Increasing Antibody Responses to Five Doses of SARS-CoV-2 mRNA Vaccine in Lung Transplant Patients
Abstract
1. Introduction
2. Material and Methods
2.1. Patients
2.2. Transplant Care
2.3. S-Specific Antibody Testing
2.4. Patient Groups
2.5. Statistical Analysis
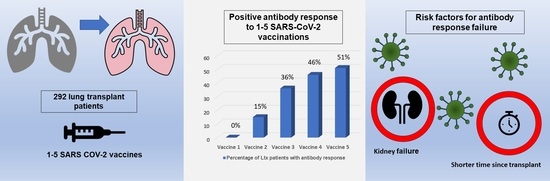
3. Results
3.1. All Patients
3.2. Responders
3.3. Non-Responders
3.4. Vaccines
3.5. Risk Factors for the Failure to Develop an Antibody Response after Vaccination
3.6. COVID
3.7. All-Cause Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CKD | chronic kidney disease |
| COPD | Chronic obstructive pulmonary disease |
| COVID-19 | coronavirus disease |
| ICU | Intensive care unit |
| ILD | Interstitial lung disease |
| LTx | Lung transplant/lung transplantation |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus-2 |
References
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Moreira, E.D.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef] [PubMed]
- Frenck, R.W.; Klein, N.P.; Kitchin, N.; Gurtman, A.; Absalon, J.; Lockhart, S.; Perez, J.L.; Walter, E.B.; Senders, S.; Bailey, R.; et al. Safety, Immunogenicity, and Efficacy of the BNT162b2 COVID-19 Vaccine in Adolescents. N. Engl. J. Med. 2021, 385, 239–250. [Google Scholar] [CrossRef]
- Walsh, E.E.; Frenck, R.W.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based COVID-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef]
- Giannella, M.; Pierrotti, L.C.; Helanterä, I.; Manuel, O. SARS-CoV-2 Vaccination in Solid-organ Transplant Recipients: What the Clinician Needs to Know. Transpl. Int. 2021, 34, 1776–1788. [Google Scholar] [CrossRef]
- Azzi, Y.; Bartash, R.; Scalea, J.; Loarte-Campos, P.; Akalin, E. COVID-19 and Solid Organ Transplantation: A Review Article. Transplantation 2021, 105, 37–55. [Google Scholar] [CrossRef]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Antibody Response to 2-Dose SARS-CoV-2 MRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA 2021, 325, 2204. [Google Scholar] [CrossRef]
- Chen, X.; Luo, D.; Mei, B.; Du, J.; Liu, X.; Xie, H.; Liu, L.; Su, S.; Mai, G. Immunogenicity of COVID-19 Vaccines in Solid Organ Transplant Recipients: A Systematic Review and Meta-Analysis. Clin. Microbiol. Infect. 2022, 29, 441–456. [Google Scholar] [CrossRef]
- Dauriat, G.; Beaumont, L.; Luong Nguyen, L.B.; Renaud Picard, B.; Penhouet, M.; Coiffard, B.; Salpin, M.; Demant, X.; Saint Raymond, C.; Carlier, N.; et al. Efficacy of Three COVID-19 Vaccine Doses in Lung Transplant Recipients: A Multicentre Cohort Study. Eur. Respir. J. 2023, 61, 2200502. [Google Scholar] [CrossRef]
- Bárczi, E.; Varga, V.; Nagy, A.; Eszes, N.; Jáky-Kováts, Z.; Müller, V.; Bohács, A. Serological Findings Following the Second and Third SARS-CoV-2 Vaccines in Lung Transplant Recipients. Immun. Inflamm. Dis. 2022, 10, e646. [Google Scholar] [CrossRef] [PubMed]
- Special Considerations in Solid Organ Transplant, Hematopoietic Cell Transplant, and Cellular Immunotherapy Candidates, Donors, and Recipients. Available online: https://www.covid19treatmentguidelines.nih.gov/special-populations/transplant/ (accessed on 10 June 2023).
- Stevens, P.E.; Levin, A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members Evaluation and Management of Chronic Kidney Disease: Synopsis of the Kidney Disease: Improving Global Outcomes 2012 Clinical Practice Guideline. Ann. Intern. Med. 2013, 158, 825–830. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Guidance from the International Society of Heart and Lung Transplantation Regarding the SARS CoV-2 Pandemic. Available online: https://www.inshlt.org/wp-content/uploads/2021/covid-19.pdf (accessed on 2 May 2023).
- Narasimhan, M.; Mahimainathan, L.; Araj, E.; Clark, A.E.; Markantonis, J.; Green, A.; Xu, J.; SoRelle, J.A.; Alexis, C.; Fankhauser, K.; et al. Clinical Evaluation of the Abbott Alinity SARS-CoV-2 Spike-Specific Quantitative IgG and IgM Assays among Infected, Recovered, and Vaccinated Groups. J. Clin. Microbiol. 2021, 59, e0038821. [Google Scholar] [CrossRef]
- Sanders, J.-S.F.; Bemelman, F.J.; Messchendorp, A.L.; Baan, C.C.; van Baarle, D.; van Binnendijk, R.; Diavatopoulos, D.A.; Frölke, S.C.; Geers, D.; GeurtsvanKessel, C.H.; et al. The RECOVAC Immune-Response Study: The Immunogenicity, Tolerability, and Safety of COVID-19 Vaccination in Patients with Chronic Kidney Disease, on Dialysis, or Living with a Kidney Transplant. Transplantation 2022, 106, 821–834. [Google Scholar] [CrossRef]
- Hoek, R.A.; Verschuuren, E.A.; de Vries, R.D.; Vonk, J.M.; van Baarle, D.; van der Heiden, M.; van Gemert, J.P.; Gore, E.J.; Niesters, H.G.; Erasmus, M.; et al. High Torque Tenovirus (TTV) Load before First Vaccine Dose Is Associated with Poor Serological Response to COVID-19 Vaccination in Lung Transplant Recipients. J. Heart Lung Transplant. 2022, 41, 765–772. [Google Scholar] [CrossRef]
- Roosma, E.; van Gemert, J.P.; de Zwart, A.E.S.; van Leer-Buter, C.C.; Hellemons, M.E.; Berg, E.M.; Luijk, B.; Hoek, R.A.S.; van Kessel, D.A.; Akkerman, O.W.; et al. The Effect of COVID-19 on Transplant Function and Development of CLAD in Lung Transplant Patients: A Multicenter Experience. J. Heart Lung Transplant. 2022, 41, 1237–1247. [Google Scholar] [CrossRef]
- Alejo, J.L.; Mitchell, J.; Chiang, T.P.-Y.; Abedon, A.T.; Boyarsky, B.J.; Avery, R.K.; Tobian, A.A.R.; Levan, M.L.; Massie, A.B.; Garonzik-Wang, J.M.; et al. Antibody Response to a Fourth Dose of a SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients: A Case Series. Transplantation 2021, 105, e280–e281. [Google Scholar] [CrossRef]
- Abedon, A.T.; Teles, M.S.; Alejo, J.L.; Kim, J.D.; Mitchell, J.; Chiang, T.P.Y.; Avery, R.K.; Tobian, A.A.R.; Levan, M.L.; Warren, D.S.; et al. Improved Antibody Response after a Fifth Dose of a SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients: A Case Series. Transplantation 2022, 106, e262–e263. [Google Scholar] [CrossRef]
- Collier, D.A.; Ferreira, I.A.T.M.; Kotagiri, P.; Datir, R.P.; Lim, E.Y.; Touizer, E.; Meng, B.; Abdullahi, A.; CITIID-NIHR BioResource COVID-19 Collaboration; Elmer, A.; et al. Age-Related Immune Response Heterogeneity to SARS-CoV-2 Vaccine BNT162b2. Nature 2021, 596, 417–422. [Google Scholar] [CrossRef]
- Shostak, Y.; Shafran, N.; Heching, M.; Rosengarten, D.; Shtraichman, O.; Shitenberg, D.; Amor, S.M.; Yahav, D.; Ben Zvi, H.; Pertzov, B.; et al. Early Humoral Response among Lung Transplant Recipients Vaccinated with BNT162b2 Vaccine. Lancet Respir. Med. 2021, 9, e52–e53. [Google Scholar] [CrossRef]
- Manothummetha, K.; Chuleerarux, N.; Sanguankeo, A.; Kates, O.S.; Hirankarn, N.; Thongkam, A.; Dioverti-Prono, M.V.; Torvorapanit, P.; Langsiri, N.; Worasilchai, N.; et al. Immunogenicity and Risk Factors Associated With Poor Humoral Immune Response of SARS-CoV-2 Vaccines in Recipients of Solid Organ Transplant: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2022, 5, e226822. [Google Scholar] [CrossRef] [PubMed]
- Zong, K.; Peng, D.; Yang, H.; Huang, Z.; Luo, Y.; Wang, Y.; Xiang, S.; Li, T.; Mou, T.; Wu, Z. Risk Factors for Weak Antibody Response of SARS-CoV-2 Vaccine in Adult Solid Organ Transplant Recipients: A Systemic Review and Meta-Analysis. Front. Immunol. 2022, 13, 888385. [Google Scholar] [CrossRef] [PubMed]
- Rozen-Zvi, B.; Yahav, D.; Agur, T.; Zingerman, B.; Ben-Zvi, H.; Atamna, A.; Tau, N.; Mashraki, T.; Nesher, E.; Rahamimov, R. Antibody Response to SARS-CoV-2 MRNA Vaccine among Kidney Transplant Recipients: A Prospective Cohort Study. Clin. Microbiol. Infect. 2021, 27, 1173.e1–1173.e4. [Google Scholar] [CrossRef] [PubMed]
- Goda, Y.; Nakajima, D.; Tanaka, S.; Yamada, Y.; Yutaka, Y.; Unagami, K.; Yoshikawa, M.; Egawa, H.; Date, H. Efficacy and Safety of the SARS-CoV-2 MRNA Vaccine in Lung Transplant Recipients: A Possible Trigger of Rejection. Gen. Thorac. Cardiovasc. Surg. 2023, 71, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Tarke, A.; Sidney, J.; Methot, N.; Yu, E.D.; Zhang, Y.; Dan, J.M.; Goodwin, B.; Rubiro, P.; Sutherland, A.; Wang, E.; et al. Impact of SARS-CoV-2 Variants on the Total CD4+ and CD8+ T Cell Reactivity in Infected or Vaccinated Individuals. Cell. Rep. Med. 2021, 2, 100355. [Google Scholar] [CrossRef]
- Jordan, S.C.; Shin, B.-H.; Gadsden, T.-A.M.; Chu, M.; Petrosyan, A.; Le, C.N.; Zabner, R.; Oft, J.; Pedraza, I.; Cheng, S.; et al. T Cell Immune Responses to SARS-CoV-2 and Variants of Concern (Alpha and Delta) in Infected and Vaccinated Individuals. Cell. Mol. Immunol. 2021, 18, 2554–2556. [Google Scholar] [CrossRef]
- Doubell, T.P.; Mannion, R.J.; Woolf, C.J. Intact Sciatic Myelinated Primary Afferent Terminals Collaterally Sprout in the Adult Rat Dorsal Horn Following Section of a Neighbouring Peripheral Nerve. J. Comp. Neurol. 1997, 380, 95–104. [Google Scholar] [CrossRef]


| Variable n, (%) | All Patients # 292 | Responders 123 (42) | Non-Responders 126 (43) | p-Value * |
|---|---|---|---|---|
| Age, years | 60 (48–66) | 58 (40–64) | 62 (54–66) | 0.004 |
| Gender, male (%) | 152 (52) | 69 (56) | 64 (51) | 0.402 |
| Caucasian, n (%) | 281 (96) | 120 (98) | 124 (98) | 0.549 |
| Transplant indication, n (%) | 0.106 | |||
| COPD | 120 (41) | 51 (42) | 53 (42) | |
| Fibrosis | 63 (21) | 23 (19) | 32 (25) | |
| Pulmonary hypertension | 35 (12) | 11 (9) | 18 (14) | |
| Cystic fibrosis | 43 (15) | 23 (19) | 17 (14) | |
| Other | 31 (11) | 15 (12) | 6 (5) | |
| Bilateral LTx, n (%) | 267 (91) | 118 (96) | 113 (90) | 0.057 |
| Time since transplant **, years | 8 (4–12) | 9 (5–13) | 7 (4–11) | 0.047 |
| Comorbidities, n (%) | ||||
| Hypertension | 137 (47) | 54 (44) | 64 (51) | 0.276 |
| Dyslipidemia | 236 (81) | 101 (82) | 100 (79) | 0.583 |
| Diabetes Mellitus | 99 (34) | 42 (34) | 46 (37) | 0.697 |
| Chronic kidney disease | 205 (70) | 77 (63) | 99 (79) | 0.006 |
| Obesitas, BMI> 30 | 33 (11) | 12 (10) | 14 (11) | 0.727 |
| Heart failure | 27 (9) | 11 (9) | 12 (10) | 0.874 |
| Immunosuppression, n (%) | ||||
| Tacrolimus | 245 (84) | 100 (81) | 105(83) | 0.372 |
| Cyclosporine | 10 (4) | 5 (4) | 3 (2) | 0.455 |
| Azathioprine | 57 (22) | 27 (22) | 22 (18) | 0.377 |
| Mycophenolate mofetil | 199 (76) | 80 (65) | 85 (68) | 0.686 |
| mTORi | 12 (5) | 5 (4) | 6 (5) | 0.781 |
| CLAD | 86 (30) | 33 (27) | 41 (33) | 0.324 |
| COVID-19, n (%) | 146 (50) | 49 (40) | 54 (43) | 0.629 |
| All-cause mortality (%) | 20 (7) | 3 (2) | 17 (14) | 0.001 |
| COVID-19 mortality (%) | 4 (1) | 0 (0) | 4 (3) | 0.046 |
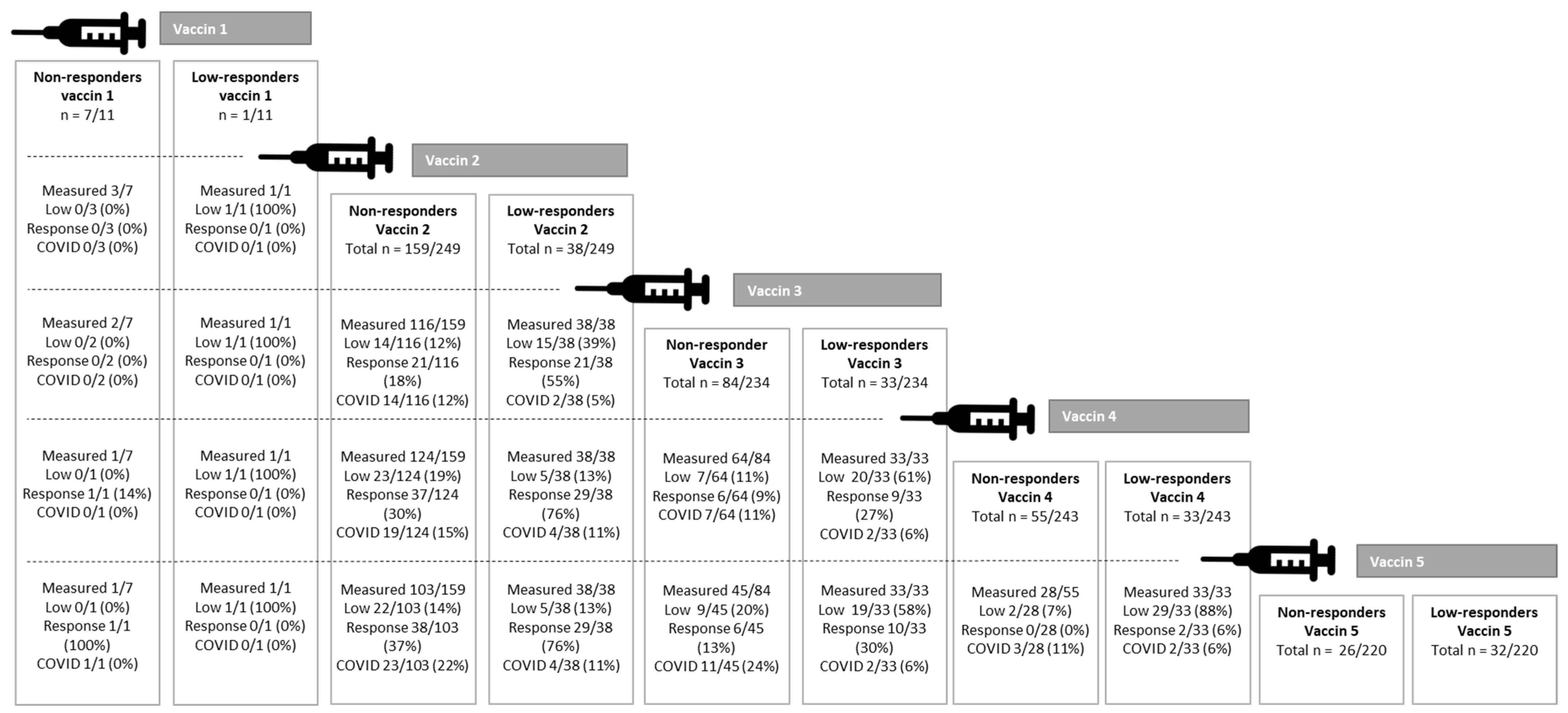
| Vaccine 1 | Vaccine 1–2 | Vaccine 1–3 | Vaccine 1–4 | Vaccine 1–5 | |
|---|---|---|---|---|---|
| Patients with antibody response assessed, n (%) | 11/292 (4) | 249/292 (85) | 234/292 (80) | 243/292 (83) | 220/292 (75) |
| Patients with pos. antibody response *, n (%) | 0 (0) | 37 (15) | 85 (36) | 112 (46) | 113 (51) |
| Patients without pos. antibody response #, n (%) | 8 (73) | 197 (79) | 117 (50) | 88 (36) | 58 (26) |
| Non-response, n Low response, n | 7 1 | 159 38 | 84 33 | 55 33 | 26 32 |
| Patients with antibody response due to COVID-19, n (%) | 3 (27) | 15 (6) | 32 (14) | 43 (18) | 49 (22) |
| Responders with COVID n = 49 | Non-Responders/Low Responders with COVID n = 54 | p-Value | |
|---|---|---|---|
| Admission, n (%) | 4 (8) | 9 (17) | 0.168 |
| Death, n (%) | 0 (0) | 4 (8) | 0.046 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Gemert, J.; Steenberg, F.; van Leer-Buter, C.; Kerstjens, H.; Steenhuis, W.; Akkerman, O.; Verschuuren, E.; Gan, T. Increasing Antibody Responses to Five Doses of SARS-CoV-2 mRNA Vaccine in Lung Transplant Patients. J. Clin. Med. 2023, 12, 4125. https://doi.org/10.3390/jcm12124125
van Gemert J, Steenberg F, van Leer-Buter C, Kerstjens H, Steenhuis W, Akkerman O, Verschuuren E, Gan T. Increasing Antibody Responses to Five Doses of SARS-CoV-2 mRNA Vaccine in Lung Transplant Patients. Journal of Clinical Medicine. 2023; 12(12):4125. https://doi.org/10.3390/jcm12124125
Chicago/Turabian Stylevan Gemert, Johanna, Fleur Steenberg, Coretta van Leer-Buter, Huib Kerstjens, Willie Steenhuis, Onno Akkerman, Erik Verschuuren, and Tji Gan. 2023. "Increasing Antibody Responses to Five Doses of SARS-CoV-2 mRNA Vaccine in Lung Transplant Patients" Journal of Clinical Medicine 12, no. 12: 4125. https://doi.org/10.3390/jcm12124125
APA Stylevan Gemert, J., Steenberg, F., van Leer-Buter, C., Kerstjens, H., Steenhuis, W., Akkerman, O., Verschuuren, E., & Gan, T. (2023). Increasing Antibody Responses to Five Doses of SARS-CoV-2 mRNA Vaccine in Lung Transplant Patients. Journal of Clinical Medicine, 12(12), 4125. https://doi.org/10.3390/jcm12124125