Abstract
Maternal smoking during pregnancy has been associated with adverse effects on foetal development, including congenital limb anomalies. This systematic review aimed to provide an updated assessment of the association between maternal smoking during pregnancy and the risk of congenital limb anomalies. A systematic search was conducted to identify relevant studies published up to February 2023. Studies reporting on the relationship between maternal smoking during pregnancy and congenital digital anomalies or congenital limb reduction defects were included. Two independent reviewers screened the studies, extracted data, and assessed the quality of the included studies. Meta-analyses were performed to estimate the pooled odds ratios with 95% confidence intervals using fixed and random-effects models. In total, 37 publications comprising 11 cohort and 26 case-control studies were included in the systematic review. The meta-analysis demonstrated a significant increased risk of congenital limb reduction defects (pooled OR: 1.27, 95% CI: 1.18–1.38) in infants born to mothers who smoked during pregnancy. Similarly, a significant relationship was observed for the development of polydactyly/syndactyly/adactyly when considered as a single group (pooled OR: 1.32, 95% CI: 1.25–1.40). Yet, in contrast, no significant association was observed when polydactyly (pooled OR: 1.06, 95% CI: 0.88–1.27) or syndactyly (pooled OR: 0.91, 95% CI: 0.77–1.08) were considered individually. This systematic review provides updated evidence of a significant relationship between maternal smoking during pregnancy and increased risk of congenital limb anomalies. These findings highlight the potential detrimental effects of smoking on foetal limb development and underscore the importance of smoking cessation interventions for pregnant women to mitigate these risks.
1. Introduction
Congenital limb defects refer to structural abnormalities that occur during foetal development and affect the limbs of newborns. These abnormalities can vary in severity, ranging from minor deformities like extra fingers or toes, to more severe malformations like complete absence of a limb. Despite being relatively rare, with an estimated prevalence of 5–27 per 10,000 live births [1,2,3,4,5,6,7,8], these anomalies can have significant impacts on the affected children and their families, including functional, cosmetic, and psychological implications [9,10,11]. There remains limited knowledge about the risk factors associated with giving birth to children with congenital limb defects, and, in most cases, these defects arise spontaneously without a clearly identifiable cause.
It has long been demonstrated that environmental exposure of the mother to various aetiological agents during pregnancy can result in significant birth defects, including limb anomalies. Among these include teratogenic medications such as thalidomide or anticonvulsants and many others [12,13,14,15,16,17,18,19]. Among the environmental factors, maternal exposure to cigarette smoke during pregnancy has been previously shown to be a significant risk factor for congenital birth defects [20,21]. Cigarette smoke is a complex mixture of over 7000 chemicals, including nicotine, carbon monoxide, and various other toxic agents, many of which can cross the placenta and potentially affect foetal development [22,23,24]. Maternal smoking during pregnancy is a well-known risk factor for various adverse health outcomes, such as low birth weight, preterm birth, and perinatal death [25,26,27,28]. Given this, the association between maternal smoking and congenital defects such as congenital limb anomalies has been an area of interest among epidemiologists and surgeons treating these conditions, however, the relationship between these two variables remains controversial. Multiple previous observational studies have revealed conflicting findings, with some showing positive associations between maternal smoking and congenital limb differences [29,30,31,32,33] and others revealing no significant association [34,35,36,37,38].
A previous systematic review published by Hackshaw et al. in 2011 suggested an association between maternal cigarette smoking during pregnancy and the risk of congenital limb defects in children [20]. For the subsection of their review focusing on congenital limb defects, they included eight studies assessing the impact of maternal smoking on congenital limb reduction defects and six on congenital digital anomalies. The obtained effect sizes were 1.26 (95% CI: 1.15–1.39) for congenital limb reduction defects and 1.18 (95% CI: 0.99–1.41) for congenital digital anomalies. However, since the publication of that review, numerous additional studies have been published, which need further consideration. Moreover, in this previous study, because their objective was stated to be inclusive and objective, no rigorous quality assessment was utilized to select studies for inclusion into their meta-analysis, leaving the results subject to increased bias. The existing literature on this topic has multiple limitations such as small sample sizes, methodological variations, and potential confounding factors, which may impact the validity of the findings. As a result, the aim of this study is to perform an updated systematic review to capture recent publications in this field, thoroughly assess the quality of the included studies, and perform a revised and reliable meta-analysis. This updated review will critically evaluate the current evidence and provide a synthesis of the relationship between maternal smoking during pregnancy and the risk of congenital limb defects in children.
2. Methods
2.1. Study Identification
This systematic review adhered to the Preferred Reporting in Systematic Review and Meta-Analysis (PRISMA) guidelines (Figure 1) [39]. A comprehensive and systematic search was conducted to identify relevant studies examining the association between maternal smoking during pregnancy and congenital limb defects in children. Multiple electronic databases including PubMed, Embase, CINAHL, and Web of Science were thoroughly searched from their inception up to the 6th of February 2023. The search strategy utilized a combination of relevant keywords and MeSH terms to ensure a comprehensive coverage of the literature. The following search terms were used: (“Maternal Smoking” OR “Prenatal Smoking” OR “Periconceptional smoking” OR “Gestational smoking”) AND (“Birth defects” OR “Congenital anomalies” OR “Congenital abnormalities” OR “Congenital defects” OR “Congenital digital anomalies” OR “Polydactyly” OR “Syndactyly” OR “Adactyly” OR “Limb Reduction”). The search was not limited by language or publication status to minimize the risk of language bias and publication bias. In addition to the electronic database searches, the reference lists of all included studies and relevant reviews were thoroughly screened for any additional studies that may have been missed in the initial search.
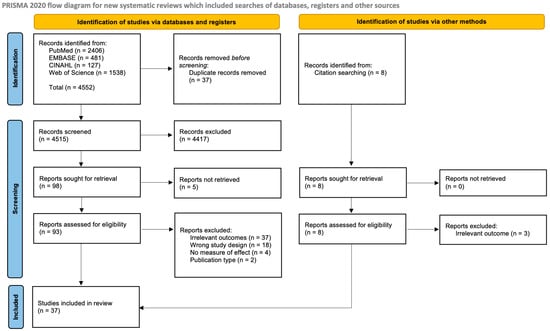
Figure 1.
PRISMA flowchart of study selection [39].
2.2. Study Inclusion and Data Extraction
Studies were included in the systematic review if they met the following predefined criteria: Firstly, study designs were limited to original randomised control, case-control, or cohort studies that evaluated the association between maternal smoking during pregnancy and congenital limb defects in children. The included outcomes were restricted to two main subgroups: congenital digital anomalies (comprising polydactyly, syndactyly, or adactyly) and congenital limb reduction defects. These studies could be conducted retrospectively or prospectively, allowing for a wide range of study designs to be included in the analysis. Secondly, only studies that provided sufficient data to calculate odds ratios (ORs) or relative risks (RRs) with 95% confidence intervals (CIs), or provided these statistics in the study, were eligible for consideration of quantitative analysis. Studies reporting outcomes which had insufficient information to obtain quantitative data were included in the presented tables and study but were unable to be considered for meta-analysis. Lastly, this review was limited to studies with human subjects, as the review focused on the impact of maternal smoking during pregnancy on congenital limb defects in human children.
On the other hand, studies were excluded from the systematic review if they were case reports, reviews, conference presentations, cross-sectional studies, editorials, letters to the editor, animal studies, or if they did not report relevant outcomes. These exclusion criteria were applied to ensure that only original research studies with relevant and reliable data were included in the analysis, while excluding studies that were not primary research or lacked relevant outcome data.
The inclusion and exclusion criteria were applied in a systematic and rigorous manner to select studies that were most relevant to the research question and ensure the robustness of the systematic review. Titles and abstracts of all identified articles in the search were reviewed by two independent reviewers (JC/OS) who conducted the study selection process, and any discrepancies were resolved through discussion and consensus or the involvement of a third reviewer where necessary (JG).
Data were extracted into data extraction tables into a shared spreadsheet in Microsoft Excel. A comprehensive and systematic approach was employed to gather relevant information from each included study. Multiple data points were carefully extracted to provide a comprehensive overview of the included studies. These data points included the title, authors, publication year, country of the study population, study design, study period, data source, details of exposed group and controls, period of smoking, cases of congenital limb defects among each group, confounding factors adjusted for, and study outcomes measured. Smoking status was considered as a binary variable, where studies had stratified patients by numbers of cigarettes per day; where possible, numbers were totalled to compare smokers and non-smokers dichotomously. Similarly, for congenital digital anomalies, the presence of any of polydactyly, syndactyly, or adactyly was considered as a case given that these were the consistent variables reported amongst included studies. The presence of other digital anomalies such as clinodactyly, brachydactyly, macrodactyly, and others were not reported amongst any found study and therefore were not assessed.
2.3. Quality Assessment
The quality of the included studies was assessed using the Newcastle–Ottawa Scale (NOS) for case-control and cohort studies [40]. The NOS is a widely used tool for assessing the methodological quality of non-randomized studies. It consists of three domains: selection of study groups, comparability of study groups, and assessment of outcome for cohort studies or exposure for case-control studies. Each domain is assessed based on a set of criteria, and the total score indicates the overall quality of the study. The NOS assigns stars to each criterion, with a higher number of stars indicating higher quality or a lower risk of bias. Quality assessment was performed independently by two reviewers (JC/OS), and any discrepancies were resolved through discussion and the involvement of a third reviewer if necessary (JG).
When assessing the comparability of each study, stars were awarded to studies if they had adjusted their analyses for one or more of maternal age/paternal age, maternal diabetes, maternal obesity, family history of limb anomaly, and maternal alcohol consumption. These confounders were chosen as previous studies have displayed evidence that these factors may potentially influence the formation of congenital limb anomalies in children [41,42,43,44,45]. It is not currently known which of these factors confers the highest risk, and therefore stars were awarded for any of the five confounders that were adjusted for in the included studies. A single star was awarded for each of the aforementioned confounding factors adjusted for, up to a maximum of two stars.
2.4. Statistical Analysis
A meta-analysis was conducted to estimate the overall effect size of the association between maternal smoking during pregnancy and congenital limb defects in children. Heterogeneity was assessed using the I2 statistic and pooled odds ratios with 95% confidence intervals were calculated using fixed-effects models when heterogeneity was low (I2 < 50%) or random-effects models when heterogeneity across studies was high. Meta-analyses were performed separately for the two subgroups of congenital digital anomalies and congenital limb reduction defects. Studies were deemed of sufficient quality to be included in the meta-analysis if they were assessed to be of “fair” or “good” quality as determined using the Newcastle–Ottawa Scale. Publication bias was assessed using funnel plots and visually inspected for asymmetry. All statistical analyses were conducted using statistical software using the Review Manager program [46].
3. Results
A total of 4552 (2406 from PubMed + 481 from EMBASE + 127 from CINAHL + 1538 from Web of Science) studies were initially identified through a comprehensive search of electronic databases and additional sources. After removing duplicates, 4515 studies were eligible for initial review. Following screening of study titles and abstracts, 106 studies were selected for full-text review. Five studies did not have available full texts. Following the full-text review, 37 publications were finally included in this systematic review based on the predefined inclusion and exclusion criteria (Figure 1).
The included studies were published between 1978 and 2022 and were conducted in various countries, including the United States, Sweden, Denmark, China, Japan, Finland, Hungary, Norway, Poland, and Australia. In total, 38 studies from 37 publications, comprising 11 cohort and 27 case-control studies, investigated the association between maternal cigarette smoking during pregnancy and the risk of congenital limb anomalies (one publication included descriptions of two case-control studies from different populations and methodologies [47]). This included 12 studies which included outcomes of congenital digital anomalies (polydactyly, syndactyly, or adactyly) and 26 studies which included outcomes on congenital limb reduction defects. In total, there were 26,787 identified cases of congenital limb anomalies comprising 16,330 cases of congenital digital anomalies and 10,457 cases of congenital limb reduction defects. Table 1 and Table 2 summarise the characteristics of the included studies.

Table 1.
Characteristics of included studies assessing impact of maternal smoking on digital anomalies.

Table 2.
Characteristics of included studies assessing impact of maternal smoking on congenital limb reduction defects.
3.1. Quality Assessment of Included Studies
The quality of the included studies was assessed using the Newcastle–Ottawa Scale for cohort and case-control studies. In total, six studies were considered of “good” quality, 14 of “fair” quality, and 18 of “poor” quality (Table 3). Most studies were deemed of poor quality due to a lack of adjustment of effect measures for confounding factors (maternal/paternal age, maternal diabetes, maternal obesity, family history of limb anomaly, and maternal alcohol consumption). This adjustment was considered only in the context of congenital limb defects and not other reported outcomes. Poor quality studies were excluded from the meta-analysis.

Table 3.
Quality assessment of included studies (Newcastle–Ottawa Scale for cohort and case-control studies).
The funnel plot (Figure 2) for congenital limb reduction defects was mostly symmetrical, however, it showed an asymmetry in the lower left corner, suggesting a lack of studies that demonstrated the protective effects of maternal smoking against defects in children, which may suggest potential publication bias towards studies with larger sample sizes. Given that quantitative data was available for less than 10 studies reporting on each outcome of congenital digital anomalies, forest plots were not performed.
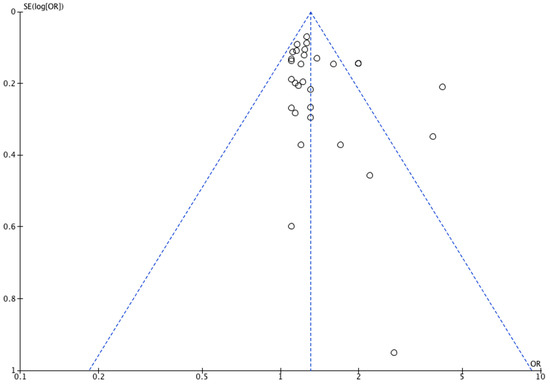
Figure 2.
Funnel plot of included studies reporting on congenital limb reduction defects.
3.2. Meta-Analyses
As previously mentioned, meta-analyses in this study were performed only on studies that were deemed of good or fair quality as assessed by the Newcastle–Ottawa Scale.
3.3. Congenital Digital Anomalies
In total, 12 studies provided data on the association between maternal cigarette smoking during pregnancy and congenital digital anomalies, including polydactyly, syndactyly, and adactyly. Nine were of sufficient quality to be included in the quantitative analysis. A meta-analysis was conducted to estimate the pooled odds ratio (OR) and 95% confidence interval (CI) for the association between maternal cigarette smoking during pregnancy and the risk of congenital digital anomalies.
The meta-analyses of the various outcomes showed no significant association between maternal cigarette smoking during pregnancy and an increased risk of polydactyly (pooled OR: 1.06, 95% CI: 0.88–1.27, Figure 3) or syndactyly (pooled OR: 0.91, 95% CI: 0.77–1.08, Figure 4) individually. The heterogeneity among the studies was low for both meta-analyses with an I2 of 46% and 39%, respectively, for polydactyly and syndactyly.
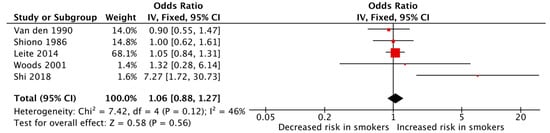
Figure 3.
Forest plot displaying the measures of effects for studies reporting polydactyly as the outcome [36,37,50,51,52].

Figure 4.
Forest plot displaying the measures of effects for studies reporting syndactyly as the outcome [36,37,49,51].
In contrast, the meta-analysis of studies that reported the outcome of polydactyly/syndactyly/adactyly was found to be significant, indicating an increased risk of children developing polydactyly or syndactyly or adactyly among mothers who smoked during pregnancy (pooled OR: 1.32, 95% CI: 1.25–1.40, Figure 5). Heterogeneity was low (I2 = 0%). However, this analysis was limited by the fact that it only included two studies.
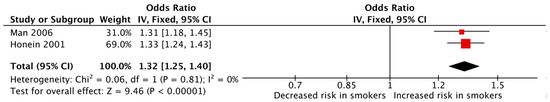
Figure 5.
Forest plot displaying the measures of effects for studies reporting polydactyly/syndactyly/adactyly as the outcome [29,30].
3.4. Congenital Limb Reduction Defects
In total, 31 studies provided data on the association between maternal cigarette smoking during pregnancy and congenital limb reduction defects, and 13 were of sufficient quality for quantitative analysis. The meta-analysis of these studies showed that maternal cigarette smoking during pregnancy was significantly associated with an increased risk of congenital limb reduction defects (pooled OR: 1.27, 95% CI: 1.18–1.38, Figure 6). The heterogeneity among the studies was low (I2 = 31%).
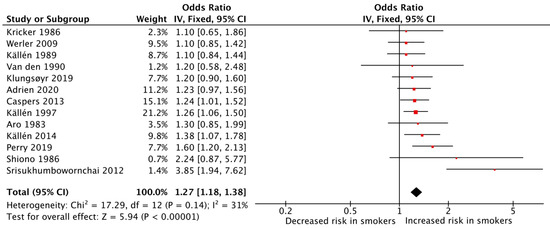
Figure 6.
Forest plot for displaying the measures of effects for studies reporting on congenital limb reduction defects [31,33,34,35,36,37,38,43,55,58,66,69,70].
4. Discussion
Despite the well-known risks, smoking remains a significant global health burden and is a leading cause of preventable deaths worldwide, resulting in an estimated 8 million deaths each year [73]. Furthermore, maternal cigarette smoking during pregnancy has been consistently shown to be a significant risk factor for adverse pregnancy outcomes and congenital birth defects. Many studies have investigated the risk of congenital birth defects among mothers who smoke during pregnancy such as congenital limb defects, congenital heart defects, neural tube defects, urogenital defects, cleft lip/palate, and others [20,21]. Herein, we have systematically reviewed the literature assessing the impact of maternal cigarette smoking during pregnancy on congenital limb defects including congenital digital anomalies and congenital limb reduction defects.
The findings of the meta-analyses in this study highlight a significant increased risk of congenital limb reduction defects (pooled OR: 1.27, 95% CI: 1.18–1.38) among offspring of smoking mothers compared to non-smoking counterparts. In contrast, the results for individual congenital digital anomalies were not significant, with meta-analyses of studies reporting outcomes of polydactyly and syndactyly individually not being found to show any significant association (polydactyly pooled OR: 1.06, 95% CI: 0.88–1.27, syndactyly pooled OR: 0.91, 95% CI: 0.77–1.08). Yet, when studies that reported the outcome of any one of polydactyly/syndactyly/adactyly were meta-analysed, a significantly increased risk of congenital digital anomalies among smokers was observed (pooled OR: 1.32, 95% CI: 1.25–1.40). However, these results must be interpreted with caution as only two studies were available for quantitative analysis for the latter meta-analysis. Overall, there seems to be an association between maternal smoking and the development of limb reduction defects among offspring; however, the results are less clear for congenital digital anomalies. It is unclear why this disparity exists, perhaps the relative paucity of the literature pertaining to congenital digital anomalies has limited the ability of this review to find a clear association if one does in fact exist.
These results are consistent with a previous meta-analysis conducted over a decade ago, which also reported a similar positive association between maternal smoking during pregnancy and the risk of congenital digital anomalies and limb reduction defects [20]. This previous review by Hackshaw et al. included eight studies assessing the impact of maternal smoking on congenital limb reduction defects and six on congenital digital anomalies comprising a total of 11 studies. The obtained effect sizes were 1.26 (95% CI: 1.15–1.39) for congenital limb reduction defects and 1.18 (95% CI: 0.99–1.41). These results are similar to the effect sizes obtained from our present review, despite finding 26 additional publications and employing a rigorous quality assessment of the included studies. By including a larger number of studies, the meta-analyses in this study provide a more comprehensive and up-to-date assessment of the association between smoking during pregnancy and congenital digital anomalies and limb reduction defects. The rigorous quality assessment of the included studies also ensured that only studies with robust methodologies and reliable data were included in the meta-analyses, enhancing the credibility of the findings.
Smoking during pregnancy has been shown to increase the risk of birth defects through various mechanisms. The toxic chemicals in tobacco smoke, such as nicotine and carbon monoxide, can cross the placenta and directly damage developing foetal tissues. Nicotine, a vasoactive agent, can also constrict blood vessels, reduce blood flow, and impair oxygen delivery to the foetus, causing disruption in development and growth [74]. Furthermore, carbon monoxide in tobacco smoke binds to haemoglobin in the blood, reducing its ability to carry oxygen, further enhancing oxygen deprivation in the developing foetus. This is thought to cause chronic hypoxia resulting in a reflex whereby blood is shunted away from the limbs (particularly the lower limbs) and preferentially redirected towards the foetal heart and brain [75]. This would inevitably cause a lack of vital oxygen and nutrients being delivered to the foetal limbs. Additionally, smoking during pregnancy can result in oxidative stress, which results in an imbalance between the production of harmful free radicals and the body’s ability to neutralise them [76,77]. This oxidative stress can damage cellular DNA and disrupt cellular processes critical for foetal development [76,77]. Moreover, tobacco smoke contains toxins such as polycyclic aromatic hydrocarbons that can also interfere with cellular processes involved in DNA synthesis, cell proliferation, and differentiation [78]. Numerous other mechanisms have been proposed, including epigenetic changes and vascular endothelial damage [79,80,81]. Overall, the mechanisms by which smoking causes birth defects are complex and multifactorial, involving a combination of direct and indirect toxic effects.
In addition to lifestyle factors such as maternal cigarette smoking or alcohol consumption, numerous other risk factors for congenital limb differences among offspring exist. Genes play a crucial role in the development and patterning of limbs during embryonic development. Mutations or alterations in specific genes can disrupt the normal limb development process, leading to congenital limb anomalies. For example, mutations in genes such as the HOX gene family, SHH gene, and TBX genes (among many others) have been associated with limb development abnormalities [82]. Furthermore, numerous genetic syndromes are connected to congenital limb differences such as Fanconi Anaemia, Roberts Syndrome, and Holt-Oram Syndrome [82,83]. Also, environmental exposure of the mother to toxins, radiation, pesticides, or other harmful substances has also been linked with the development of congenital limb difference [84,85,86].
This systematic review has several strengths. Firstly, this review employed a broad and comprehensive search strategy to identify relevant studies, which can help minimize the risk of missing important evidence. Additionally, the study selection process was rigorous, involving careful screening of studies based on predefined inclusion and exclusion criteria. Furthermore, the review included a methodical assessment of study quality, ensuring that only studies of sufficiently high quality were included in the analysis.
However, there are several limitations to consider. One limitation is the lack of high-quality studies assessing the relationship between cigarette smoking and congenital digital anomalies. While we found 12 studies assessing this relationship, the various outcomes reported in each study differ, making meaningful meta-analysis of these outcomes difficult. Hence, the relationship observed between maternal smoking and the development of polydactyly/syndactyly/adactyly was based on only two studies in the meta-analysis, making this relationship less reliable. Another limitation is the potential for recall bias in case-control studies that used interviews as a method of investigation. Interviews rely on self-reporting by participants, which can be subject to recall bias and may not always accurately capture exposure information, such as smoking during pregnancy. Additionally, despite efforts to only include studies in the meta-analysis that had adjusted for confounding variables, residual confounding may still be present in the included studies. For example, while the review mentioned adjustment for significant confounding variables such as maternal age, diabetes, obesity, family history of limb anomaly, and alcohol consumption, there may be other unmeasured or unknown confounding factors that could impact the relationship between maternal smoking and congenital limb anomalies. A study protocol was not registered prior to the conduct of this study. Furthermore, publication bias may be a limitation, as indicated by the asymmetric funnel plot in congenital limb reduction defects. Additionally, unfortunately we were unable to assess a dose–response relationship between studies due to inconsistent reporting of number of cigarettes smoked by mothers in the included studies. Lastly, there was no consideration of the effect of passive smoking or second-hand smoke exposure in the meta-analysis. Maternal exposure to second-hand smoke during pregnancy has been shown to be connected with congenital defects and limb deficiencies, and could potentially confound the relationship between maternal smoking and congenital limb anomalies as the non-smoking mothers may experience similar detrimental effects of cigarette smoke from second-hand smoking [31,57,87].
5. Conclusions
This systematic review provides updated evidence of the relationship between maternal cigarette smoking during pregnancy and congenital limb anomalies, specifically congenital digital anomalies and congenital limb reduction defects. The detrimental effects of smoking on foetal development are well-established, and our review further reinforces the harmful impact of maternal smoking on limb development. Further research is warranted to better understand the underlying mechanisms linking maternal smoking and congenital limb anomalies, particularly congenital digital anomalies, and to explore potential strategies to mitigate these risks. This review underscores the critical need for effective smoking cessation interventions for pregnant women to ensure optimal foetal development.
Author Contributions
Conceptualization, J.C. and W.M.R.; methodology, J.C.; validation, J.C. and W.M.R.; formal analysis, J.C., O.S. and J.G.; investigation, J.C., O.S. and J.G.; data curation, J.C., O.S. and J.G.; writing—original draft preparation, J.C.; writing—review and editing, W.M.R., O.S., J.G. and J.C.; supervision, W.M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Correction Statement
This article has been republished with a minor correction to resolve spelling and grammatical errors. This change does not affect the scientific content of the article.
References
- Parker, S.E.; Mai, C.T.; Canfield, M.A.; Rickard, R.; Wang, Y.; Meyer, R.E.; Anderson, P.; Mason, C.A.; Collins, J.S.; Kirby, R.S.; et al. Updated National Birth Prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Res. A Clin. Mol. Teratol. 2010, 88, 1008–1016. [Google Scholar] [CrossRef]
- Goldfarb, C.A.; Shaw, N.; Steffen, J.A.; Wall, L.B. The Prevalence of Congenital Hand and Upper Extremity Anomalies Based upon the New York Congenital Malformations Registry. J. Pediatr. Orthop. 2017, 37, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Ekblom, A.G.; Laurell, T.; Arner, M. Epidemiology of congenital upper limb anomalies in 562 children born in 1997 to 2007: A total population study from stockholm, sweden. J. Hand Surg. Am. 2010, 35, 1742–1754. [Google Scholar] [CrossRef]
- Vasluian, E.; van der Sluis, C.K.; van Essen, A.J.; Bergman, J.E.; Dijkstra, P.U.; Reinders-Messelink, H.A.; de Walle, H.E. Birth prevalence for congenital limb defects in the northern Netherlands: A 30-year population-based study. BMC Musculoskelet. Disord. 2013, 14, 323. [Google Scholar] [CrossRef] [PubMed]
- Giele, H.; Giele, C.; Bower, C.; Allison, M. The incidence and epidemiology of congenital upper limb anomalies: A total population study. J. Hand Surg. Am. 2001, 26, 628–634. [Google Scholar] [CrossRef]
- Jaruratanasirikul, S.; Tangtrakulwanich, B.; Rachatawiriyakul, P.; Sriplung, H.; Limpitikul, W.; Dissaneevate, P.; Khunnarakpong, N.; Tantichantakarun, P. Prevalence of congenital limb defects: Data from birth defects registries in three provinces in Southern Thailand. Congenit. Anom. 2016, 56, 203–208. [Google Scholar] [CrossRef]
- Koskimies, E.; Lindfors, N.; Gissler, M.; Peltonen, J.; Nietosvaara, Y. Congenital upper limb deficiencies and associated malformations in Finland: A population-based study. J. Hand Surg. Am. 2011, 36, 1058–1065. [Google Scholar] [CrossRef]
- Wynne-Davies, R.; Lamb, D.W. Congenital upper limb anomalies: An etiologic grouping of clinical, genetic, and epidemiologic data from 387 patients with “absence” defects, constriction bands, polydactylies, and syndactylies. J. Hand Surg. Am. 1985, 10, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Michielsen, A.; Van Wijk, I.; Ketelaar, M. Participation and quality of life in children and adolescents with congenital limb deficiencies: A narrative review. Prosthet. Orthot. Int. 2010, 34, 351–361. [Google Scholar] [CrossRef]
- Montesinos-Magraner, L.; Issa-Benítez, D.; Pagès-Bolíbar, E.; Meléndez-Plumed, M.; González-Viejo, M.A.; Castellano-Tejedor, C. Physical and Psychosocial Functions of Adults with Lower Limb Congenital Deficiencies and Amputations in Childhood. Rehabil. Res. Pract. 2016, 2016, 8109365. [Google Scholar] [CrossRef]
- McDougall, L.; Kennedy, J.; Coombs, C.; Penington, A. The psychosocial impact of congenital hand and upper limb differences on children: A qualitative study. J. Hand Surg. Eur. Vol. 2021, 46, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Lenz, W. Malformations caused by drugs in pregnancy. Am. J. Dis. Child. 1966, 112, 99–106. [Google Scholar] [CrossRef]
- Smithells, R.W.; Newman, C.G. Recognition of thalidomide defects. J. Med. Genet. 1992, 29, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Shaul, W.L.; Hall, J.G. Multiple congenital anomalies associated with oral anticoagulants. Am. J. Obstet. Gynecol. 1977, 127, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Therapontos, C.; Erskine, L.; Gardner, E.R.; Figg, W.D.; Vargesson, N. Thalidomide induces limb defects by preventing angiogenic outgrowth during early limb formation. Proc. Natl. Acad. Sci. USA 2009, 106, 8573–8578. [Google Scholar] [CrossRef]
- Vargesson, N. Thalidomide-induced teratogenesis: History and mechanisms. Birth Defects Res. C Embryo Today 2015, 105, 140–156. [Google Scholar] [CrossRef]
- Sharony, R.; Garber, A.; Viskochil, D.; Schreck, R.; Platt, L.D.; Ward, R.; Buehler, B.A.; Graham, J.M., Jr. Preaxial ray reduction defects as part of valproic acid embryofetopathy. Prenat. Diagn. 1993, 13, 909–918. [Google Scholar] [CrossRef]
- Soprano, D.R.; Soprano, K.J. Retinoids as teratogens. Annu. Rev. Nutr. 1995, 15, 111–132. [Google Scholar] [CrossRef]
- Andrade, C. Valproate in Pregnancy: Recent Research and Regulatory Responses. J. Clin. Psychiatry 2018, 79, 22082. [Google Scholar] [CrossRef]
- Hackshaw, A.; Rodeck, C.; Boniface, S. Maternal smoking in pregnancy and birth defects: A systematic review based on 173 687 malformed cases and 11.7 million controls. Hum. Reprod. Update 2011, 17, 589–604. [Google Scholar] [CrossRef]
- Nicoletti, D.; Appel, L.D.; Siedersberger Neto, P.; Guimarães, G.W.; Zhang, L. Maternal smoking during pregnancy and birth defects in children: A systematic review with meta-analysis. Cad. Saude Publica 2014, 30, 2491–2529. [Google Scholar] [CrossRef] [PubMed]
- Soleimani, F.; Dobaradaran, S.; De-la-Torre, G.E.; Schmidt, T.C.; Saeedi, R. Content of toxic components of cigarette, cigarette smoke vs cigarette butts: A comprehensive systematic review. Sci. Total. Environ. 2022, 813, 152667. [Google Scholar] [CrossRef]
- Talbot, P. In vitro assessment of reproductive toxicity of tobacco smoke and its constituents. Birth Defects Res. C Embryo Today 2008, 84, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.M. Tobacco and pregnancy. Reprod. Toxicol. 2009, 28, 152–160. [Google Scholar] [CrossRef]
- Blatt, K.; Moore, E.; Chen, A.; Van Hook, J.; DeFranco, E.A. Association of reported trimester-specific smoking cessation with fetal growth restriction. Obstet. Gynecol. 2015, 125, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Reeves, S.; Bernstein, I. Effects of maternal tobacco-smoke exposure on fetal growth and neonatal size. Expert. Rev. Obstet. Gynecol. 2008, 3, 719–730. [Google Scholar] [CrossRef]
- Marufu, T.C.; Ahankari, A.; Coleman, T.; Lewis, S. Maternal smoking and the risk of still birth: Systematic review and meta-analysis. BMC Public Health 2015, 15, 239. [Google Scholar] [CrossRef]
- Pineles, B.L.; Hsu, S.; Park, E.; Samet, J.M. Systematic Review and Meta-Analyses of Perinatal Death and Maternal Exposure to Tobacco Smoke During Pregnancy. Am. J. Epidemiol. 2016, 184, 87–97. [Google Scholar] [CrossRef]
- Honein, M.A.; Paulozzi, L.J.; Watkins, M.L. Maternal smoking and birth defects: Validity of birth certificate data for effect estimation. Public Health Rep. 2001, 116, 327–335. [Google Scholar] [CrossRef]
- Man, L.X.; Chang, B. Maternal cigarette smoking during pregnancy increases the risk of having a child with a congenital digital anomaly. Plast. Reconstr. Surg. 2006, 117, 301–308. [Google Scholar] [CrossRef]
- Caspers, K.M.; Romitti, P.A.; Lin, S.; Olney, R.S.; Holmes, L.B.; Werler, M.M. Maternal periconceptional exposure to cigarette smoking and congenital limb deficiencies. Paediatr. Perinat. Epidemiol. 2013, 27, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Källén, K. Multiple malformations and maternal smoking. Paediatr. Perinat. Epidemiol. 2000, 14, 227–233. [Google Scholar] [CrossRef]
- Perry, M.F.; Mulcahy, H.; DeFranco, E.A. Influence of periconception smoking behavior on birth defect risk. Am. J. Obstet. Gynecol. 2019, 220, 588.e1–588.e7. [Google Scholar] [CrossRef]
- Källén, B. Epidemiology of Human Congenital Malformations; Springer: Cham, Switzerland, 2014. [Google Scholar]
- Kricker, A.; Elliott, J.W.; Forrest, J.M.; McCredie, J. Congenital limb reduction deformities and use of oral contraceptives. Am. J. Obstet. Gynecol. 1986, 155, 1072–1078. [Google Scholar] [CrossRef]
- Shiono, P.H.; Klebanoff, M.A.; Berendes, H.W. Congenital malformations and maternal smoking during pregnancy. Teratology 1986, 34, 65–71. [Google Scholar] [CrossRef]
- Van den Eeden, S.K.; Karagas, M.R.; Daling, J.R.; Vaughan, T.L. A case-control study of maternal smoking and congenital malformations. Paediatr. Perinat. Epidemiol. 1990, 4, 147–155. [Google Scholar] [CrossRef]
- Werler, M.M.; Bosco, J.L.; Shapira, S.K. Maternal vasoactive exposures, amniotic bands, and terminal transverse limb defects. Birth Defects Res. A Clin. Mol. Teratol. 2009, 85, 52–57. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. 2000. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 23 April 2023).
- Syvänen, J.; Nietosvaara, Y.; Hurme, S.; Perheentupa, A.; Gissler, M.; Raitio, A.; Helenius, I. Maternal risk factors for congenital limb deficiencies: A population-based case-control study. Paediatr. Perinat. Epidemiol. 2021, 35, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, B.; Kong, F.; Li, X. Prenatal limb defects: Epidemiologic characteristics and an epidemiologic analysis of risk factors. Medicine 2018, 97, e11471. [Google Scholar] [CrossRef] [PubMed]
- Aro, T. Maternal diseases, alcohol consumption and smoking during pregnancy associated with reduction limb defects. Early Hum. Dev. 1983, 9, 49–57. [Google Scholar] [CrossRef]
- Martínez-Frías, M.L.; Bermejo, E.; Rodríguez-Pinilla, E.; Frías, J.L. Risk for congenital anomalies associated with different sporadic and daily doses of alcohol consumption during pregnancy: A case-control study. Birth Defects Res. A Clin. Mol. Teratol. 2004, 70, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Polednak, A.P. Paternal age in relation to selected birth defects. Hum. Biol. 1976, 48, 727–739. [Google Scholar]
- Review Manager (RevMan) [Computer Program], Version 5.4; The Cochrane Collaboration: London, UK, 2020.
- Martínez-Frías, M.L.; Czeizel, A.E.; Rodríguez-Pinilla, E.; Bermejo, E. Smoking during pregnancy and Poland sequence: Results of a population-based registry and a case-control registry. Teratology 1999, 59, 35–38. [Google Scholar] [CrossRef]
- Kelsey, J.L.; Dwyer, T.; Holford, T.R.; Bracken, M.B. Maternal smoking and congenital malformations: An epidemiological study. J. Epidemiol. Community Health 1978, 32, 102–107. [Google Scholar] [CrossRef]
- Carr, B.K. Congenital Limb Reduction Defects in Infants: A Look at Possible Associations with Maternal Smoking and Hypertension; Washington University: Seattle, WA, USA, 1997. [Google Scholar]
- Woods, S.E.; Raju, U. Maternal smoking and the risk of congenital birth defects: A cohort study. J. Am. Board. Fam. Pract. 2001, 14, 330–334. [Google Scholar]
- Leite, M.; Albieri, V.; Kjaer, S.K.; Jensen, A. Maternal smoking in pregnancy and risk for congenital malformations: Results of a Danish register-based cohort study. Acta Obstet. Gynecol. Scand. 2014, 93, 825–834. [Google Scholar] [CrossRef]
- Shi, J.; Tian, Y.; Lei, Y.; Kang, H. Active and passive maternal smoking during pregnancy and risk of having a child with polydactyly: A case-control study. Zhonghua Liu Xing Bing Xue Za Zhi 2018, 39, 1482–1485. [Google Scholar]
- Tsuchida, A.; Hamazaki, K.; Kigawa, M.; Tanaka, T.; Ito, M.; Inadera, H. Association between maternal smoking history and congenital anomalies in children: Results from the Japan Environment and Children’s Study. Congenit. Anom. 2021, 61, 159–168. [Google Scholar] [CrossRef]
- Aro, T.; Haapakoski, J.; Heinonen, O.P. A multivariate analysis of the risk indicators of reduction limb defects. Int. J. Epidemiol. 1984, 13, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Källén, B. A prospective study of some aetiological factors in limb reduction defects in Sweden. J. Epidemiol. Community Health 1989, 43, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Czeizel, A.E.; Kodaj, I.; Lenz, W. Smoking during pregnancy and congenital limb deficiency. BMJ 1994, 308, 1473–1476. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, C.R.; Shaw, G.M.; O’Malley, C.D.; Tolarova, M.M.; Lammer, E.J. Parental cigarette smoking and risk for congenital anomalies of the heart, neural tube, or limb. Teratology 1996, 53, 261–267. [Google Scholar] [CrossRef]
- Källén, K. Maternal smoking during pregnancy and limb reduction malformations in Sweden. Am. J. Public Health 1997, 87, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Shaw, G.M.; Wasserman, C.R.; O’Malley, C.D.; Nelson, V.; Jackson, R.J. Maternal pesticide exposure from multiple sources and selected congenital anomalies. Epidemiology 1999, 10, 60–66. [Google Scholar] [CrossRef]
- Shaw, G.M.; Nelson, V.; Carmichael, S.L.; Lammer, E.J.; Finnell, R.H.; Rosenquist, T.H. Maternal periconceptional vitamins: Interactions with selected factors and congenital anomalies? Epidemiology 2002, 13, 625–630. [Google Scholar] [CrossRef]
- Carmichael, S.L.; Shaw, G.M.; Yang, W.; Lammer, E.J.; Zhu, H.; Finnell, R.H. Limb deficiency defects, MSX1, and exposure to tobacco smoke. Am. J. Med. Genet. A 2004, 125A, 285–289. [Google Scholar] [CrossRef]
- Czeizel, A.E.; Petik, D.; Puho, E. Smoking and alcohol drinking during pregnancy. The reliability of retrospective maternal self-reported information. Cent. Eur. J. Public Health 2004, 12, 179–183. [Google Scholar]
- Carmichael, S.L.; Shaw, G.M.; Yang, W.; Iovannisci, D.M.; Lammer, E. Risk of limb deficiency defects associated with NAT1, NAT2, GSTT1, GSTM1, and NOS3 genetic variants, maternal smoking, and vitamin supplement intake. Am. J. Med. Genet. A 2006, 140, 1915–1922. [Google Scholar] [CrossRef]
- Carmichael, S.L.; Shaw, G.M.; Iovannisci, D.M.; Yang, W.; Finnell, R.H.; Cheng, S.; Lammer, E.J. Risks of human limb deficiency anomalies associated with 29 SNPs of genes involved in homocysteine metabolism, coagulation, cell-cell interactions, inflammatory response, and blood pressure regulation. Am. J. Med. Genet. A 2006, 140, 2433–2440. [Google Scholar] [CrossRef]
- Robitaille, J.; Carmichael, S.L.; Shaw, G.M.; Olney, R.S. Maternal nutrient intake and risks for transverse and longitudinal limb deficiencies: Data from the National Birth Defects Prevention Study, 1997–2003. Birth Defects Res. A Clin. Mol. Teratol. 2009, 85, 773–779. [Google Scholar] [CrossRef]
- Srisukhumbowornchai, S.; Krikov, S.; Feldkamp, M.L. Self-reported maternal smoking during pregnancy by source in Utah, 2003–2007. Birth Defects Res. A Clin. Mol. Teratol. 2012, 94, 996–1003. [Google Scholar] [CrossRef] [PubMed]
- Pace, N.D.; Desrosiers, T.A.; Carmichael, S.L.; Shaw, G.M.; Olshan, A.F.; Siega-Riz, A.M. Antioxidant Consumption is Associated with Decreased Odds of Congenital Limb Deficiencies. Paediatr. Perinat. Epidemiol. 2018, 32, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.; Stingone, J.A.; Desrosiers, T.A.; Olshan, A.F.; Nembhard, W.N.; Shaw, G.M.; Pruitt, S.; Romitti, P.A.; Yazdy, M.M.; Browne, M.L.; et al. Maternal exposure to outdoor air pollution and congenital limb deficiencies in the National Birth Defects Prevention Study. Environ. Res. 2019, 179, 108716. [Google Scholar] [CrossRef]
- Klungsøyr, K.; Nordtveit, T.I.; Kaastad, T.S.; Solberg, S.; Sletten, I.N.; Vik, A.K. Epidemiology of limb reduction defects as registered in the Medical Birth Registry of Norway, 1970–2016: Population based study. PLoS ONE 2019, 14, e0219930. [Google Scholar] [CrossRef]
- Adrien, N.; Petersen, J.M.; Parker, S.E.; Werler, M.M. Vasoactive exposures and risk of amniotic band syndrome and terminal transverse limb deficiencies. Birth Defects Res. 2020, 112, 1074–1084. [Google Scholar] [CrossRef]
- Materna-Kiryluk, A.; Wisniewska, K.; Wieckowska, B.; Wierzba, J.; Jazdzewska, A.; Jaroszewska-Swiatek, B.; Skotnicka, K.; Latos-Bielenska, A. Maternal Risk Factors Associated with Limb Reduction Defects: Data from the Polish Registry of Congenital Malformations (PRCM). Children 2021, 8, 138. [Google Scholar] [CrossRef]
- Yang, L.; Wang, H.; Yang, L.; Zhao, M.; Guo, Y.; Bovet, P.; Xi, B. Maternal cigarette smoking before or during pregnancy increases the risk of birth congenital anomalies: A population-based retrospective cohort study of 12 million mother-infant pairs. BMC Med. 2022, 20, 4. [Google Scholar] [CrossRef] [PubMed]
- Cafà, E.V. World Health Organization: Tobacco. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/tobacco (accessed on 10 March 2023).
- Machaalani, R.; Ghazavi, E.; Hinton, T.; Waters, K.A.; Hennessy, A. Cigarette smoking during pregnancy regulates the expression of specific nicotinic acetylcholine receptor (nAChR) subunits in the human placenta. Toxicol. Appl. Pharmacol. 2014, 276, 204–212. [Google Scholar] [CrossRef]
- Akalin-Sel, T.; Campbell, S. Understanding the pathophysiology of intra-uterine growth retardation: The role of the ‘lower limb reflex’ in redistribution of blood flow. Eur. J. Obstet. Gynecol. Reprod. Biol. 1992, 46, 79–86. [Google Scholar] [CrossRef]
- Chełchowska, M.; Gajewska, J.; Ambroszkiewicz, J.; Mazur, J.; Ołtarzewski, M.; Maciejewski, T.M. Influence of Oxidative Stress Generated by Smoking during Pregnancy on Glutathione Status in Mother-Newborn Pairs. Antioxidants 2021, 10, 1866. [Google Scholar] [CrossRef]
- Noakes, P.S.; Thomas, R.; Lane, C.; Mori, T.A.; Barden, A.E.; Devadason, S.G.; Prescott, S.L. Association of maternal smoking with increased infant oxidative stress at 3 months of age. Thorax 2007, 62, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Lupo, P.J.; Langlois, P.H.; Reefhuis, J.; Lawson, C.C.; Symanski, E.; Desrosiers, T.A.; Khodr, Z.G.; Agopian, A.J.; Waters, M.A.; Duwe, K.N.; et al. Maternal occupational exposure to polycyclic aromatic hydrocarbons: Effects on gastroschisis among offspring in the National Birth Defects Prevention Study. Environ. Health Perspect. 2012, 120, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Quinton, A.E.; Cook, C.M.; Peek, M.J. The relationship between cigarette smoking, endothelial function and intrauterine growth restriction in human pregnancy. BJOG 2008, 115, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Rauschert, S.; Melton, P.E.; Burdge, G.; Craig, J.M.; Godfrey, K.M.; Holbrook, J.D.; Lillycrop, K.; Mori, T.A.; Beilin, L.J.; Oddy, W.H.; et al. Maternal Smoking During Pregnancy Induces Persistent Epigenetic Changes Into Adolescence, Independent of Postnatal Smoke Exposure and Is Associated With Cardiometabolic Risk. Front. Genet. 2019, 10, 770. [Google Scholar] [CrossRef]
- Nakamura, A.; François, O.; Lepeule, J. Epigenetic Alterations of Maternal Tobacco Smoking during Pregnancy: A Narrative Review. Int. J. Environ. Res. Public Health 2021, 18, 5083. [Google Scholar] [CrossRef]
- Sun, L.; Huang, Y.; Zhao, S.; Zhong, W.; Lin, M.; Guo, Y.; Yin, Y.; Wu, N.; Wu, Z.; Tian, W. Advances in understanding the genetics of syndromes involving congenital upper limb anomalies. Ann. Jt. 2019, 4, 30. [Google Scholar] [CrossRef]
- Bergman, J.E.H.; Löhner, K.; van der Sluis, C.K.; Rump, P.; de Walle, H.E.K. Etiological diagnosis in limb reduction defects and the number of affected limbs: A population-based study in the Northern Netherlands. Am. J. Med. Genet. A 2020, 182, 2909–2918. [Google Scholar] [CrossRef]
- Alexander, P.G.; Clark, K.L.; Tuan, R.S. Prenatal exposure to environmental factors and congenital limb defects. Birth Defects Res. C Embryo Today 2016, 108, 243–273. [Google Scholar] [CrossRef]
- Lin, S.; Marshall, E.G.; Davidson, G.K. Potential parental exposure to pesticides and limb reduction defects. Scand. J. Work. Environ. Health 1994, 20, 166–179. [Google Scholar] [CrossRef]
- Wang, B.; Ohyama, H.; Haginoya, K.; Odaka, T.; Yamada, T.; Hayata, I. Prenatal radiation-induced limb defects mediated by Trp53-dependent apoptosis in mice. Radiat. Res. 2000, 154, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Hoyt, A.T.; Canfield, M.A.; Romitti, P.A.; Botto, L.D.; Anderka, M.T.; Krikov, S.V.; Tarpey, M.K.; Feldkamp, M.L. Associations between maternal periconceptional exposure to secondhand tobacco smoke and major birth defects. Am. J. Obstet. Gynecol. 2016, 215, 613.e611. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).