Fibrous Dysplasia of the Jaw: Advances in Imaging and Treatment
Abstract
:1. Introduction
2. Epidemiology and Clinical Appearance
3. Pathophysiology and Mutational Analysis
4. Diagnostic Findings
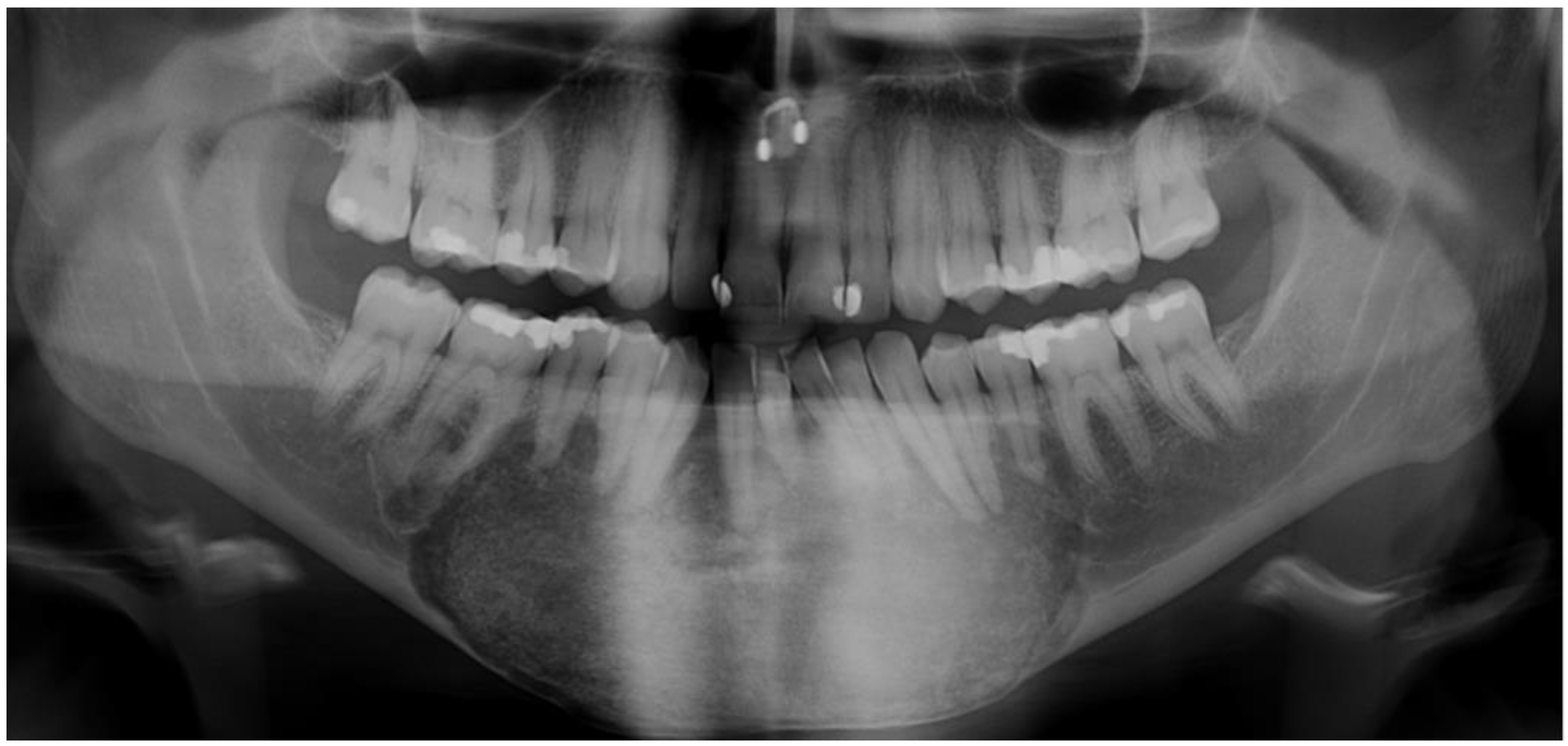
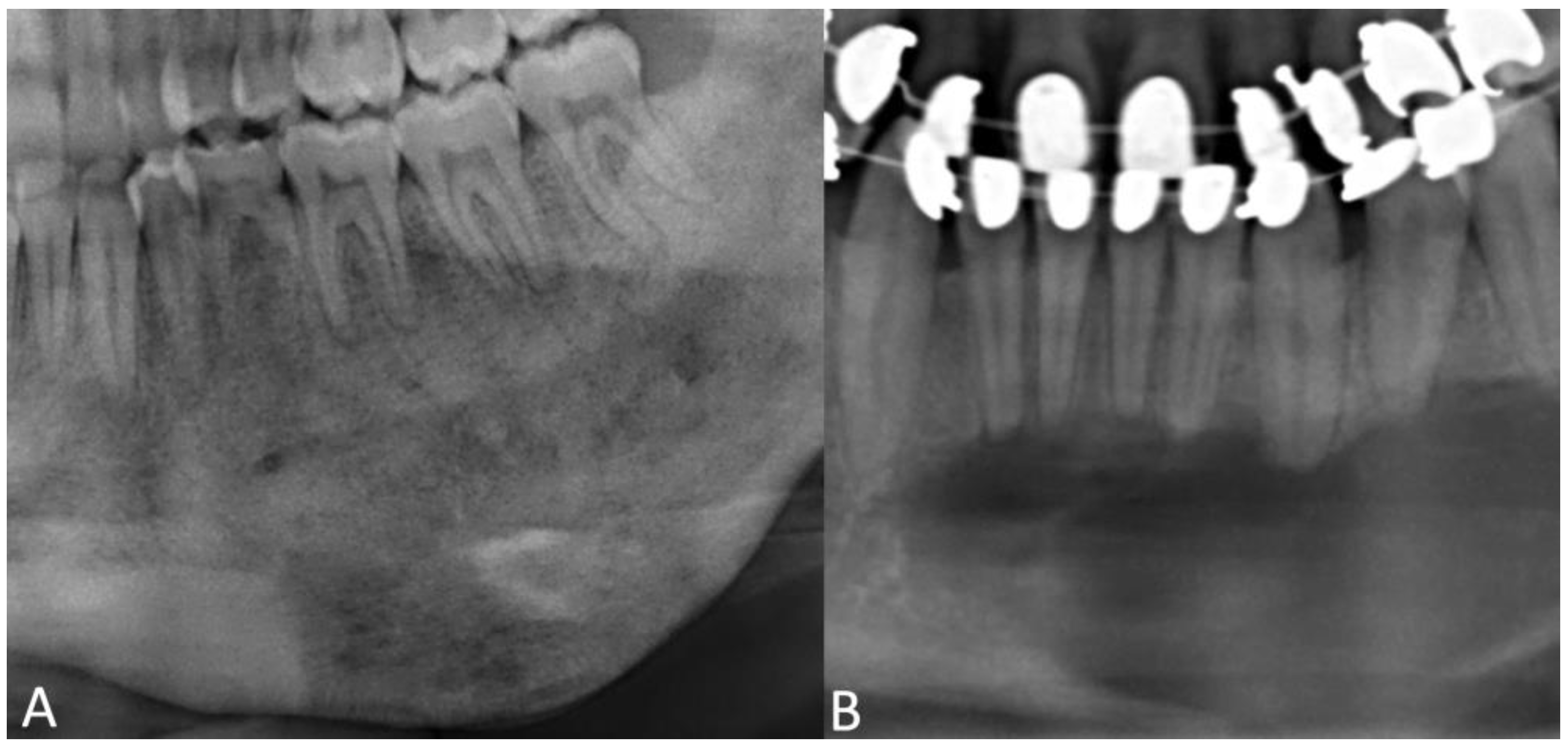
4.1. Radiographic Features
4.2. Histomorphology
4.3. Differential Diagnosis and Malignant Transformation
5. Treatment Options
5.1. Surgical Approaches
5.2. Nonsurgical Approaches
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kruse, A.; Pieles, U.; Riener, M.O.; Zunker, C.; Bredell, M.G.; Grätz, K.W. Craniomaxillofacial fibrous dysplasia: A 10-year database 1996–2006. Br. J. Oral Maxillofac. Surg. 2009, 47, 302–305. [Google Scholar] [CrossRef] [Green Version]
- MacDonald, D.S. Maxillofacial fibro-osseous lesions. Clin. Radiol. 2015, 70, 25–36. [Google Scholar] [CrossRef]
- Alawi, F. Benign fibro-osseous diseases of the maxillofacial bones. A review and differential diagnosis. Am. J. Clin. Pathol. 2002, 118, S50–S70. [Google Scholar] [CrossRef]
- Davidova, L.A.; Bhattacharyya, I.; Islam, M.N.; Cohen, D.M.; Fitzpatrick, S.G. An Analysis of Clinical and Histopathologic Features of Fibrous Dysplasia of the Jaws: A Series of 40 Cases and Review of Literature. Head Neck Pathol. 2020, 14, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Alsharif, M.J.; Sun, Z.J.; Chen, X.M.; Wang, S.P.; Zhao, Y.F. Benign fibro-osseous lesions of the jaws: A study of 127 Chinese patients and review of the literature. Int. J. Surg. Pathol. 2009, 17, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Macdonald-Jankowski, D.S.; Li, T.K. Fibrous dysplasia in a Hong Kong community: The clinical and radiological features and outcomes of treatment. Dentomaxillofac. Radiol. 2009, 38, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; Venkatswamy, S.; Ramu, V.; Banu, K.; Ehtaih, S.; Kashyap, V.M. Craniofacial fibrous dysplasia: Surgery and literature review. Ann. Maxillofac. Surg. 2013, 3, 66–71. [Google Scholar] [CrossRef] [Green Version]
- Lustig, L.R.; Holliday, M.J.; McCarthy, E.F.; Nager, G.T. Fibrous dysplasia involving the skull base and temporal bone. Arch. Otolaryngol. Head Neck Surg. 2001, 127, 1239–1247. [Google Scholar] [CrossRef] [Green Version]
- Dalle Carbonare, M.; Manisali, M. Surgical management of syndromic versus non-syndromic craniofacial fibrous dysplasia: A systematic review and meta-analysis. Br. J. Oral Maxillofac. Surg. 2022, 60, 1166–1175. [Google Scholar] [CrossRef]
- Kolomvos, N.; Theologie-Lygidakis, N.; Christopoulos, P.; Iatrou, I. Benign fibro-osseous lesions of the jaws in children. A 12-year retrospective study. J. Craniomaxillofac. Surg. 2013, 41, 574–580. [Google Scholar] [CrossRef]
- Worawongvasu, R.; Songkampol, K. Fibro-osseous lesions of the jaws: An analysis of 122 cases in Thailand. J. Oral Pathol. Med. 2010, 39, 703–708. [Google Scholar] [CrossRef]
- Lee, J.S.; FitzGibbon, E.J.; Chen, Y.R.; Kim, H.J.; Lustig, L.R.; Akintoye, S.O.; Collins, M.T.; Kaban, L.B. Clinical guidelines for the management of craniofacial fibrous dysplasia. Orphanet J. Rare Dis. 2012, 7, S2. [Google Scholar] [CrossRef] [Green Version]
- Gupta, D.; Garg, P.; Mittal, A. Computed Tomography in Craniofacial Fibrous Dysplasia: A Case Series with Review of Literature and Classification Update. Open Dent. J. 2017, 11, 384–403. [Google Scholar] [CrossRef] [Green Version]
- Lisle, D.A.; Monsour, P.A.; Maskiell, C.D. Imaging of craniofacial fibrous dysplasia. J. Med. Imaging Radiat. Oncol. 2008, 52, 325–332. [Google Scholar] [CrossRef]
- Valentini, V.; Cassoni, A.; Terenzi, V.; Della Monaca, M.; Fadda, M.T.; Rajabtork Zadeh, O.; Raponi, I.; Anelli, A.; Iannetti, G. Our experience in the surgical management of craniofacial fibrous dysplasia: What has changed in the last 10 years? Acta Otorhinolaryngol. Ital. 2017, 37, 436–443. [Google Scholar] [CrossRef]
- Yetiser, S.; Gonul, E.; Tosun, F.; Tasar, M.; Hidir, Y. Monostotic craniofacial fibrous dysplasia: The Turkish experience. J. Craniofacial Surg. 2006, 17, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Ogunsalu, C.O.; Lewis, A.; Doonquah, L. Benign fibro-osseous lesions of the jaw bones in Jamaica: Analysis of 32 cases. Oral Dis. 2001, 7, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraj, C.P.; Srinivas, C.V. Craniofacial and monostotic variants of fibrous dysplasia affecting the maxillofacial region. J. Oral Maxillofac. Surg. Med. Pathol. 2014, 26, 424–431. [Google Scholar] [CrossRef]
- MacDonald-Jankowski, D. Fibrous dysplasia: A systematic review. Dentomaxillofac. Radiol. 2009, 38, 196–215. [Google Scholar] [CrossRef]
- Akintoye, S.O.; Lee, J.S.; Feimster, T.; Booher, S.; Brahim, J.; Kingman, A.; Riminucci, M.; Robey, P.G.; Collins, M.T. Dental characteristics of fibrous dysplasia and McCune-Albright syndrome. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 96, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Jiang, Y.; Chen, L. The Status of Affected Infraorbital Nerve and Inferior Alveolar Nerve in Patients With Jaw Fibrous Dysplasia: A Clinical and Radiographic Evaluation. J. Craniofac. Surg. 2017, 28, e566–e569. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.M., Jr.; Howell, R.E. Etiology of fibrous dysplasia and McCune-Albright syndrome. Int. J. Oral Maxillofac. Surg. 1999, 28, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Tabareau-Delalande, F.; de Pinieux, G. Molecular Markers of Fibro-Osseous Lesions and Osteosarcomas of the Craniofacial Complex—Current Situation and Recent Advances. Curr. Oral Health Rep. 2016, 3, 102–110. [Google Scholar] [CrossRef]
- Meier, M.E.; Clerkx, S.N.; Winter, E.M.; Pereira, A.M.; van de Ven, A.C.; van de Sande, M.A.J.; Appelman-Dijkstra, N.M. Safety of therapy with and withdrawal from denosumab in fibrous dysplasia and McCune-Albright syndrome: An observational study. J. Bone Min. Res. 2021, 36, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Perdigão, P.F.; Pimenta, F.J.; Castro, W.H.; De Marco, L.; Gomez, R.S. Investigation of the GSalpha gene in the diagnosis of fibrous dysplasia. Int. J. Oral Maxillofac. Surg. 2004, 33, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Isobe, Y.; Takahashi, K.; Kiso, H.; Nakao, K.; Ikeno, M.; Koyama, N.; Sugai, M.; Shimizu, A.; Haga, H.; Bessho, K. Direct evidence for the age-dependent demise of GNAS-mutated cells in oral fibrous dysplasia. Arch. Oral Biol. 2018, 93, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Shi, R.R.; Li, X.F.; Zhang, R.; Chen, Y.; Li, T.J. GNAS mutational analysis in differentiating fibrous dysplasia and ossifying fibroma of the jaw. Mod. Pathol. 2013, 26, 1023–1031. [Google Scholar] [CrossRef] [Green Version]
- Xue, J.; Jia, K.; Li, T.; Zhang, J.; An, J. GNAS mutation analysis assists in differentiating chronic diffuse sclerosing osteomyelitis from fibrous dysplasia in the jaw. Mod. Pathol. 2022, 35, 1334–1340. [Google Scholar] [CrossRef]
- Pick, E.; Schäfer, T.; Al-Haj Husain, A.; Rupp, N.J.; Hingsammer, L.; Valdec, S. Clinical, Radiological, and Pathological Diagnosis of Fibro-Osseous Lesions of the Oral and Maxillofacial Region: A Retrospective Study. Diagnostics 2022, 12, 238. [Google Scholar] [CrossRef]
- Akashi, M.; Matsuo, K.; Shigeoka, M.; Kakei, Y.; Hasegawa, T.; Tachibana, A.; Furudoi, S.; Komori, T. A Case Series of Fibro-Osseous Lesions of the Jaws. Kobe J. Med. Sci. 2017, 63, E73–E79. [Google Scholar]
- Speight, P.M.; Carlos, R. Maxillofacial fibro-osseous lesions. Curr. Diagn. Pathol. 2006, 12, 1–10. [Google Scholar] [CrossRef]
- Cordeiro, M.S.; Backes, A.R.; Júnior, A.F.; Gonçalves, E.H.; de Oliveira, J.X. Fibrous Dysplasia Characterization Using Lacunarity Analysis. J. Digit. Imaging 2016, 29, 134–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacDonald-Jankowski, D.S. Fibro-osseous lesions of the face and jaws. Clin. Radiol. 2004, 59, 11–25. [Google Scholar] [CrossRef] [PubMed]
- White, D.K.; Street, C.C.; Jenkins, W.S.; Clark, A.R.; Ford, J.E. Panoramic radiograph in pathology. Atlas Oral Maxillofac. Surg. Clin. North Am. 2003, 11, 1–53. [Google Scholar] [CrossRef]
- Chen, Y.R.; Wong, F.H.; Hsueh, C.; Lo, L.J. Computed tomography characteristics of non-syndromic craniofacial fibrous dysplasia. Change Gung Med. J. 2002, 25, 1–8. [Google Scholar]
- Özgür, A.; Kara, E.; Arpacı, R.; Arpacı, T.; Esen, K.; Kara, T.; Duce, M.N.; Apaydın, F.D. Nonodontogenic mandibular lesions: Differentiation based on CT attenuation. Diagn. Interv. Radiol. 2014, 20, 475–480. [Google Scholar] [CrossRef] [Green Version]
- Soluk-Tekkesin, M.; Sinanoglu, A.; Selvi, F.; Karabas, H.C.; Aksakalli, N. The importance of clinical and radiological findings for the definitive histopathologic diagnosis of benign fibro-osseous lesions of the jaws: Study of 276 cases. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.; Gomes, C.C.; Brennan, P.A.; Fonseca, F.P.; Gomez, R.S. Fibrous dysplasia of the jaws: Integrating molecular pathogenesis with clinical, radiological, and histopathological features. J. Oral Pathol. Med. 2019, 48, 3–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, R.I.; de Almeida, S.M.; Bóscolo, F.N.; Santos, A.O.; Camargo, E.E. Bone scintigraphy as an adjunct for the diagnosis of oral diseases. J. Dent. Educ. 2002, 66, 1381–1387. [Google Scholar] [CrossRef]
- MacDonald-Jankowski, D.S.; Yeung, R.; Li, T.K.; Lee, K.M. Computed tomography of fibrous dysplasia. Dentomaxillofac. Radiol. 2004, 33, 114–118. [Google Scholar] [CrossRef]
- Harmon, M.; Arrigan, M.; Toner, M.; O’Keeffe, S.A. A radiological approach to benign and malignant lesions of the mandible. Clin. Radiol. 2015, 70, 335–350. [Google Scholar] [CrossRef]
- Shmuly, T.; Allon, D.M.; Vered, M.; Chaushu, G.; Shlomi, B.; Kaplan, I. Can Differences in Vascularity Serve as a Diagnostic Aid in Fibro-Osseous Lesions of the Jaws? J. Oral Maxillofac. Surg. 2017, 75, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, R.R.; Sarvade, S.D.; Boaz, K.; Srikant, N.; Nandita, K.P.; Lewis, A.J. Polarizing and light microscopic analysis of mineralized components and stromal elements in fibrous ossifying lesions. J. Clin. Diagn. Res. 2014, 8, ZC42–ZC45. [Google Scholar] [CrossRef]
- Elias, L.S.; Costa, R.F.; Carvalho, M.A.; Batista, A.C.; Silva, T.A.; Leles, C.R.; Mendonça, E.F. Markers of bone remodeling in neoplastic and bone-related lesions. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 110, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Toyosawa, S.; Yuki, M.; Kishino, M.; Ogawa, Y.; Ueda, T.; Murakami, S.; Konishi, E.; Iida, S.; Kogo, M.; Komori, T.; et al. Ossifying fibroma vs fibrous dysplasia of the jaw: Molecular and immunological characterization. Mod. Pathol. 2007, 20, 389–396. [Google Scholar] [CrossRef] [Green Version]
- WHO Classification of Tumours Editorial Board. Head and Neck Tumours, 5th ed.; WHO Classification of Tumours Series; International Agency for Research on Cancer: Lyon, France, 2022; Volume 9, Available online: https://publications.iarc.fr/ (accessed on 16 May 2023).
- Pannone, G.; Nocini, R.; Santoro, A.; Spirito, F.; Nocini, P.F.; Papagerakis, S.; Franceschi, R.T.; Di Domenico, M.; Di Carlo, A.; Danelia, N.; et al. Expression of Beta-Catenin, Cadherins and P-Runx2 in Fibro-Osseous Lesions of the Jaw: Tissue Microarray Study. Biomolecules 2022, 12, 587. [Google Scholar] [CrossRef]
- Horvai, A.E.; Jordan, R.C. Fibro-osseous lesions of the craniofacial bones: β-catenin immunohistochemical analysis and CTNNB1 and APC mutation analysis. Head Neck Pathol. 2014, 8, 291–297. [Google Scholar] [CrossRef] [Green Version]
- Ma, M.; Liu, L.; Shi, R.; Zhang, J.; Li, X.; Li, X.; Bai, J.; Wang, J.; Huang, Y.; Li, T. Copy number alteration profiling facilitates differential diagnosis between ossifying fibroma and fibrous dysplasia of the jaws. Int. J. Oral Sci. 2021, 13, 21. [Google Scholar] [CrossRef]
- Jia, K.; Li, X.; An, J.; Zhang, Y. Comparing Clinical and Radiographic Characteristics of Chronic Diffuse Sclerosing Osteomyelitis and Craniofacial Fibrous Dysplasia in the Mandible. J. Oral Maxillofac. Surg. 2021, 79, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, E.F. Fibro-osseous lesions of the maxillofacial bones. Head Neck Pathol. 2013, 7, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Delozier, J.B., 3rd; Egger, M.E.; Bottomy, M.B. Infantile fibrous dysplasia of the mandible. J. Craniofac. Surg. 2004, 15, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Kueper, J.; Tsimbal, C.; Olsen, B.R.; Kaban, L.; Liao, E.C. SH3BP2-related fibro-osseous disorders of the maxilla and mandible: A systematic review. Int. J. Oral Maxillofac. Surg. 2022, 51, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Li, X.; Zhang, J.; Chen, F.; Ma, M.; Feng, Y.; Li, T. Clinicopathological and genetic study of a rare occurrence: Malignant transformation of fibrous dysplasia of the jaws. Mol. Genet. Genom. Med. 2022, 10, e1861. [Google Scholar] [CrossRef]
- Yap, F.H.X.; Amanuel, B.; Van Vliet, C.; Thomas, M.; Wong, D. Malignant transformation of fibrous dysplasia into osteosarcoma confirmed with TP53 somatic mutation and mutational analysis of GNAS gene. Pathology 2021, 53, 652–654. [Google Scholar] [CrossRef] [PubMed]
- Brannon, R.B.; Fowler, C.B. Benign fibro-osseous lesions: A review of current concepts. Adv. Anat. Pathol. 2001, 8, 126–143. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.R.; Noordhoff, M.S. Treatment of craniomaxillofacial fibrous dysplasia: How early and how extensive? Plast. Reconstr. Surg. 1991, 87, 799–800. [Google Scholar] [CrossRef] [PubMed]
- Valentini, V.; Cassoni, A.; Marianetti, T.M.; Terenzi, V.; Fadda, M.T.; Iannetti, G. Craniomaxillofacial fibrous dysplasia: Conservative treatment or radical surgery? A retrospective study on 68 patients. Plast. Reconstr. Surg. 2009, 123, 653–660. [Google Scholar] [CrossRef]
- Fattah, A.; Khechoyan, D.; Phillips, J.H.; Forrest, C.R. Paediatric craniofacial fibrous dysplasia: The Hospital for Sick Children experience and treatment philosophy. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, 1346–1355. [Google Scholar] [CrossRef]
- Pacino, G.A.; Cocuzza, S.; Tonoli, G.; Boscolo Rizzo, P.; Tirelli, G.; Tofanelli, M.; Ciprandi, G.; La Mantia, I.; Maniaci, A.; Da Mosto, M.C.; et al. Jawbone fibrous dysplasia: Retrospective evaluation in a cases series surgically treated and short review of the literature. Acta Biomed. 2020, 92, e2021018. [Google Scholar] [CrossRef]
- Connolly, C.; Sabarigirivasan, V.; Cottone, L.; Flanagan, A.M.; Tirabosco, R. An overview and update on bone lesion in craniofacial bones. Diagn. Histopathol. 2021, 27, 216–225. [Google Scholar] [CrossRef]
- Huang, D.; Chen, M.; He, D.; Yang, C.; Yuan, J.; Bai, G.; Wang, Y.; Wei, W.; Chen, Z. Preservation of the inferior alveolar neurovascular bundle in the osteotomy of benign lesions of the mandible using a digital template. Br. J. Oral Maxillofac. Surg. 2015, 53, 637–641. [Google Scholar] [CrossRef]
- Yu, H.; Shen, S.G.; Wang, X.; Zhang, L.; Zhang, S. The indication and application of computer-assisted navigation in oral and maxillofacial surgery-Shanghai’s experience based on 104 cases. J. Craniomaxillofac. Surg. 2013, 41, 770–774. [Google Scholar] [CrossRef]
- Ni, Y.; Yang, Z.; Xu, H.; Lu, P.; Wang, W.; Liu, F. Assessment of preoperative and postoperative quality of life of 24 patients with fibrous dysplasia of the mandible. Br. J. Oral Maxillofac. Surg. 2019, 57, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Glied, A. Medication Management of Selected Pathological Jaw Lesions. Oral Maxillofac. Surg. Clin. North. Am. 2022, 34, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Boyce, A.M.; Tosi, L.L.; Paul, S.M. Bisphosphonate treatment for children with disabling conditions. PM R 2014, 6, 427–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majoor, B.C.; Appelman-Dijkstra, N.M.; Fiocco, M.; van de Sande, M.A.; Dijkstra, P.S.; Hamdy, N.A. Outcome of Long-Term Bisphosphonate Therapy in McCune-Albright Syndrome and Polyostotic Fibrous Dysplasia. J. Bone Min. Res. 2017, 32, 264–276. [Google Scholar] [CrossRef] [Green Version]
- Pan, K.S.; Boyce, A.M. Denosumab Treatment for Giant Cell Tumors, Aneurysmal Bone Cysts, and Fibrous Dysplasia—Risks and Benefits. Curr. Osteoporos. Rep. 2021, 19, 141–150. [Google Scholar] [CrossRef]
- Simm, P.J.; Biggin, A.; Zacharin, M.R.; Rodda, C.P.; Tham, E.; Siafarikas, A.; Jefferies, C.; Hofman, P.L.; Jensen, D.E.; Woodhead, H.; et al. Consensus guidelines on the use of bisphosphonate therapy in children and adolescents. J. Paediatr. Child Health 2018, 54, 223–233. [Google Scholar] [CrossRef]
- Kos, M.; Luczak, K.; Godzinski, J.; Klempous, J. Treatment of monostotic fibrous dysplasia with pamidronate. J. Craniomaxillofac. Surg. 2004, 32, 10–15. [Google Scholar] [CrossRef]
- Chapurlat, R.; Legrand, M.A. Bisphosphonates for the treatment of fibrous dysplasia of bone. Bone 2021, 143, 115784. [Google Scholar] [CrossRef]
- Otto, S.; Troeltzsch, M.; Burian, E.; Mahaini, S.; Probst, F.; Pautke, C.; Ehrenfeld, M.; Smolka, W. Ibandronate treatment of diffuse sclerosing osteomyelitis of the mandible: Pain relief and insight into pathogenesis. J. Craniomaxillofac. Surg. 2015, 43, 1837–1842. [Google Scholar] [CrossRef]
- Nadella, S.; Mupparapu, M.; Akintoye, S.O. Risk of developing spontaneous MRONJ in fibrous dysplasia patients treated with bisphosphonates: A systematic review of the literature. Quintessence Int. 2022, 53, 616–623. [Google Scholar] [CrossRef]
- Tessaris, D.; Matarazzo, P.; Lala, R.; Defabianis, P. Odontoiatric perspectives and osteonecrosis of the jaw as a possible adverse effect of bisphosphonates therapy in fibrous dysplasia and McCune-Albright syndrome. J. Pediatr. Endocrinol. Metab. 2016, 29, 333–336. [Google Scholar] [CrossRef]
- Ngan, K.K.; Bowe, J.; Goodger, N. The risk of bisphosphonate-related osteonecrosis of the jaw in children. A case report and literature review. Dent. Update 2013, 40, 733–734, 736–738. [Google Scholar] [CrossRef]
- Hess, L.M.; Jeter, J.M.; Benham-Hutchins, M.; Alberts, D.S. Factors associated with osteonecrosis of the jaw among bisphosphonate users. Am. J. Med. 2008, 121, 475–483.e473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mäkitie, A.A.; Törnwall, J.; Mäkitie, O. Bisphosphonate treatment in craniofacial fibrous dysplasia--a case report and review of the literature. Clin. Rheumatol. 2008, 27, 809–812. [Google Scholar] [CrossRef]
- Polyzos, S.A.; Makras, P.; Tournis, S.; Anastasilakis, A.D. Off-label uses of denosumab in metabolic bone diseases. Bone 2019, 129, 115048. [Google Scholar] [CrossRef]
- Palmisano, B.; Spica, E.; Remoli, C.; Labella, R.; Di Filippo, A.; Donsante, S.; Bini, F.; Raimondo, D.; Marinozzi, F.; Boyde, A.; et al. RANKL Inhibition in Fibrous Dysplasia of Bone: A Preclinical Study in a Mouse Model of the Human Disease. J. Bone Min. Res. 2019, 34, 2171–2182. [Google Scholar] [CrossRef]
- Boyce, A.M.; Chong, W.H.; Yao, J.; Gafni, R.I.; Kelly, M.H.; Chamberlain, C.E.; Bassim, C.; Cherman, N.; Ellsworth, M.; Kasa-Vubu, J.Z.; et al. Denosumab treatment for fibrous dysplasia. J. Bone Min. Res. 2012, 27, 1462–1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ukarapong, S.; Seeherunvong, T.; Berkovitz, G. Current and Emerging Therapies for Pediatric Bone Diseases. Clin. Rev. Bone Miner. Metab. 2020, 18, 31–42. [Google Scholar] [CrossRef]
- Akintoye, S.O.; Otis, L.L.; Atkinson, J.C.; Brahim, J.; Kushner, H.; Robey, P.G.; Collins, M.T. Analyses of variable panoramic radiographic characteristics of maxillo-mandibular fibrous dysplasia in McCune-Albright syndrome. Oral Dis. 2004, 10, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.E. Structure and mechanism of alkaline phosphatase. Annu. Rev. Biophys. Biomol. Struct. 1992, 21, 441–483. [Google Scholar] [CrossRef] [PubMed]
- Onyebuchi, E.P.; Ajike, S.O.; Yusuf, R.; Fomete, B. Alkaline Phosphatase Profile of Patients with Fibro-Osseous Lesions. Iran. J. Otorhinolaryngol. 2022, 34, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Park, B.Y.; Cheon, Y.W.; Kim, Y.O.; Pae, N.S.; Lee, W.J. Prognosis for craniofacial fibrous dysplasia after incomplete resection: Age and serum alkaline phosphatase. Int. J. Oral Maxillofac. Surg. 2010, 39, 221–226. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obermeier, K.T.; Hartung, J.T.; Hildebrandt, T.; Dewenter, I.; Smolka, W.; Hesse, E.; Fegg, F.; Otto, S.; Malenova, Y.; Abdullah, A. Fibrous Dysplasia of the Jaw: Advances in Imaging and Treatment. J. Clin. Med. 2023, 12, 4100. https://doi.org/10.3390/jcm12124100
Obermeier KT, Hartung JT, Hildebrandt T, Dewenter I, Smolka W, Hesse E, Fegg F, Otto S, Malenova Y, Abdullah A. Fibrous Dysplasia of the Jaw: Advances in Imaging and Treatment. Journal of Clinical Medicine. 2023; 12(12):4100. https://doi.org/10.3390/jcm12124100
Chicago/Turabian StyleObermeier, Katharina Theresa, Jens Tobias Hartung, Tim Hildebrandt, Ina Dewenter, Wenko Smolka, Eric Hesse, Florian Fegg, Sven Otto, Yoana Malenova, and Anusha Abdullah. 2023. "Fibrous Dysplasia of the Jaw: Advances in Imaging and Treatment" Journal of Clinical Medicine 12, no. 12: 4100. https://doi.org/10.3390/jcm12124100
APA StyleObermeier, K. T., Hartung, J. T., Hildebrandt, T., Dewenter, I., Smolka, W., Hesse, E., Fegg, F., Otto, S., Malenova, Y., & Abdullah, A. (2023). Fibrous Dysplasia of the Jaw: Advances in Imaging and Treatment. Journal of Clinical Medicine, 12(12), 4100. https://doi.org/10.3390/jcm12124100





