnostic, nr (%) | <0.001 | ||
| Stage 1: | 99 (19.88) | 10 (18.18) | |
| Stage 2: | 156 (31.33) | 14 (25.45) | |
| Stage 3: | 150 (30.12) | 8 (14.55) | |
| Stage 4: | 93 (18.67) | 23 (41.82) | |
| Prosthetic design, no (%) | 0.024 | ||
| Cemented: | 168 (30.55) | 24 (36.92) | |
| OD: | 40 (7.27) | 10 (15.38) | |
| Screw: | 342 (62.18) | 31 (47.69) | |
| Healing (open vs. closed), nr (%) | 133 (24.18) | 23 (35.38) | 0.05 |
| Recall at a specialized clinic, no (%) | 269 (48.91) | 43 (66.15) | 0.009 |
| Periodontal check-up at specialized clinic, no (%) | 254 (46.18) | 40 (61.54) | 0.019 |
| A. Marginal Bone Level Associated with the Periodontal Diagnostic at Baseline | ||||||
| Periodontal diagnostic baseline: | Stage 1 (n = 48) (a) | Stage 2 (n = 89) (b) | Stage 3 (n = 83) (c) | Stage 4 (n = 66) (d) | p {(a,b)/(a,c)/(a,d)/(b,c)/(b,d)/(c,d)} * | |
| Marginal bone level (mm), median (IQR) | 0.1 (0.1–0.2) | 0.1 (0–0.2) | 0.1 (0.05–0.3) | 0.15 (0–0.3) | 0.359 {0.661/1/0.972/0.531/0.398/0.969} * | |
| B. Marginal bone level associated with treated periodontitis | ||||||
| Treated periodontitis: | Yes (n = 192) | No (n = 129) | Difference (95% CI) | p | ||
| Marginal bone level (mm), median (IQR) | 0.1 (0–0.2) | 0.2 (0–0.3) | 0.1 (−0.1–0) | 0.029 | ||
| C. Marginal bone level associated with periodontitis status | ||||||
| Periodontitis status: | Stage 1,2 not treated (n = 43) (a) | Stage 1,2 treated (n = 94) (b) | Stage 3,4 not treated (n = 51) (c) | Stage 3,4 treated (n = 98) (d) | p {(a,b)/(a,c)/(a,d)/(b,c)/(b,d)/(c,d)} * | |
| Marginal bone level (mm), median (IQR) | 0.2 (0–0.3) | 0.1 (0–0.2) | 0.2 (0.1–0.3) | 0.1 (0–0.2) | 0.006 {0.581/0.504/0.848/0.005/0.993/0.035} | |
| D. Marginal bone level associated with the Implant type | ||||||
| Implant type: | BEGO (n = 27) | MEGAGEN (n = 294) | Difference (95% CI) | p | ||
| Marginal bone level (mm), median (IQR) | 0.4 (0.3–0.5) | 0.1 (0–0.2) | 0.3 (0.2–0.3) | <0.001 | ||
| E. Marginal bone level associated with the number of prosthetic units per implant | ||||||
| Number of prosthetic units/implant ≤ 3: | Yes (n = 318) | No (n = 3) | Difference (95% CI) | p | ||
| Marginal bone level (mm), median (IQR) | 0.1 (0–0.2) | 0.1 (0.05–0.15) | 0 (−0.1–0.2) | 0.602 | ||
| F. Marginal bone level associated with the type of prosthesis | ||||||
| Type of prosthetic work | Cemented (n = 85) (a) | Overdenture (n = 27) (b) | Screw-retained (n = 209) (c) | p {(a,b)/(a,c)/(b,c)} * | ||
| Marginal bone level (mm), median (IQR) | 0 (0–0.2) | 0.1 (0–0.2) | 0.1 (0.1–0.2) | 0.001 {0.868/0.005/0.398} | ||
| G. Marginal bone level associated with implant position | ||||||
| Implant position: | Anterior mandible (n = 9) (a) | Posterior mandible (n = 147) (b) | Anterior maxilla (n = 29) (c) | Posterior maxilla (n = 136) (d) | p {(a,b)/(a,c)/(a,d)/(b,c)/(b,d)/(c,d)} * | |
| Marginal bone level (mm), median (IQR) | 0.2 (0.2–0.3) | 0.1 (0–0.25) | 0.2 (0.1–0.3) | 0.1 (0–0.2) | 0.042 {0.071/0.747/0.007/0.757/0.49/0.281} | |
| H. Marginal bone level associated with healing type | ||||||
| Healing: | Open (n = 88) | Closed (n = 233) | Difference (95% CI) | p | ||
| Marginal bone level (mm), median (IQR) | 0.1 (0–0.2) | 0.1 (0–0.2) | 0 (0–0) | 0.611 | ||
| I. Marginal bone level associated with recalls at specialized clinics | ||||||
| Recall at a specialized clinic: | Yes (n = 219) | No (n = 102) | Difference (95% CI) | p | ||
| Marginal bone level (mm), median (IQR) | 0.1 (0–0.2) | 0.2 (0.1–0.3) | 0.1 (−0.1–−0.1) | <0.001 | ||
| J. Marginal bone level associated with periodontal check-ups at specialized clinics | ||||||
| Periodontal check-ups at a specialized clinic: | Yes (n = 211) | No (n = 110) | Difference (95% CI) | p | ||
| Marginal bone level (mm), median (IQR) | 0.1 (0–0.2) | 0.2 (0.1–0.3) | 0.1 (−0.1–−0.1) | <0.001 | ||
| K. Marginal bone level associated with the prosthetic loading time | ||||||
| Prosthetic loading: | Conventional (n = 561) (a) | Early (n = 107) (b) | Immediate (n = 119) (c) | p {(a,b)/(a,c)/(b,c)} * | ||
| Marginal bone level (mm), median (IQR) | 0.1 (0–0.2) | 0.1 (0.05–0.3) | 0.1 (0–0.2) | 0.017{0.113/0.193/0.011} | ||
| Characteristic | Estimate | 95% CI | p-Value |
|---|---|---|---|
| (Intercept) | 0.185 | (0.112–0.258) | |
| Gender (M vs. F) | 0.018 | (−0.009–0.045) | 0.186 |
| Osteoporosis | −0.034 | (−0.084–0.017) | 0.190 |
| Periodontal diagnosis baseline/grouped | 0.0037 | ||
| Stage 1,2 vs. healthy, edentulous | 0.027 | (−0.027–0.081) | |
| Stage 3,4 vs. healthy, edentulous | 0.067 | (0.014–0.119) | |
| Previously treated periodontitis | −0.011 | (−0.043–0.021) | 0.4975 |
| Implant type MEGAGEN vs. BEGO | −0.201 | (−0.241–−0.16) | <0.001 |
| Prosthetic | 0.019 | ||
| OD | 0.027 | (−0.024–0.078) | |
| Screw-retained | 0.035 | (0.011–0.06) | |
| Implant position | 0.4195 | ||
| Posterior mandible | 0.018 | (−0.02–0.056) | |
| Anterior maxilla | 0.032 | (−0.012–0.076) | |
| Posterior maxilla | 0.015 | (−0.025–0.056) | |
| Loading | 0.2228 | ||
| Early | 0.038 | (−0.005–0.081) | |
| Immediate | 0.006 | (−0.031–0.042) | |
| Recall at a specialized clinic | 0.042 | (0.013–0.072) | 0.005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomina, D.C.; Petruțiu, Ș.A.; Crișan, B.; Leucuța, D.-C.; Dinu, C.M. Influence of Periodontal Status and Prosthetic Treatment on Survival and Success Rates in Implant Therapy: A 5-Year Retrospective Follow-Up Study. J. Clin. Med. 2023, 12, 4275. https://doi.org/10.3390/jcm12134275
Tomina DC, Petruțiu ȘA, Crișan B, Leucuța D-C, Dinu CM. Influence of Periodontal Status and Prosthetic Treatment on Survival and Success Rates in Implant Therapy: A 5-Year Retrospective Follow-Up Study. Journal of Clinical Medicine. 2023; 12(13):4275. https://doi.org/10.3390/jcm12134275
Chicago/Turabian StyleTomina, Darius Cătălin, Ștefan Adrian Petruțiu, Bogdan Crișan, Daniel-Corneliu Leucuța, and Cristian Mihail Dinu. 2023. "Influence of Periodontal Status and Prosthetic Treatment on Survival and Success Rates in Implant Therapy: A 5-Year Retrospective Follow-Up Study" Journal of Clinical Medicine 12, no. 13: 4275. https://doi.org/10.3390/jcm12134275
APA StyleTomina, D. C., Petruțiu, Ș. A., Crișan, B., Leucuța, D.-C., & Dinu, C. M. (2023). Influence of Periodontal Status and Prosthetic Treatment on Survival and Success Rates in Implant Therapy: A 5-Year Retrospective Follow-Up Study. Journal of Clinical Medicine, 12(13), 4275. https://doi.org/10.3390/jcm12134275







