The Impact of Frailty on Postoperative Complications in Total En Bloc Spondylectomy for Spinal Tumors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Frailty Index
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tomita, K.; Kawahara, N.; Baba, H.; Tsuchiya, H.; Nagata, S.; Toribatake, Y. Total en bloc spondylectomy for solitary spinal metastases. Int. Orthop. 1994, 18, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Tomita, K.; Kawahara, N.; Baba, H.; Tsuchiya, H.; Fujita, T.; Toribatake, Y. Total en bloc spondylectomy: A new surgical technique for primary malignant vertebral tumors. Spine 1997, 22, 324–333. [Google Scholar] [CrossRef]
- Matsumoto, M.; Tsuji, T.; Iwanami, A.; Watanabe, K.; Hosogane, N.; Ishii, K.; Nakamura, M.; Morioka, H.; Toyama, Y. Total en bloc spondylectomy for spinal metastasis of differentiated thyroid cancers: A long-term follow-up. J. Spinal Disord. Tech. 2013, 26, e137–e142. [Google Scholar] [CrossRef]
- Boriani, S.; Bandiera, S.; Casadei, R.; Boriani, L.; Donthineni, R.; Gasbarrini, A.; Pignotti, E.; Biagini, R.; Schwab, J.H. Giant cell tumor of the mobile spine: A review of 49 cases. Spine 2012, 37, E37–E45. [Google Scholar] [CrossRef]
- Murakami, H.; Kawahara, N.; Demura, S.; Kato, S.; Yoshioka, K.; Tomita, K. Total en bloc spondylectomy for lung cancer metastasis to the spine. J. Neurosurg. Spine 2010, 13, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Melcher, I.; Disch, A.C.; Khodadadyan-Klostermann, C.; Tohtz, S.; Smolny, M.; Stöckle, U.; Haas, N.P.; Schaser, K.D. Primary malignant bone tumors and solitary metastases of the thoracolumbar spine: Results by management with total en bloc spondylectomy. Eur. Spine J. 2007, 16, 1193–1202. [Google Scholar] [CrossRef]
- Kato, S.; Murakami, H.; Demura, S.; Yoshioka, K.; Kawahara, N.; Tomita, K.; Tsuchiya, H. More than 10-year follow-up after total en bloc spondylectomy for spinal tumors. Ann. Surg. Oncol. 2014, 21, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Paulino Pereira, N.R.; Pedlow, F.X.; Wain, J.C.; Yoon, S.S.; Hornicek, F.J.; Schwab, J.H. Modified en bloc spondylectomy for tumors of the thoracic and lumbar spine: Surgical technique and outcomes. J. Bone Jt. Surg. Am. 2017, 99, 1476–1484. [Google Scholar] [CrossRef]
- Tomita, K.; Kawahara, N.; Murakami, H.; Demura, S. Total en bloc spondylectomy for spinal tumors: Improvement of the technique and its associated basic background. J. Orthop. Sci. 2006, 11, 3–12. [Google Scholar] [CrossRef]
- Yokogawa, N.; Murakami, H.; Demura, S.; Kato, S.; Yoshioka, K.; Shimizu, T.; Oku, N.; Kitagawa, R.; Tsuchiya, H. Total spondylectomy for Enneking stage III giant cell tumor of the mobile spine. Eur. Spine J. 2018, 27, 3084–3091. [Google Scholar] [CrossRef]
- Kato, S.; Demura, S.; Shinmura, K.; Yokogawa, N.; Yonezawa, N.; Shimizu, T.; Oku, N.; Kitagawa, R.; Murakami, H.; Kawahara, N.; et al. Clinical outcomes and survivals after total en bloc spondylectomy for metastatic leiomyosarcoma in the spine. Eur. Spine J. 2020, 29, 3237–3244. [Google Scholar] [CrossRef]
- Kato, S.; Demura, S.; Murakami, H.; Yoshioka, K.; Shinmura, K.; Yokogawa, N.; Shimizu, T.; Kawahara, N.; Tsuchiya, H. Clinical outcomes and prognostic factors following the surgical resection of renal cell carcinoma spinal metastases. Cancer Sci. 2021, 112, 2416–2425. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Demura, S.; Kitagawa, R.; Yokogawa, N.; Shimizu, T.; Kobayashi, M.; Yamada, Y.; Nagatani, S.; Murakami, H.; Kawahara, N.; et al. Clinical outcomes following total en bloc spondylectomy for spinal metastases from lung cancer. J. Orthop. Sci. 2023; in press. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Demura, S.; Yokogawa, N.; Shimizu, T.; Kobayashi, M.; Yamada, Y.; Murakami, H.; Tsuchiya, H. Metastasectomy of spinal lesions from thyroid carcinomas. Bone Jt. J. 2023, 105-B, 575–582. [Google Scholar] [CrossRef]
- Igarashi, T.; Murakami, H.; Demura, S.; Kato, S.; Yoshioka, K.; Yokogawa, N.; Tsuchiya, H. Risk factors for local recurrence after total en bloc spondylectomy for metastatic spinal tumors: A retrospective study. J. Orthop. Sci. 2018, 23, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Demura, S.; Kato, S.; Yoshioka, K.; Shinmura, K.; Yokogawa, N.; Yonezawa, N.; Handa, M.; Annen, R.; Yamada, Y.; et al. Prevalence and risk factors for the development of venous thromboembolism after spinal tumor surgery. World Neurosurg. 2022, 164, e177–e182. [Google Scholar] [CrossRef] [PubMed]
- Boriani, S.; Gasbarrini, A.; Bandiera, S.; Ghermandi, R.; Lador, R. Predictors for surgical complications of en bloc resections in the spine: Review of 220 cases treated by the same team. Eur. Spine J. 2016, 25, 3932–3941. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, N.; Tomita, K.; Murakami, H.; Demura, S.; Yoshioka, K.; Kato, S. Total en bloc spondylectomy of the lower lumbar spine: A surgical techniques of combined posterior-anterior approach. Spine 2011, 36, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Sciubba, D.M.; De la Garza Ramos, R.; Goodwin, C.R.; Xu, R.; Bydon, A.; Witham, T.F.; Gokaslan, Z.L.; Wolinsky, J.P. Total en bloc spondylectomy for locally aggressive and primary malignant tumors of the lumbar spine. Eur. Spine J. 2016, 25, 4080–4087. [Google Scholar] [CrossRef]
- Howell, E.P.; Williamson, T.; Karikari, I.; Abd-El-Barr, M.; Erickson, M.; Goodwin, M.L.; Reynolds, J.; Sciubba, D.M.; Goodwin, C.R. Total en bloc resection of primary and metastatic spine tumors. Ann. Transl. Med. 2019, 7, 226. [Google Scholar] [CrossRef]
- Demura, S.; Kato, S.; Shinmura, K.; Yokogawa, N.; Shimizu, T.; Handa, M.; Annen, R.; Kobayashi, M.; Yamada, Y.; Murakami, H.; et al. Perioperative complications of total en bloc spondylectomy for spinal tumours. Bone Jt. J. 2021, 103-B, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Amendola, L.; Cappuccio, M.; De Iure, F.; Bandiera, S.; Gasbarrini, A.; Boriani, S. En bloc resections for primary spinal tumors in 20 years of experience: Effectiveness and safety. Spine J. 2014, 14, 2608–2617. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G.; Iliffe, S.; Walters, K. Frailty index as a predictor of mortality: A systematic review and meta-analysis. Age Ageing 2018, 47, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Dent, E.; Martin, F.C.; Bergman, H.; Woo, J.; Romero-Ortuno, R.; Walston, J.D. Management of frailty: Opportunities, challenges, and future directions. Lancet 2019, 394, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Saeki, C.; Kanai, T.; Nakano, M.; Oikawa, T.; Torisu, Y.; Abo, M.; Saruta, M.; Tsubota, A. Relationship between osteosarcopenia and frailty in patients with chronic liver disease. J. Clin. Med. 2020, 9, 2381. [Google Scholar] [CrossRef] [PubMed]
- Makary, M.A.; Segev, D.L.; Pronovost, P.J.; Syin, D.; Bandeen-Roche, K.; Patel, P.; Takenaga, R.; Devgan, L.; Holzmueller, C.G.; Tian, J.; et al. Frailty as a predictor of surgical outcomes in older patients. J. Am. Coll. Surg. 2010, 210, 901–908. [Google Scholar] [CrossRef]
- Yagi, M.; Michikawa, T.; Hosogane, N.; Fujita, N.; Okada, E.; Suzuki, S.; Tsuji, O.; Nagoshi, N.; Asazuma, T.; Tsuji, T.; et al. The 5-item modified frailty index is predictive of severe adverse events in patients undergoing surgery for adult spinal deformity. Spine 2019, 44, E1083–E1091. [Google Scholar] [CrossRef]
- Cui, P.; Wang, P.; Wang, J.; Liu, X.; Kong, C.; Lu, S. The impact of frailty on perioperative outcomes in patients receiving short-level posterior lumbar interbody fusion: A stepwise propensity score matching analysis. Clin. Interv. Aging 2022, 17, 1297–1306. [Google Scholar] [CrossRef]
- Veronesi, F.; Borsari, V.; Martini, L.; Visani, A.; Gasbarrini, A.; Brodano, G.B.; Fini, M. The impact of frailty on spine surgery: Systematic review on 10 years clinical studies. Aging Dis. 2021, 12, 625–645. [Google Scholar] [CrossRef]
- Ethun, C.G.; Bilen, M.A.; Jani, A.B.; Maithel, S.K.; Ogan, K.; Master, V.A. Frailty and cancer: Implications for oncology surgery, medical oncology, and radiation oncology. CA Cancer J. Clin. 2017, 67, 362–377. [Google Scholar] [CrossRef]
- Handforth, C.; Clegg, A.; Young, C.; Simpkins, S.; Seymour, M.T.; Selby, P.J.; Young, J. The prevalence and outcomes of frailty in older cancer patients: A systematic review. Ann. Oncol. 2015, 26, 1091–1101. [Google Scholar] [CrossRef]
- Lu, J.; Cao, L.L.; Zheng, C.H.; Li, P.; Xie, J.W.; Wang, J.B.; Lin, J.X.; Chen, Q.Y.; Lin, M.; Tu, R.H.; et al. The preoperative frailty versus inflammation-based prognostic score: Which is better as an objective predictor for gastric cancer patients 80 years and older? Ann. Surg. Oncol. 2017, 24, 754–762. [Google Scholar] [CrossRef]
- Komici, K.; Cappuccio, M.; Scacchi, A.; Vaschetti, R.; Delli Carpini, G.; Picerno, V.; Avella, P.; Brunese, M.C.; Rengo, G.; Guerra, G.; et al. The prevalence and the impact of frailty in hepato-biliary pancreatic cancers: A systematic review and meta-analysis. J. Clin. Med. 2022, 11, 1116. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Demura, S.; Shinmura, K.; Yokogawa, N.; Shimizu, T.; Murakami, H.; Kawahara, N.; Tomita, K.; Tsuchiya, H. Surgical Metastasectomy in the spine: A review article. Oncologist 2021, 26, e1833–e1843. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, M.F.; Glassman, S.D.; Dimar, J.R., 2nd; Puno, R.M.; Johnson, J.R. Perioperative complications of anterior procedures on the spine. J. Bone Jt. Surg. Am. 1996, 78, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Aalberg, J.J.; Soriano, R.P.; Divino, C.M. New 5-factor modified frailty index using American College of Surgeons NSQIP data. J. Am. Coll. Surg. 2018, 226, 173–181.e8. [Google Scholar] [CrossRef]
- Bandiera, S.; Noli, L.E.; Griffoni, C.; Tosini, G.; Carretta, E.; Pasini, S.; Pesce, E.; Ruinato, A.D.; Barbanti Brodano, G.; Tedesco, G.; et al. Complications and risk factors in en bloc resection of spinal tumors: A retrospective analysis on 298 patients treated in a single institution. Curr. Oncol. 2022, 29, 7842–7857. [Google Scholar] [CrossRef]
- Li, Z.; Guo, L.; Zhang, P.; Wang, J.; Wang, X.; Yao, W. A systematic review of perioperative complications in en bloc resection for spinal tumors. Glob. Spine J. 2023, 13, 812–822. [Google Scholar] [CrossRef]
- Kurokawa, Y.; Murakami, H.; Demura, S.; Kato, S.; Yokogawa, N.; Shinmura, K.; Shimizu, T.; Tsuchiya, H. Risk factors for poor outcomes of early rehabilitation after total en bloc spondylectomy: A retrospective chart review of 140 patients. Spinal Cord 2020, 58, 900–907. [Google Scholar] [CrossRef]
- Liao, J.C.; Chen, W.J.; Chen, L.H. Surgery for metastatic epidural spinal cord compression in thoracic spine, anterior or posterior approach? Biomed. J. 2022, 45, 370–376. [Google Scholar] [CrossRef]
- O’Sullivan, B.; Davis, A.M.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Wunder, J.; Kandel, R.; Goddard, K.; Sadura, A.; et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: A randomised trial. Lancet 2002, 359, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Cannon, C.P.; Ballo, M.T.; Zagars, G.K.; Mirza, A.N.; Lin, P.P.; Lewis, V.O.; Yasko, A.W.; Benjamin, R.S.; Pisters, P.W. Complications of combined modality treatment of primary lower extremity soft-tissue sarcomas. Cancer 2006, 107, 2455–2461. [Google Scholar] [CrossRef]
- Holm, T.; Singnomklao, T.; Rutqvist, L.E.; Cedermark, B. Adjuvant preoperative radiotherapy in patients with rectal carcinoma. Adverse effects during long term follow-up of two randomized trials. Cancer 1996, 78, 968–976. [Google Scholar] [CrossRef]
- Yokogawa, N.; Murakami, H.; Demura, S.; Kato, S.; Yoshioka, K.; Yamamoto, M.; Iseki, S.; Tsuchiya, H. Effects of radiation on spinal dura mater and surrounding tissue in mice. PLoS ONE 2015, 10, e0133806. [Google Scholar] [CrossRef] [PubMed]
- Yokogawa, N.; Murakami, H.; Demura, S.; Kato, S.; Yoshioka, K.; Hayashi, H.; Ishii, T.; Igarashi, T.; Fang, X.; Tsuchiya, H. Perioperative complications of total en bloc spondylectomy: Adverse effects of preoperative irradiation. PLoS ONE 2014, 9, e98797. [Google Scholar] [CrossRef] [PubMed]
- Vergote, I.; Amant, F.; Kristensen, G.; Ehlen, T.; Reed, N.S.; Casado, A. Primary surgery or neoadjuvant chemotherapy followed by interval debulking surgery in advanced ovarian cancer. Eur. J. Cancer 2011, 47 (Suppl. S3), S88–S92. [Google Scholar] [CrossRef] [PubMed]
- Gazendam, A.M.; Schneider, P.; Spiguel, A.; Ghert, M. Neoadjuvant chemotherapy and endoprosthetic reconstruction for lower extremity sarcomas: Does timing impact complication rates? Ann. Surg. Oncol. 2022, 29, 7312–7317. [Google Scholar] [CrossRef] [PubMed]
- Adamson, K.; Chavez-MacGregor, M.; Caudle, A.; Smith, B.; Baumann, D.; Liu, J.; Schaverien, M. Neoadjuvant chemotherapy does not Increase complications in oncoplastic breast-conserving surgery. Ann. Surg. Oncol. 2019, 26, 2730–2737. [Google Scholar] [CrossRef]
- Hanlon, P.; Nicholl, B.I.; Jani, B.D.; Lee, D.; McQueenie, R.; Mair, F.S. Frailty and pre-frailty in middle-aged and older adults and its association with multimorbidity and mortality: A prospective analysis of 493 737 UK Biobank participants. Lancet Public Health 2018, 3, e323–e332. [Google Scholar] [CrossRef]
- George, E.L.; Hall, D.E.; Youk, A.; Chen, R.; Kashikar, A.; Trickey, A.W.; Varley, P.R.; Shireman, P.K.; Shinall, M.C., Jr.; Massarweh, N.N.; et al. Association between patient frailty and postoperative mortality across multiple noncardiac surgical specialties. JAMA Surg. 2021, 156, e205152. [Google Scholar] [CrossRef]
- Muscedere, J.; Waters, B.; Varambally, A.; Bagshaw, S.M.; Boyd, J.G.; Maslove, D.; Sibley, S.; Rockwood, K. The impact of frailty on intensive care unit outcomes: A systematic review and meta-analysis. Intensive Care Med. 2017, 43, 1105–1122. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.L.L.; Chan, Y.H.; O’Neill, G.K.; Murphy, D.; Merchant, R.A. Frailty, length of stay and cost in hip fracture patients. Osteoporos. Int. 2023, 34, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Chan, V.; Wilson, J.R.F.; Ravinsky, R.; Badhiwala, J.H.; Jiang, F.; Anderson, M.; Yee, A.; Wilson, J.R.; Fehlings, M.G. Frailty adversely affects outcomes of patients undergoing spine surgery: A systematic review. Spine J. 2021, 21, 988–1000. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshian, J.; Shahrestani, S.; Buser, Z.; Hah, R.; Hsieh, P.C.; Liu, J.C.; Wang, J.C. The performance of frailty in predictive modeling of short-term outcomes in the surgical management of metastatic tumors to the spine. Spine J. 2022, 22, 605–615. [Google Scholar] [CrossRef]
- Dos Santos, N.L.O.; Pegorari, M.S.; Silva, C.F.R.; Jamami, M.; Matos, A.P.; Pinto, A.C.P.N.; Ohara, D.G. Pulmonary function as a predictor of frailty syndrome in community-dwelling older adults. J. Geriatr. Phys. Ther. 2023, 46, 64–70. [Google Scholar] [CrossRef]
- Lee, D.U.; Kwon, J.; Han, J.; Fan, G.H.; Hastie, D.J.; Lee, K.J.; Karagozian, R. The clinical impact of frailty on the postoperative outcomes of patients undergoing gastrectomy for gastric cancer: A propensity-score matched database study. Gastric Cancer 2022, 25, 450–458. [Google Scholar] [CrossRef]
- Lin, H.S.; Watts, J.N.; Peel, N.M.; Hubbard, R.E. Frailty and post-operative outcomes in older surgical patients: A systematic review. BMC Geriatr. 2016, 16, 157. [Google Scholar] [CrossRef]
- Tuddenham, S.A.; Gearhart, S.L.; Wright, E.J., 3rd; Handa, V.L. Frailty and postoperative urinary tract infection. BMC Geriatr. 2022, 22, 828. [Google Scholar] [CrossRef]
- Chen, X.; Mao, G.; Leng, S.X. Frailty syndrome: An overview. Clin. Interv. Aging 2014, 9, 433–441. [Google Scholar] [CrossRef]
- Ni Lochlainn, M.; Cox, N.J.; Wilson, T.; Hayhoe, R.P.G.; Ramsay, S.E.; Granic, A.; Isanejad, M.; Roberts, H.C.; Wilson, D.; Welch, C.; et al. Nutrition and frailty: Opportunities for prevention and treatment. Nutrients 2021, 13, 2349. [Google Scholar] [CrossRef]
- Das, U.N. Nutritional factors in the prevention and management of coronary artery disease and heart failure. Nutrition 2015, 31, 283–291. [Google Scholar] [CrossRef] [PubMed]
| Variable | Total (n = 169) | Non-Complications (n = 83) | Complications (n = 86) | p-Value |
|---|---|---|---|---|
| Age (years), mean ± SD | 53.3 (14.3) | 55.0 (14.1) | 51.5 (14.4) | 0.113 |
| Sex | 0.079 | |||
| - Male, n (%) | 99 (58.6) | 43 (51.8) | 56 (65.1) | |
| - Female, n (%) | 70 (41.4) | 40 (48.2) | 30 (34.9) | |
| BMI (mean ± SD) | 22.4 (3.6) | 21.9 (3.5) | 22.8 (3.7) | 0.137 |
| Type of tumors, n (%) | 0.124 | |||
| - Primary | 44 (26.0) | 26 (31.3) | 18 (20.9) | |
| - Metastatic | 125 (74.0) | 57 (68.7) | 68 (79.1) | |
| Location of tumors, n (%) | 0.41 | |||
| - Thoracic spine (T1 to T12) | 119 (70.4) | 56 (67.5) | 63 (73.3) | |
| - Lumbar spine (L1 to L5) | 50 (29.6) | 27 (32.5) | 23 (26.7) | |
| ASA-PS, n (%) | 0.005 * | |||
| - I | 4 (2.4) | 3 (3.6) | 1 (1.1) | |
| - II | 43 (84.6) | 75 (90.4) | 68 (79.1) | |
| - III | 22 (13.0) | 5 (6.0) | 17 (19.8) | |
| mFI-5, n (%) | <0.001 * | |||
| - 0 | 97 (57.4) | 63 (75.9) | 34 (39.5) | |
| - 1 | 52 (30.8) | 16 (19.3) | 36 (41.9) | |
| - 2 | 15 (8.9) | 4 (4.8) | 11 (12.8) | |
| - 3 | 5 (2.9) | 0 | 5 (5.8) | |
| NLR, median (IQR) | 2.64 (1.87, 3.95) | 2.22 (1.70, 3.34) | 3.21 (1.93, 4.61) | 0.015 * |
| CAR, median (IQR) | 0.027 (0.010, 0.110) | 0.024 (0.002, 0.050) | 0.048 (0.004, 0.163) | <0.001 * |
| Preoperative chemotherapy, n (%) | 53 (31.4) | 28 (33.7) | 25 (29.1) | 0.513 |
| Preoperative radiotherapy, n (%) | 38 (22.5) | 12 (14.5) | 26 (30.2) | 0.014 |
| Surgical approach, n (%) | 0.36 | |||
| - Posterior only | 140 (82.8) | 71 (85.5) | 69 (80.2) | |
| - Anterior–posterior combined | 29 (17.2) | 12 (14.5) | 17 (19.8) | |
| The number of resected vertebrae, n (%) | 0.007 * | |||
| - 1 | 129 (76.3) | 72 (86.7) | 57 (66.3) | |
| - 2 | 18 (10.1) | 5 (6.0) | 13 (15.1) | |
| - 3 | 22 (13.6) | 6 (7.3) | 16 (18.6) | |
| Operative time (min), median (IQR) | 447 (360, 559) | 399 (338, 535) | 476 (396, 567) | 0.002 * |
| Estimated blood loss (mL), median (IQR) | 420 (235, 590) | 400 (200, 600) | 430 (265, 585) | 0.338 |
| Type of Complications | Total | Major | Minor |
|---|---|---|---|
| Total, n (%) | 161 | 72 (44.7) | 89 (55.3) |
| Respiratory | 37 (41.4) | 12 (16.2) | 25 (27.8) |
| Operative wound | 28 (17.3) | 13 (17.6) | 15 (16.7) |
| Neurological | 25 (15.4) | 12 (16.2) | 13 (14.4) |
| CSF leakage | 24 (14.8) | 11 (14.9) | 13 (14.4) |
| Cardiovascular | 16 (9.9) | 7 (9.5) | 9 (10.0) |
| Genitourinary | 11 (6.8) | 1 (1.4) | 10 (11.1) |
| Gastrointestinal | 7 (4.3) | 5 (6.8) | 2 (2.2) |
| Others | 13 (8.0) | 11 (14.9) | 2 (2.2) |
| OR | p-Value | 95% CI | |
|---|---|---|---|
| Total complications | |||
| - mFI-5 score | 2.99 | <0.001 | 1.76–5.09 |
| - number of resected vertebrae | 1.87 | 0.018 | 1.11–3.14 |
| Major complications | |||
| - mFI-5 score | 1.83 | 0.010 | 1.16–2.91 |
| - number of resected vertebrae | 2.39 | <0.001 | 1.48–3.87 |
| Variable | Non-Frailty (mFI-5 = 0, n = 97) | Pre-Frailty/Frailty (mFI-5 ≥ 1, n = 72) | p-Value |
|---|---|---|---|
| Age (years), mean ± SD | 47.9 (14.4) | 60.6 (10.6) | <0.001 * |
| Sex | <0.001 * | ||
| - Male, n (%) | 44 (45.4) | 55 (76.4) | |
| - Female, n (%) | 53 (54.6) | 17 (23.6) | |
| BMI, mean ± SD | 22.0 (3.8) | 22.8 (3.3) | 0.143 |
| Type of tumors, n (%) | <0.001 * | ||
| - Primary | 35 (36.1) | 9 (12.5) | |
| - Metastatic | 62 (63.9) | 63 (87.5) | |
| Location of the tumor, n (%) | 0.071 | ||
| - Thoracic spine (T1 to T12) | 63 (64.9) | 56 (77.8) | |
| - Lumbar spine (L1 to L5) | 34 (35.1) | 16 (22.2) | |
| ASA-PS, n (%) | <0.001 * | ||
| - I | 4 (4.1) | 0 | |
| - II | 89 (91.8) | 54 (75.0) | |
| - III | 4 (4.1) | 18 (25.0) | |
| NLR, median (IQR) | 2.25 (1.72, 3.43) | 3.36 (2.06, 4.88) | 0.002 * |
| CAR, median (IQR) | 0.024 (0.004, 0.527) | 0.049 (0.022, 0.250) | 0.001 * |
| Preoperative chemotherapy, n (%) | 30 (30.1) | 23 (31.9) | 0.888 |
| Preoperative radiotherapy, n (%) | 17 (17.5) | 21 (29.2) | 0.073 |
| Surgical approach, n (%) | 0.166 | ||
| - Posterior only | 77 (79.4) | 63 (87.5) | |
| - Anterior–posterior combined | 20 (20.6) | 9 (12.5) | |
| The number of resected vertebrae, n (%) | 0.227 | ||
| - 1 | 78 (80.4) | 51 (70.8) | |
| - 2 | 10 (10.3) | 8 (11.1) | |
| - 3 | 9 (9.3) | 13 (18.1) | |
| Total operative time (min), median (IQR) | 455 (355, 576) | 445 (359, 547) | 0.724 |
| Estimated blood loss (mL), median (IQR) | 400 (215, 600) | 430 (240, 570) | 0.675 |
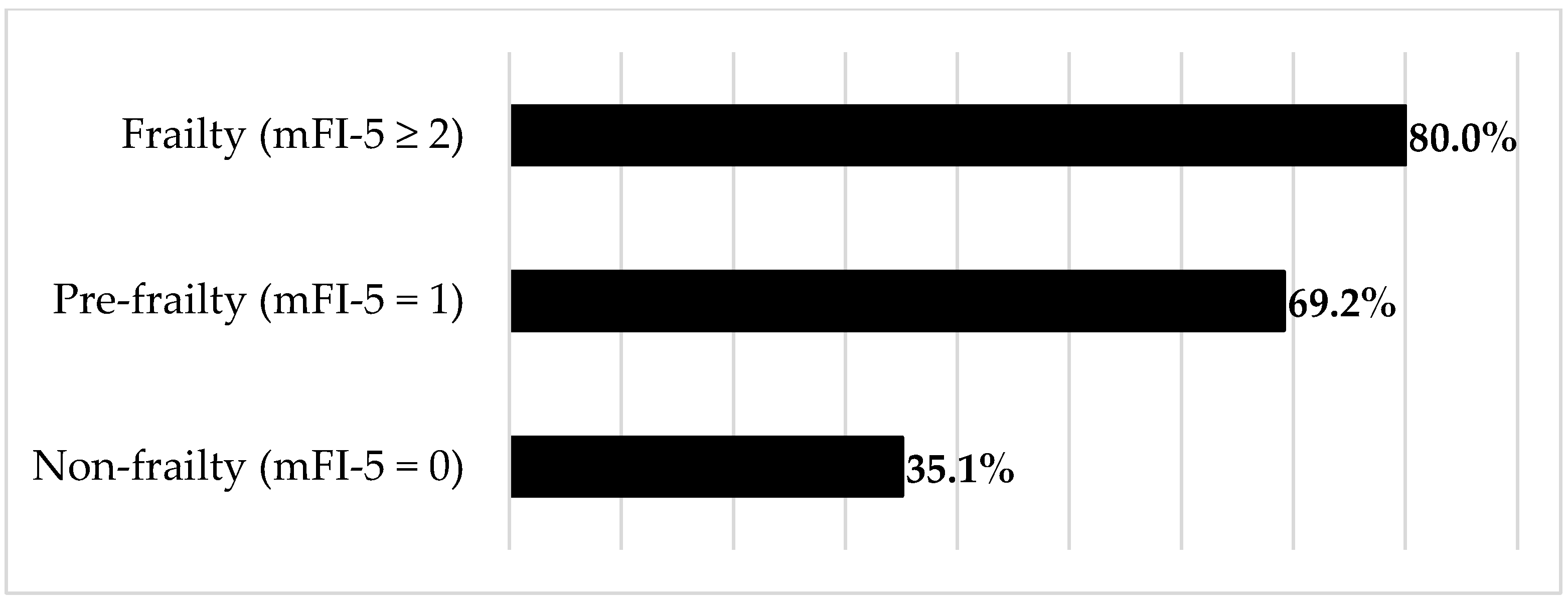
| Complications, n (%) | |||
| - Total | 34 (35.1) | 52 (72.2) | <0.001 * |
| - Major | 20 (20.6) | 35 (48.6) | <0.001 * |
| - Respiratory | 11 (11.3) | 22 (30.6) | 0.002 * |
| - Operative wound | 8 (8.2) | 19 (26.4) | 0.001 * |
| - Neurological | 12 (12.4) | 13 (18.1) | 0.303 |
| - CSF leakage | 6 (6.2) | 18 (25.0) | <0.001 * |
| - Cardiovascular | 6 (6.2) | 8 (11.1) | 0.251 |
| - Genitourinary | 1 (1.0) | 10 (13.9) | <0.01 * |
| - Gastrointestinal | 2 (2.1) | 5 (6.9) | 0.119 |
| - Others | 2 (2.1) | 8 (11.1) | 0.016 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawai, M.; Demura, S.; Kato, S.; Yokogawa, N.; Shimizu, T.; Kurokawa, Y.; Kobayashi, M.; Yamada, Y.; Nagatani, S.; Uto, T.; et al. The Impact of Frailty on Postoperative Complications in Total En Bloc Spondylectomy for Spinal Tumors. J. Clin. Med. 2023, 12, 4168. https://doi.org/10.3390/jcm12124168
Kawai M, Demura S, Kato S, Yokogawa N, Shimizu T, Kurokawa Y, Kobayashi M, Yamada Y, Nagatani S, Uto T, et al. The Impact of Frailty on Postoperative Complications in Total En Bloc Spondylectomy for Spinal Tumors. Journal of Clinical Medicine. 2023; 12(12):4168. https://doi.org/10.3390/jcm12124168
Chicago/Turabian StyleKawai, Masafumi, Satoru Demura, Satoshi Kato, Noriaki Yokogawa, Takaki Shimizu, Yuki Kurokawa, Motoya Kobayashi, Yohei Yamada, Satoshi Nagatani, Takaaki Uto, and et al. 2023. "The Impact of Frailty on Postoperative Complications in Total En Bloc Spondylectomy for Spinal Tumors" Journal of Clinical Medicine 12, no. 12: 4168. https://doi.org/10.3390/jcm12124168
APA StyleKawai, M., Demura, S., Kato, S., Yokogawa, N., Shimizu, T., Kurokawa, Y., Kobayashi, M., Yamada, Y., Nagatani, S., Uto, T., & Murakami, H. (2023). The Impact of Frailty on Postoperative Complications in Total En Bloc Spondylectomy for Spinal Tumors. Journal of Clinical Medicine, 12(12), 4168. https://doi.org/10.3390/jcm12124168







