The Development and Validation of a Predictive Model for Voriconazole-Related Liver Injury in Hospitalized Patients in China
Abstract
1. Introduction
2. Materials and Methods
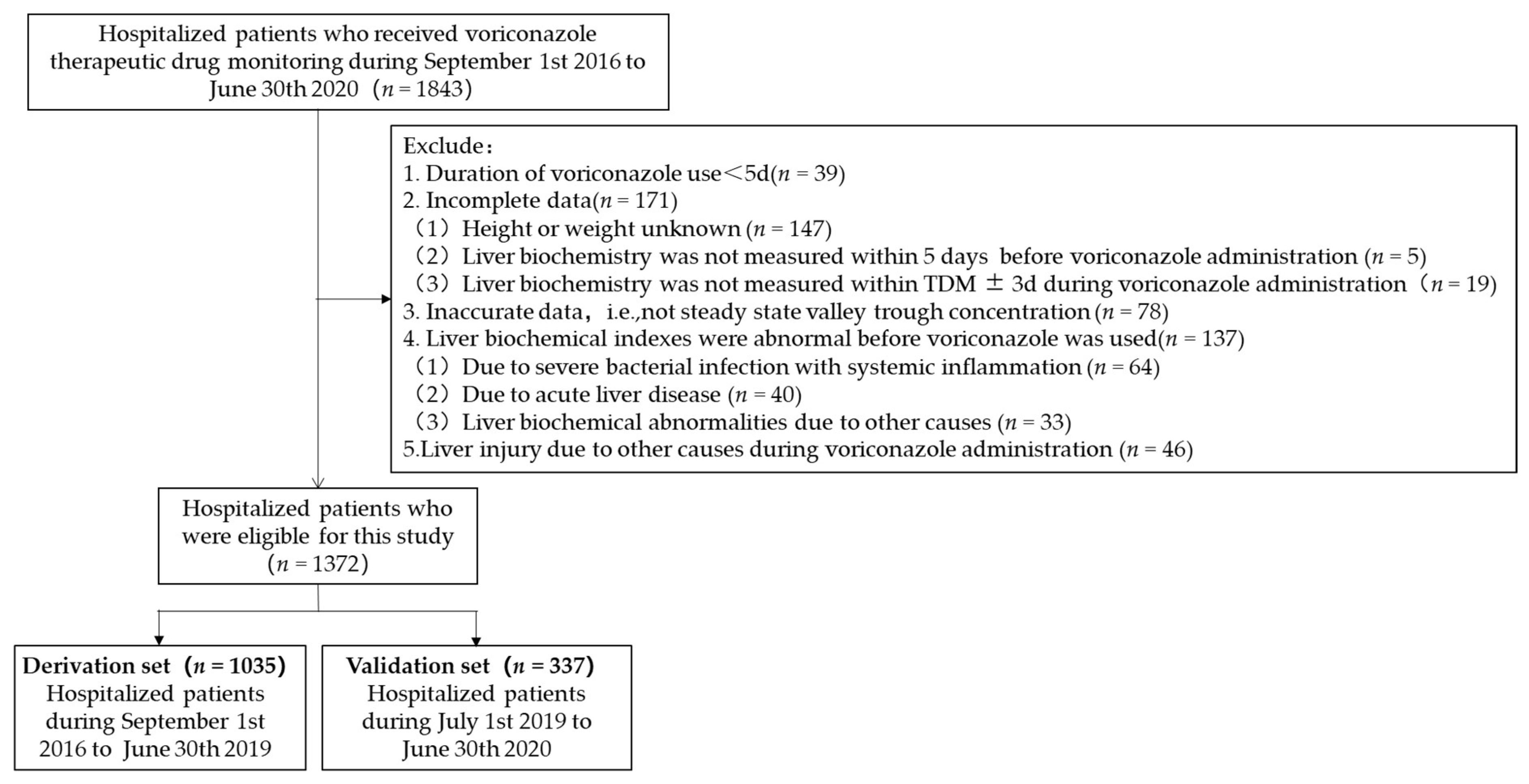
2.1. Study Design and Patient Selection
2.2. Variable Definitions
2.3. Statistical Methods
3. Results
3.1. Clinical Characteristics of Patients
3.2. Incidence of Voriconazole-Related Liver Injury
3.3. Disposal Cost of Voriconazole-Related Liver Injury
3.4. Predictors of Voriconazole-Related Liver Injury
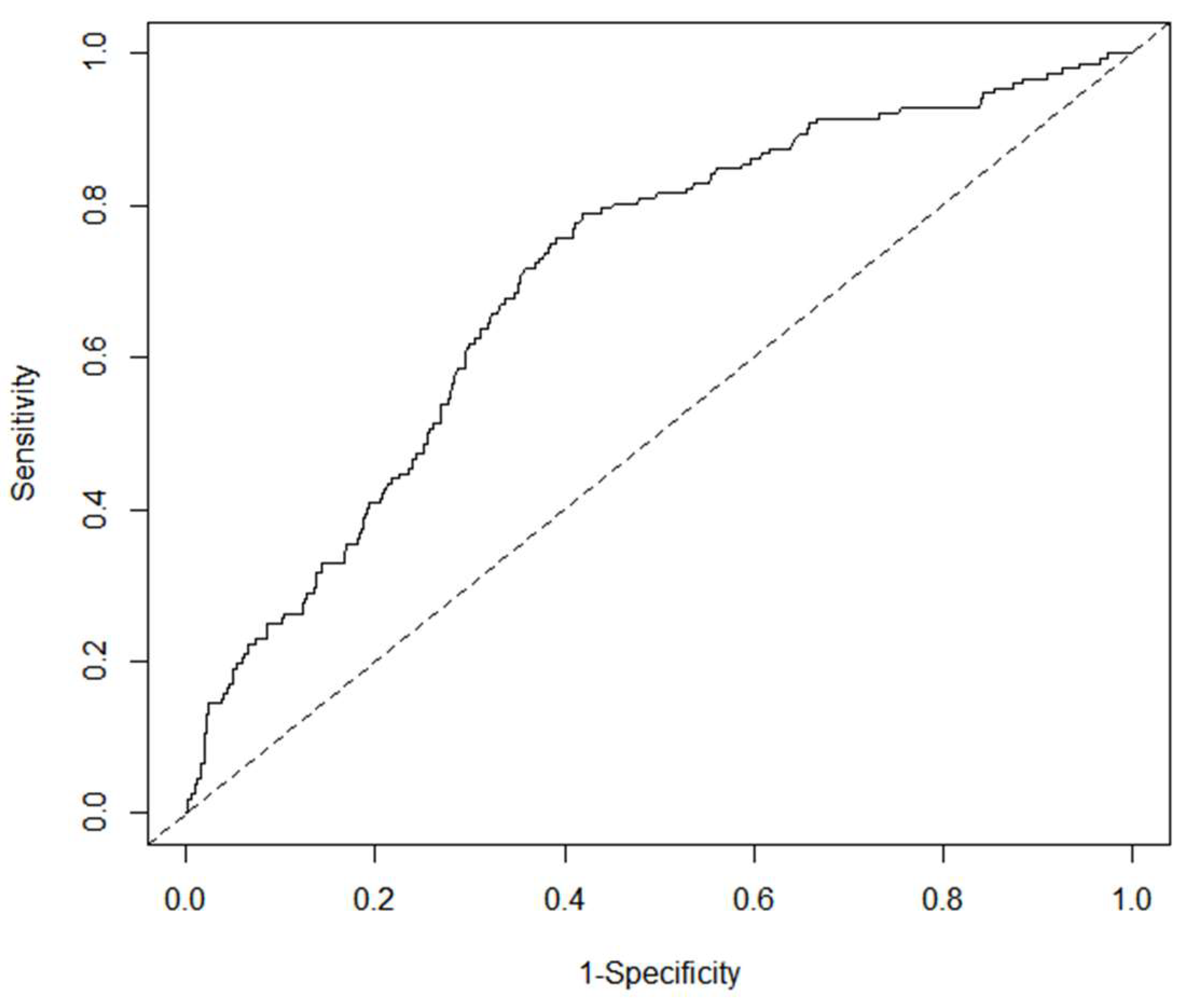
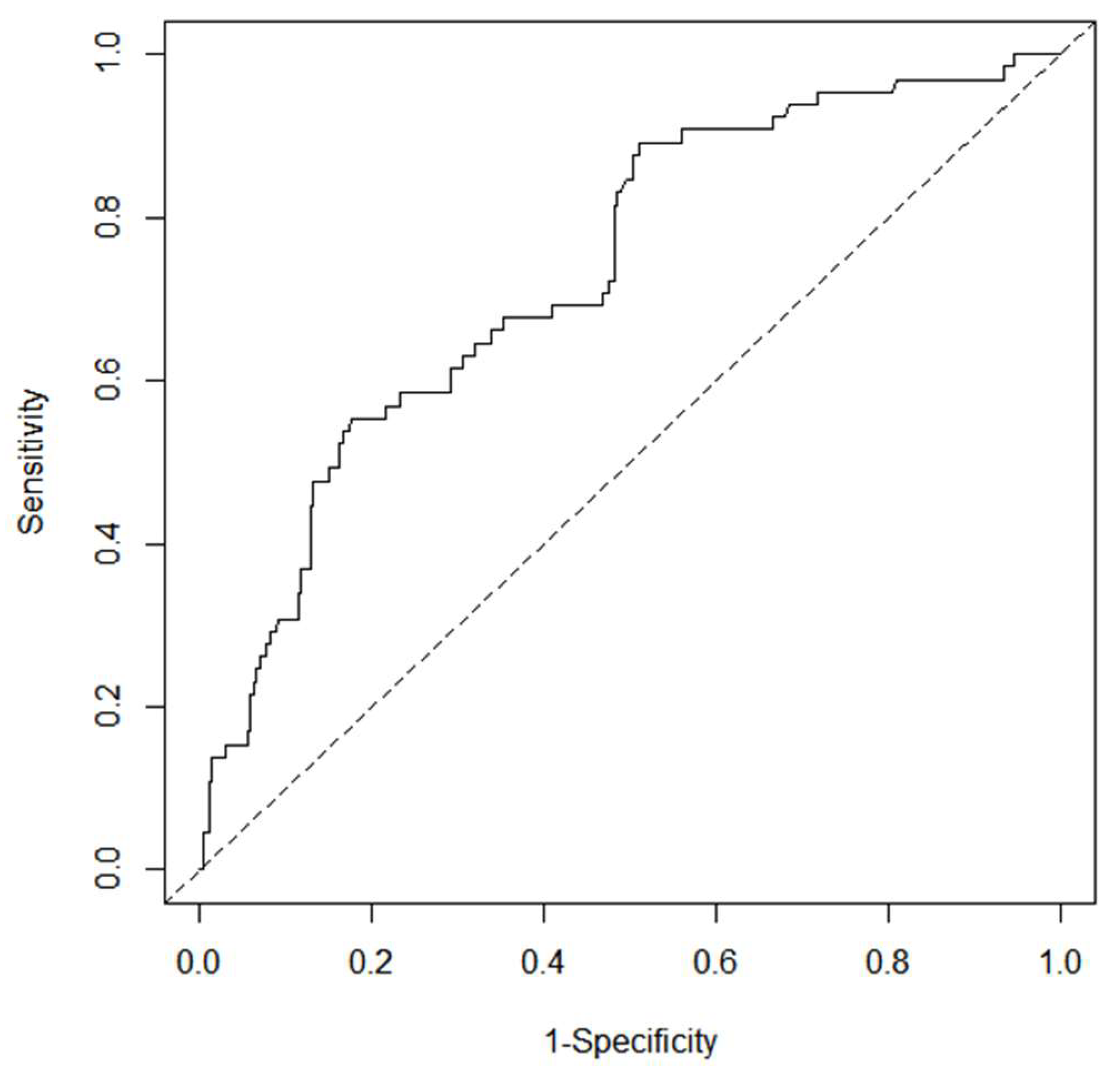
3.5. Development and Validation of Nomogram
3.6. Yodon’s Index
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patterson, T.F.; Thompson, G.R., III; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef] [PubMed]
- Warris, A.; Lehrnbecher, T.; Roilides, E.; Castagnola, E.; Bruggemann, R.J.M.; Groll, A.H. ESCMID-ECMM guideline: Diagnosis and management of invasive aspergillosis in neonates and children. Clin. Microbiol. Infect. 2019, 25, 1096–1113. [Google Scholar] [CrossRef] [PubMed]
- Seyoum, E.; Bitew, A.; Mihret, A. Distribution of Candida albicans and non-albicans Candida species isolated in different clinical samples and their in vitro antifungal suscetibity profile in Ethiopia. BMC Infect. Dis. 2020, 20, 231. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Xu, X.; Wu, Y.; Zhang, W.; Zeng, Q.; Lu, Y.; Yang, T.; Zhou, G.; Yu, J.; Lan, K.; et al. Comparison of amphotericin B deoxycholate in combination with either flucytosine or fluconazole, and voriconazole plus flucytosine for the treatment of HIV-associated cryptococcal meningitis: A prospective multicenter study in China. BMC Infect. Dis. 2022, 22, 677. [Google Scholar] [CrossRef] [PubMed]
- Maertens, J.A.; Rahav, G.; Lee, D.-G.; Ponce-De-León, A.; Sánchez, I.C.R.; Klimko, N.; Sonet, A.; Haider, S.; Vélez, J.D.; Raad, I.; et al. Posaconazole versus voriconazole for primary treatment of invasive aspergillosis: A phase 3, randomised, controlled, non-inferiority trial. Lancet 2021, 397, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Olum, R.; Baluku, J.B.; Kazibwe, A.; Russell, L.; Bongomin, F. Tolerability of oral itraconazole and voriconazole for the treatment of chronic pulmonary aspergillosis: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0240374. [Google Scholar] [CrossRef]
- Ren, X.L.; Zhang, X.; Zhan, Y.Q. Safety study of voriconazole clinical application: Datas based on 10-year spontaneous reports in Beijing. Chin. J. Hosp. Pharm. 2022, 42, 439–442. [Google Scholar] [CrossRef]
- Bogler, Y.; Stern, A.; Su, Y.; Lee, Y.J.; Seo, S.K.; Shaffer, B.; Perales, M.-A.; Papanicolaou, G.A.; Neofytos, D. Efficacy and safety of isavuconazole compared with voriconazole as primary antifungal prophylaxis in allogeneic hematopoietic cell transplant recipients. Med. Mycol. 2021, 59, 970–979. [Google Scholar] [CrossRef]
- Bongomin, F.; Otu, A.; Harris, C.; Foden, P.; Kosmidis, C.; Denning, D.W. Risk factors for relapse of chronic pulmonary aspergillosis after discontinuation of antifungal therapy. Clin. Infect. Pract. 2020, 5, 100015. [Google Scholar] [CrossRef]
- Hanai, Y.; Hamada, Y.; Kimura, T.; Matsumoto, K.; Takahashi, Y.; Fujii, S.; Nishizawa, K.; Miyazaki, Y.; Takesue, Y. Favorable Effects of Voriconazole Trough Concentrations Exceeding 1 μg/mL on Treatment Success and All-Cause Mortality: A Systematic Review and Meta-Analysis. J. Fungi 2021, 7, 306. [Google Scholar] [CrossRef]
- Li, X.; Yu, C.; Wang, T.; Chen, K.; Zhai, S.; Tang, H. Effect of cytochrome P450 2C19 polymorphisms on the clinical outcomes of voriconazole: A systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 2016, 72, 1185–1193. [Google Scholar] [CrossRef]
- Song, Y.; Jia, M.-X.; Yang, G.; Feng, X.-Y.; Yin, D.-H.; Kang, J.-B.; Zhao, Q.; Duan, J.-J. Association of CYP2C19 and UGT1A4 polymorphisms with voriconazole-induced liver injury. Pers. Med. 2020, 17, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.; Brayshaw, N.; Tomaszewski, K.; Troke, P.; Wood, N. Investigation of the Potential Relationships Between Plasma Voriconazole Concentrations and Visual Adverse Events or Liver Function Test Abnormalities. J. Clin. Pharmacol. 2006, 46, 235–243. [Google Scholar] [CrossRef]
- Hanai, Y.; Hamada, Y.; Kimura, T.; Matsumoto, K.; Takahashi, Y.; Fujii, S.; Nishizawa, K.; Takesue, Y. Optimal trough concentration of voriconazole with therapeutic drug monitoring in children: A systematic review and meta-analysis. J. Infect. Chemother. 2020, 27, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Strohbehn, G.W.; Pan, W.; Petrilli, C.M.; Heidemann, L.; Larson, S.; Aaronson, K.D.; Johnson, M.; Ellies, T.; Heung, M. Large-Scale Variability of Inpatient Tacrolimus Therapeutic Drug Monitoring at an Academic Transplant Center: A Retrospective Study. Ther. Drug Monit. 2018, 40, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Chee-How, E.L.; Acquisto, N.M.; Zhang, Y.V. Appropriateness of tacrolimus therapeutic drug monitoring timing in the emergency department. Am. J. Emerg. Med. 2020, 45, 233–236. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, X.; Ke, X.; Du, G.; Yang, K.; Zhai, S. Individualized Medication of Voriconazole: A Practice Guideline of the Division of Therapeutic Drug Monitoring, Chinese Pharmacological Society. Ther. Drug Monit. 2018, 40, 663–674. [Google Scholar] [CrossRef]
- Bénichou, C. Criteria of drug-induced liver disorders. Report of an international consensus meeting. J. Hepatol. 1990, 11, 272–276. [Google Scholar] [CrossRef]
- Fontana, R.J.; Watkins, P.B.; Bonkovsky, H.L.; Chalasani, N.; Davern, T.J.; Serrano, J.; Rochon, J. Drug-Induced Liver Injury Network (DILIN) Prospective Study. Drug Saf. 2009, 32, 55–68. [Google Scholar] [CrossRef]
- Aithal, G.P.; Watkins, P.B.; Andrade, R.J.; Larrey, D.; Molokhia, M.; Takikawa, H.; Hunt, C.M.; Wilke, R.A.; Avigan, M.; Kaplowitz, N.; et al. Case Definition and Phenotype Standardization in Drug-Induced Liver Injury. Clin. Pharmacol. Ther. 2011, 89, 806–815. [Google Scholar] [CrossRef]
- Tan, E.H.; Ling, Z.J.; Ang, P.S.; Sung, C.; Dan, Y.Y.; Tai, B.C. Comparison of laboratory threshold criteria in drug-induced liver injury detection algorithms for use in pharmacovigilance. Pharmacoepidemiol. Drug Saf. 2020, 29, 1480–1488. [Google Scholar] [CrossRef]
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5; US Department of Health and Human Services: Washington, DC, USA; National Institutes of Health: New York, NY, USA; National Cancer Institute: Bethesda, MD, USA, 2017. Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. (accessed on 31 March 2023).
- Guoen, L. China Guidelines for Pharmaoeconomic Evaluations; China Market Press: Beijing, China, 2020. [Google Scholar]
- Samanta, P.; Clancy, C.J.; Marini, R.V.; Rivosecchi, R.M.; McCreary, E.K.; Shields, R.K.; Falcione, B.A.; Viehman, A.; Sacha, L.; Kwak, E.J.; et al. Isavuconazole Is as Effective as and Better Tolerated Than Voriconazole for Antifungal Prophylaxis in Lung Transplant Recipients. Clin. Infect. Dis. 2020, 73, 416–426. [Google Scholar] [CrossRef]
- Chan, S.Y.; Hughes, R.M.; Woo, K.; Perales, M.-A.; Neofytos, D.; Papanicolaou, G. Reasons for voriconazole prophylaxis discontinuation in allogeneic hematopoietic cell transplant recipients: A real-life paradigm. Med. Mycol. 2020, 58, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.P.; Yang, H.; Huang, Y.H.; Da, C.L.; Jin, Y.Z.; Bai, Z.Q. Retrospective analysis of targeted treatment for drug-induced liver injury. Chin. J. Hosp. Pharm. 2019, 39, 2424–2427. [Google Scholar] [CrossRef]
- Andrade, R.J.; Aithal, G.P.; Björnsson, E.S.; Kaplowitz, N.; Kullak-Ublick, G.A.; Larrey, D.; Karlsen, T.H. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Drug-induced liver injury. J. Hepatol. 2019, 70, 1222–1261. [Google Scholar] [CrossRef] [PubMed]
- Luong, M.-L.; Hosseini-Moghaddam, S.M.; Singer, L.G.; Chaparro, C.; Azad, S.; Lazar, N.; Boutros, P.C.; Keshavjee, S.; Rotstein, C.; Husain, S. Risk Factors for Voriconazole Hepatotoxicity at 12 Weeks in Lung Transplant Recipients. Am. J. Transplant. 2012, 12, 1929–1935. [Google Scholar] [CrossRef]
- Re, V.L.; Carbonari, D.M.; Lewis, J.D.; Forde, K.A.; Goldberg, D.S.; Reddy, K.R.; Haynes, K.; Roy, J.A.; Sha, D.; Marks, A.R.; et al. Oral Azole Antifungal Medications and Risk of Acute Liver Injury, Overall and by Chronic Liver Disease Status. Am. J. Med. 2015, 129, 283–291.e5. [Google Scholar] [CrossRef]
- Jin, H.; Wang, T.; Falcione, B.A.; Olsen, K.M.; Chen, K.; Tang, H.; Hui, J.; Zhai, S. Trough concentration of voriconazole and its relationship with efficacy and safety: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2016, 71, 1772–1785. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T.; Xie, J.; Yang, Q.; Zheng, X.; Dong, W.; Xing, J.; Wang, X.; Dong, Y. Risk Factors for Voriconazole-Associated Hepatotoxicity in Patients in the Intensive Care Unit. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2016, 36, 757–765. [Google Scholar] [CrossRef]
- Hirata, A.; Noto, K.; Ota, R.; Yokoyama, S.; Hosomi, K.; Takada, M.; Matsuoka, H. Voriconazole trough concentration and hepatotoxicity in patients with low serum albumin. Int. J. Clin. Pharmacol. Ther. 2019, 57, 135–143. [Google Scholar] [CrossRef]
- Hamada, Y.; Ueda, T.; Miyazaki, Y.; Nakajima, K.; Fukunaga, K.; Miyazaki, T.; Nakada-Motokawa, N.; Nagao, M.; Kawamura, H.; Shigemi, A.; et al. Effects of antifungal stewardship using therapeutic drug monitoring in voriconazole therapy on the prevention and control of hepatotoxicity and visual symptoms: A multicentre study conducted in Japan. Mycoses 2020, 63, 779–786. [Google Scholar] [CrossRef]
- Solís-Muñoz, P.; López, J.C.; Bernal, W.; Willars, C.; Verma, A.; Heneghan, M.A.; Wendon, J.; Auzinger, G. Voriconazole hepatotoxicity in severe liver dysfunction. J. Infect. 2012, 66, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hou, K.; Liu, F.; Luo, X.; He, S.; Hu, L.; Yang, C.; Huang, L.; Feng, Y. The influence of CYP2C19 polymorphisms on voriconazole trough concentrations: Systematic review and meta-analysis. Mycoses 2021, 64, 860–873. [Google Scholar] [CrossRef]
- Xu, N.; Zhang, Q.; Wu, G.; Lv, D.; Zheng, Y. Derivation and Validation of a Risk Prediction Model for Vancomycin-Associated Acute Kidney Injury in Chinese Population. Ther. Clin. Risk Manag. 2020, 16, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Guo, D.; Yao, C.; Zhu, Y.; Liu, S.; Kong, X. Development and Validation of a Nomogram for Predicting Drug-Induced Acute Kidney Injury in Hospitalized Patients: A Case-Control Study Based on Propensity-Score Matching. Front. Pharmacol. 2021, 12, 657853. [Google Scholar] [CrossRef] [PubMed]
- Benitez, L.L.; Carver, P.L. Adverse Effects Associated with Long-Term Administration of Azole Antifungal Agents. Drugs 2019, 79, 833–853. [Google Scholar] [CrossRef]


| Variables | Total (n = 1372) | Groups | |||
|---|---|---|---|---|---|
| Derivation Set (n = 1035) | Validation Set (n = 337) | Statistical Value | p | ||
| Basic information | |||||
| Age (y) | 48.55 ± 17.14 | 48.19 ± 17.40 | 49.66 ± 16.29 | −1.410 a | 0.159 |
| Male (n, %) | 939 (68.44) | 715 (69.08) | 224 (66.47) | 0.804 b | 0.370 |
| Height (cm) | 164.82 ± 7.10 | 164.92 ± 7.14 | 164.52 ± 6.96 | 0.911 a | 0.363 |
| Weight (kg) | 59.76 ± 10.99 | 58.89 ± 10.35 | 62.45 ± 12.39 | −4.761 a | <0.001 |
| BMI (kg/m2) | 17.66 ± 3.51 | 17.93 ± 3.61 | 16.84 ± 3.04 | 5.437 a | <0.001 |
| Fungal disease type (n, %) | |||||
| Aspergillosis | 539 (39.29) | 443 (42.80) | 96 (28.49) | 21.842 b | <0.001 |
| Cryptococcosis | 97 (7.07) | 80 (7.73) | 17 (5.04) | 2.789 b | 0.095 |
| Candidiasis | 52 (3.79) | 35 (3.38) | 17 (5.04) | 1.928 b | 0.165 |
| Talaromycosis | 4 (0.29) | 1 (0.10) | 3 (0.89) | 0.048 c | |
| Unknown pathogen | 680 (49.56) | 476 (45.99) | 204 (60.53) | 128.199 b | <0.001 |
| Major comorbidities (n, %) | |||||
| Bacterial infection | 492 (35.86) | 402 (38.84) | 90 (26.71) | 16.275 b | <0.001 |
| Chronic viral hepatitis B (normal liver biochemistry) | 91 (6.63) | 73 (7.05) | 18 (5.34) | 1.203 b | 0.273 |
| Decompensated cirrhosis (normal liver biochemistry) | 60 (4.37) | 42 (4.06) | 18 (5.34) | 1.101 b | 0.317 |
| Chronic renal failure | 213 (15.52) | 166 (16.04) | 47 (13.95) | 0.848 b | 0.357 |
| Abnormal renal function | 41 (2.99) | 30 (2.90) | 11 (3.26) | 0.117 b | 0.732 |
| Cancer | 246 (17.93) | 211 (20.39) | 35 (10.39) | 17.279 b | <0.001 |
| Renal transplantation | 138 (10.06) | 120 (11.59) | 18 (5.34) | 10.988 b | 0.001 |
| Stem cell transplantation | 75 (5.47) | 62 (5.99) | 13 (3.86) | 2.238 b | 0.135 |
| Lung transplantation | 29 (2.11) | 23 (2.22) | 6 (1.78) | 0.240 b | 0.624 |
| Liver transplantation | 5 (0.36) | 4 (0.39) | 1 (0.30) | 0.400 c | 1.000 |
| Autoimmune disease | 93 (6.78) | 67 (6.47) | 26 (7.72) | 0.620 d | 0.431 |
| Diabetes | 314 (22.89) | 227 (21.93) | 87 (25.82) | 2.173 b | 0.140 |
| Chronic obstructive pulmonary disease | 51 (3.72) | 49 (4.73) | 2 (0.59) | 12.179 b | <0.001 |
| Asthma | 22 (1.60) | 12 (1.16) | 10 (2.97) | 5.267 b | 0.022 |
| Hypertension | 487 (35.50) | 337 (32.56) | 110 (32.64) | 0.001 b | 0.978 |
| Heart disease | 166 (12.10) | 122 (11.79) | 44 (13.06) | 0.385 b | 0.535 |
| Hyperlipidemia | 40 (2.92) | 28 (2.71) | 12 (3.56) | 0.657 b | 0.417 |
| Anemia | 327 (23.83) | 226 (21.84) | 101 (29.97) | 9.267 b | 0.002 |
| Hypoproteinemia | 340 (24.78) | 192 (18.55) | 148 (43.92) | 87.756 b | <0.001 |
| Voriconazole dose (mg/kg) | 3.31 ± 0.51 | 3.34 ± 0.48 | 3.22 ± 0.58 | 3.573 a | <0.001 |
| Voriconazole trough concentration (mg/L) | |||||
| Cmin, M (P25, P75) | 2.66 (1.20,5.00) | 2.62 (1.25,5.16) | 2.62 (1.10,4.54) | 2.472 e | 0.013 |
| Cmin < 1.0 mg/L (n, %) | 279 (20.34) | 227 (21.93) | 52 (15.43) | 6.634 b | 0.010 |
| Cmin [1.0, 5.5] mg/L (n, %) | 797 (58.09) | 596 (57.58) | 201 (59.64) | 0.725 b | 0.394 |
| Cmin > 5.5mg/L (n, %) | 296 (21.57) | 212 (20.48) | 84 (24.93) | 2.966 b | 0.085 |
| Liver biochemical indicator | |||||
| ALT [M(P25,P75), IU/L] | 22 (12,39) | 22 (12,39) | 23 (12,39) | 0.947 e | 0.344 |
| AST [M(P25,P75), IU/L] | 24 (17,40) | 24 (17,40) | 24 (18,40) | 2.832 e | 0.005 |
| TBil [M(P25,P75), μmol/L] | 7.7 (5.3,11.8) | 7.9 (5.4,12.1) | 7.4 (5.2,11.2) | 4.047 e | <0.001 |
| DBil [M(P25,P75), μmol/L] | 3.5 (2.3,6.2) | 3.5 (2.3,6.3) | 3.4 (2.1,5.6) | 4.442 e | <0.001 |
| ALP [M(P25,P75), IU/L] | 99 (72,144) | 99 (72,146) | 101 (72,138) | 5.553 e | <0.001 |
| Group | n | Disposal Cost (Chinese Yuan) | ||
|---|---|---|---|---|
| Range | Mean | Median (P25, P75) | ||
| non VCZ-LI | 1155 | 0.00~4823.43 | 58.19 | 0.00 (0.00, 0.00) |
| VCZ-LI | 217 | 0.00~8372.65 | 599.23 | 101.90 (0.00, 786.48) |
| General VCZ-LI | 175 | 0.00~4540.50 | 483.23 | 0.00 (0.00, 410.48) |
| Severe VCZ-LI | 42 | 0.00~8372.65 | 1082.58 | 993.59 (361.70, 1451.76) |
| Group | Discount Rate of 0% | Discount Rate of 8% | ||
|---|---|---|---|---|
| Mean | Median (P25, P75) | Mean | Median (P25, P75) | |
| non VCZ-LI (n = 1155) | 46.06 | 0.00 (0.00, 0.00) | 66.64 | 0.00 (0.00, 0.00) |
| VCZ-LI (n = 217) | 466.29 | 79.84 (0.00, 617.40) | 693.05 | 117.31 (0.00, 900.29) |
| General VCZ-LI (n = 175) | 373.48 | 0.00 (0.00, 321.62) | 561.04 | 0.00 (0.00, 472.57) |
| Severe VCZ-LI (n = 42) | 853.02 | 770.00 (294.08, 1124.14) | 1243.09 | 1128.31 (409.63, 1689.02) |
| Variables | VCZ-LI Group (n = 152) | Non VCZ-LI Group (n = 883) | Statistical Value | p |
|---|---|---|---|---|
| Basic information | ||||
| Age (y) | 49.55 ± 15.90 | 47.96 ± 17.64 | 1.117 a | 0.265 |
| Male (n, %) | 99 (65.13) | 616 (69.76) | 1.302 b | 0.254 |
| Height (㎝) | 164.25 ± 7.18 | 165.04 ± 7.14 | −1.255 a | 0.210 |
| Weight (kg) | 58.54 ± 9.56 | 58.95 ± 10.48 | −0.445 a | 0.657 |
| BMI (kg/m2) | 17.74 ± 3.43 | 17.96 ± 3.64 | −0.679 a | 0.497 |
| Fungal disease (n, %) | ||||
| Aspergillosis | 67 (44.08) | 376 (42.58) | 0.119 b | 0.730 |
| Cryptococcosis | 4 (2.63) | 76 (8.61) | 6.492 b | 0.011 |
| Candidiasis | 5 (3.29) | 30 (3.40) | 0.005 b | 0.946 |
| Unknown pathogen | 76 (50.00) | 400 (45.30) | 1.153 b | 0.283 |
| Major comorbidities (n, %) | ||||
| Bacterial infection | 62 (40.79) | 340 (38.51) | 0.285 b | 0.594 |
| Chronic viral hepatitis B (normal liver biochemistry) | 6 (3.95) | 67 (7.59) | 2.621 b | 0.105 |
| Decompensated cirrhosis (normal liver biochemistry) | 10 (6.58) | 32 (3.62) | 2.908 b | 0.088 |
| Chronic renal failure | 21 (13.82) | 145 (16.42) | 0.654 b | 0.419 |
| Abnormal renal function | 4 (2.63) | 26 (2.94) | 0.045 b | 0.832 |
| Cancer | 38 (25.00) | 173 (19.59) | 2.336 b | 0.126 |
| Transplantation | 19 (12.50) | 190 (21.52) | 6.543 b | 0.011 |
| Autoimmune disease | 12 (7.89) | 55 (6.23) | 0.594 b | 0.441 |
| Diabetes | 32 (21.05) | 195 (22.08) | 0.081 b | 0.777 |
| Chronic obstructive pulmonary disease or Asthma | 10 (6.58) | 51 (5.78) | 0.151 b | 0.698 |
| Hypertension | 47 (30.92) | 330 (37.37) | 2.331 b | 0.127 |
| Heart disease | 26 (17.11) | 96 (10.87) | 4.846 b | 0.028 |
| Hyperlipidemia | 4 (2.63) | 24 (2.72) | 0.004 b | 0.952 |
| Anemia | 41 (26.97) | 185 (20.95) | 2.756 b | 0.097 |
| Hypoproteinemia | 47 (30.92) | 145 (16.42) | 18.044 b | <0.001 |
| Voriconazole dose (mg/kg) | 3.36 ± 0.46 | 3.34 ± 0.48 | 0.448 a | 0.654 |
| Cmin, [M (P25, P75), mg/L] | 4.96 (2.78, 7.46) | 2.33 (1.01, 4.34) | −8.317 c | <0.001 |
| Predictors | β | S.E | Wald χ2 | p | OR | 95%CI |
|---|---|---|---|---|---|---|
| Cmin (mg/L) | 0.094 | 0.019 | 23.940 | <0.001 | 1.099 | (1.058, 1.140) |
| hypoproteinemia | 0.544 | 0.217 | 6.275 | 0.012 | 1.723 | (1.126, 2.636) |
| transplantation | −0.589 | 0.273 | 4.645 | 0.031 | 0.555 | (0.325, 0.948) |
| Constant | −2.219 | 0.160 | 192.567 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, G.; Liu, Y.; Chen, Y.; He, Z.; Wen, Y.; Hu, M. The Development and Validation of a Predictive Model for Voriconazole-Related Liver Injury in Hospitalized Patients in China. J. Clin. Med. 2023, 12, 4254. https://doi.org/10.3390/jcm12134254
Xiao G, Liu Y, Chen Y, He Z, Wen Y, Hu M. The Development and Validation of a Predictive Model for Voriconazole-Related Liver Injury in Hospitalized Patients in China. Journal of Clinical Medicine. 2023; 12(13):4254. https://doi.org/10.3390/jcm12134254
Chicago/Turabian StyleXiao, Guirong, Yiyao Liu, Yanhua Chen, Zhiyao He, Yan Wen, and Ming Hu. 2023. "The Development and Validation of a Predictive Model for Voriconazole-Related Liver Injury in Hospitalized Patients in China" Journal of Clinical Medicine 12, no. 13: 4254. https://doi.org/10.3390/jcm12134254
APA StyleXiao, G., Liu, Y., Chen, Y., He, Z., Wen, Y., & Hu, M. (2023). The Development and Validation of a Predictive Model for Voriconazole-Related Liver Injury in Hospitalized Patients in China. Journal of Clinical Medicine, 12(13), 4254. https://doi.org/10.3390/jcm12134254








