Electrocardiogram Changes in the Postictal Phase of Epileptic Seizure: Results from a Prospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Enrollment and Clinical Data
2.2. Comparison of ECGs and Selection Criteria for ECG Changes
- BEP: increase in ST voltage of ST voltage ≥2 mV or prolongation of ST pattern >40 ms.
- QTc prolongation: QTc increase ≥40 ms.
- ERP: ST voltage increase ≥2 mV.
- Right precordial abnormalities: BBD prolongation ≥40 ms, delta-wave voltage increase ≥2 mV, increase in QRS fragmentation wide ≥40 ms.
2.3. Blood Sample Collection, DNA Extraction and Exome Sequencing
2.4. Statistical Analysis
3. Results
3.1. Study Population
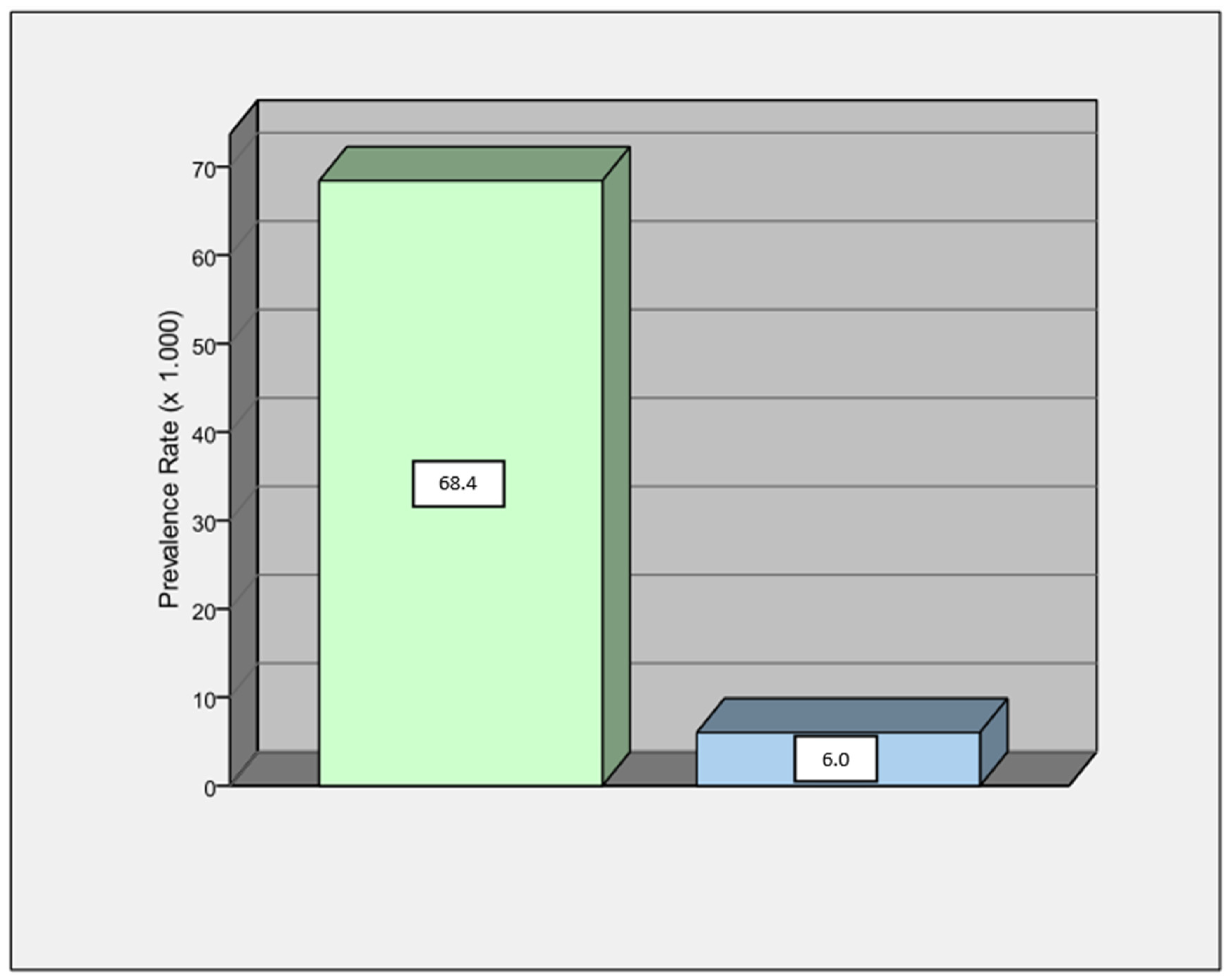
3.2. ECG Alterations
3.3. Post-Ictal and Basal ECG Comparisons
3.4. NGS Results
4. Discussion
4.1. Main Findings
- Patients with epilepsy have a high prevalence of ECG abnormalities, especially QTc alterations.
- Overall, the post-ictal ECG showed more marked abnormalities than the basal ECG (p = 0.003).
- A BEP of any type was significantly higher in the post-ictal ECG in comparison with the basal ECG (p = 0.04).
- There was a relationship between seizure onset during sleep and the presence of BEP on the post-ictal ECG (p = 0.04).
- In patients with ECG alterations diagnostic for myocardial channelopathy (BrS and ERP), only in early ECG, a pathogenic gene variant was identified.
4.2. Arrhythmogenic Diseases in Patients with Epilepsy
4.3. The Integrative Role of Post-Ictal ECG in Patients with Epilepsy
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samuels, M.A. The Brain–Heart Connection. Circulation 2007, 116, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Costagliola, G.; Orsini, A.; Coll, M.; Brugada, R.; Parisi, P.; Striano, P. The brain-heart interaction in epilepsy: Implications for diagnosis, therapy, and SUDEP prevention. Ann. Clin. Transl. Neurol. 2021, 8, 1557–1568. [Google Scholar] [CrossRef] [PubMed]
- Sevcencu, C.; Struijk, J.J. Autonomic alterations and cardiac changes in epilepsy. Epilepsia 2010, 51, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Asadi-Pooya, A.A.; Emami, M.; Sperling, M.R. Age of onset in idiopathic (genetic) generalized epilepsies: Clinical and EEG findings in various age groups. Seizure 2012, 21, 417–421. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.B. Channelopathies. Korean J. Pediatr. 2014, 57, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Ohno, S.; Nagaoka, I.; Fukuyama, M.; Kimura, H.; Itoh, H.; Makiyama, T.; Shimizu, A.; Horie, M. Age-Dependent Clinical and Genetic Characteristics in Japanese Patients with Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia. Circ. J. 2013, 77, 1534–1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nashef, L. Sudden unexpected death in epilepsy: Terminology and definitions. Epilepsia 1997, 38, S6–S8. [Google Scholar] [CrossRef]
- Eckhardt, L.L. Monomorphic ventricular tachycardia in Brugada syndrome: True-true but related? Heart Rhythm. 2016, 13, 683–685. [Google Scholar] [CrossRef] [Green Version]
- Nashef, L.; Ryvlin, P. Sudden unexpected death in epilepsy (SUDEP): Update and reflections. Neurol. Clin. 2009, 27, 1063–1074. [Google Scholar] [CrossRef]
- Van Gorp, V.; Danschutter, D.; Huyghens, L.; Hachimi-Idrissi, S.; Sarkozy, A.; Chierchia, G.B.; Henkens, S.; Brugada, P. Monitoring the safety of antiepileptic medication in a child with Brugada syndrome. Int. J. Cardiol. 2010, 145, e64–e67. [Google Scholar] [CrossRef]
- Parisi, P.; Oliva, A.; Coll Vidal, M.; Partemi, S.; Campuzano, O.; Iglesias, A.; Pisani, D.; Pascali, V.L.; Paolino, M.C.; Villa, M.P.; et al. Coexistence of epilepsy and Brugada syndrome in a family with SCN5A mutation. Epilepsy Res. 2013, 105, 415–418. [Google Scholar] [CrossRef]
- Antzelevitch, C.; Brugada, P.; Borggrefe, M.; Brugada, J.; Brugada, R.; Corrado, D.; Gussak, I.; LeMarec, H.; Nademanee, K.; Perez Riera, A.R.; et al. Brugada syndrome: Report of the second consensus conference: Endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation 2005, 111, 659–670. [Google Scholar] [CrossRef] [Green Version]
- Makielski, J.C. SIDS: Genetic and environmental influences may cause arrhythmia in this silent killer. J. Clin. Investig. 2006, 116, 297–299. [Google Scholar] [CrossRef]
- Keilson, M.J.; Hauser, W.A.; Magrill, J.P.; Goldman, M. ECG abnormalities in patients with epilepsy. Neurology 1987, 37, 1624. [Google Scholar] [CrossRef]
- Keilson, M.J.; Hauser, W.A.; Magrill, J.P. Electrocardiographic Changes During Electrographic Seizures. Arch. Neurol. 1989, 46, 1169–1170. [Google Scholar] [CrossRef]
- Gigli, L.; Bertero, G.; Vidal, M.C.; Iglesias, A.; Campuzano, O.; Striano, P.; Oliva, A.; Brugada, R. Juvenile myoclonic epilepsy and Brugada type 1 ECG pattern associated with (a novel) plakophillin 2 mutation. J. Neurol. 2017, 264, 792–795. [Google Scholar] [CrossRef] [PubMed]
- Brotherstone, R.; Blackhall, B.; McLellan, A. Lengthening of corrected QT during epileptic seizures. Epilepsia 2010, 51, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Mazzaccara, C.; Lombardi, R.; Mirra, B.; Barretta, F.; Esposito, M.V.; Uomo, F.; Caiazza, M.; Monda, E.; Losi, M.A.; Limongelli, G.; et al. Next-Generation Sequencing Gene Panels in Inheritable Cardiomyopathies and Channelopathies: Prevalence of Pathogenic Variants and Variants of Unknown Significance in Uncommon Genes. Biomolecules 2022, 12, 1417. [Google Scholar] [CrossRef] [PubMed]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart. J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef]
- Alfares, A.A.; Kelly, M.A.; McDermott, G.; Funke, B.H.; Lebo, M.S.; Baxter, S.B.; Shen, J.; McLaughlin, H.M.; Clark, E.H.; Babb, L.J.; et al. Results of clinical genetic testing of 2,912 probands with hypertrophic cardiomyopathy: Expanded panels offer limited additional sensitivity. Genet. Med. 2015, 17, 880–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brion, M.; Sobrino, B.; Martinez, M.; Blanco-Verea, A.; Carracedo, A. Massive parallel sequencing applied to the molecular autopsy in sudden cardiac death in the young. Forensic. Sci. Int. Genet 2015, 18, 160–170. [Google Scholar] [CrossRef]
- King, A.P.; Eckersley, R.J. (Eds.) Statistics for Biomedical Engineers and Scientists; Academic Press: Cambridge, MA, USA, 2019; pp. 147–171. [Google Scholar]
- Rochon, J.; Gondan, M.; Kieser, M. To test or not to test: Preliminary assessment of normality when comparing two independent samples. BMC Med. Res. Methodol. 2012, 12, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vutthikraivit, W.; Rattanawong, P.; Putthapiban, P.; Sukhumthammarat, W.; Vathesatogkit, P.; Ngarmukos, T.; Thakkinstian, A. Worldwide Prevalence of Brugada Syndrome: A Systematic Review and Meta-Analysis. Acta Cardiol. Sin. 2018, 34, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Sadrnia, S.; Yousefi, P.; Jalali, L. Correlation between seizure in children and prolonged QT interval. ARYA Atheroscler. 2013, 9, 7–10. [Google Scholar] [PubMed]
- Feldman, A.E.; Gidal, B.E. QTc prolongation by antiepileptic drugs and the risk of torsade de pointes in patients with epilepsy. Epilepsy Behav. 2013, 26, 421–426. [Google Scholar] [CrossRef]
- Brugada, J.; Campuzano, O.; Arbelo, E.; Sarquella-Brugada, G.; Brugada, R. Present Status of Brugada Syndrome. J. Am. Coll. Cardiol. 2018, 72, 1046–1059. [Google Scholar] [CrossRef]
- Bezzina, C.R.; Lahrouchi, N.; Priori, S.G. Genetics of Sudden Cardiac Death. Circ. Res. 2015, 116, 1919–1936. [Google Scholar] [CrossRef]
- Ufongene, C.; El Atrache, R.; Loddenkemper, T.; Meisel, C. Electrocardiographic changes associated with epilepsy beyond heart rate and their utilization in future seizure detection and forecasting methods. Clin. Neurophysiol. 2020, 131, 866–879. [Google Scholar] [CrossRef]
- Kishk, N.A.; Sharaf, Y.; Ebraheim, A.M.; Baghdady, Y.; Alieldin, N.; Afify, A.; Eldamaty, A. Interictal cardiac repolarization abnormalities in people with epilepsy. Epilepsy Behav. 2018, 79, 106–111. [Google Scholar] [CrossRef]
- Zijlmans, M.; Flanagan, D.; Gotman, J. Heart rate changes and ECG abnormalities during epileptic seizures: Prevalence and definition of an objective clinical sign. Epilepsia 2002, 43, 847–854. [Google Scholar] [CrossRef] [Green Version]
- Opherk, C.; Coromilas, J.; Hirsch, L.J. Heart rate and EKG changes in 102 seizures: Analysis of influencing factors. Epilepsy Res. 2002, 52, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Druschky, A.; Hilz, M.J.; Hopp, P.; Platsch, G.; Radespiel-Tröger, M.; Druschky, K.; Kuwert, T.; Stefan, H.; Neundörfer, B. Interictal cardiac autonomic dysfunction in temporal lobe epilepsy demonstrated by [(123)I]metaiodobenzylguanidine-SPECT. Brain 2001, 124, 2372–2382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravindran, K.; Powell, K.L.; Todaro, M.; O’Brien, T.J. The pathophysiology of cardiac dysfunction in epilepsy. Epilepsy Res. 2016, 127, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Toffa, D.H.; Pana, R.; Nguyen, D.K. Ictal asystole with intercurrent cardiopathy: A complex combination leading to delayed diagnosis. Epileptic Disord. 2019, 21, 598–602. [Google Scholar] [CrossRef]
- Seo, M.H.; Sung, W.Y. A case of near-sudden unexpected death in epilepsy due to ventricular fibrillation. Open Access Emerg. Med. 2019, 11, 161–166. [Google Scholar] [CrossRef] [Green Version]
- Mori, S.; Hori, A.; Turker, I.; Inaji, M.; Bello-Pardo, E.; Miida, T.; Otomo, Y.; Ai, T. Abnormal Cardiac Repolarization After Seizure Episodes in Structural Brain Diseases: Cardiac Manifestation of Electrical Remodeling in the Brain? J. Am. Heart Assoc. 2021, 10, e019778. [Google Scholar] [CrossRef]
- Chahal, C.A.A.; Salloum, M.N.; Alahdab, F.; Gottwald, J.A.; Tester, D.J.; Anwer, L.A.; So, E.L.; Murad, M.H.; Louis, E.K.S.; Ackerman, M.J.; et al. Systematic Review of the Genetics of Sudden Unexpected Death in Epilepsy: Potential Overlap with Sudden Cardiac Death and Arrhythmia-Related Genes. J. Am. Heart Assoc. 2020, 9, e012264. [Google Scholar] [CrossRef]
- Behere, S.P.; Weindling, S.N. Inherited arrhythmias: The cardiac channelopathies. Ann. Pediatr. Cardiol. 2015, 8, 210–220. [Google Scholar] [CrossRef]
- Campuzano, O.; Beltrán-Álvarez, P.; Iglesias, A.; Scornik, F.; Pérez, G.; Brugada, R. Genetics and cardiac channelopathies. Genet. Med. 2010, 12, 260–267. [Google Scholar] [CrossRef] [Green Version]
- Nei, M. Cardiac effects of seizures. Epilepsy Curr. 2009, 9, 91–95. [Google Scholar] [CrossRef]
- Jansen, K.; Lagae, L. Cardiac changes in epilepsy. Seizure 2010, 19, 455–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, K.; Kohno, R.; Akamatsu, N.; Benditt, D.G.; Abe, H. Abnormal repolarization: A common electrocardiographic finding in patients with epilepsy. J. Cardiovasc. Electrophysiol. 2019, 30, 109–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stawicki, T.M.; Zhou, K.; Yochem, J.; Chen, L.; Jin, Y. TRPM channels modulate epileptic-like convulsions via systemic ion homeostasis. Curr. Biol. 2011, 21, 883–888. [Google Scholar] [CrossRef] [Green Version]
- Al-Khleaf, A.; Babi, A.; Jarjanazi, M.; Haddad, W. Sporadic and rapidly progressive arrhythmogenic right ventricular cardiomyopathy in a 12-year-old boy who was diagnosed with epilepsy. Oxf. Med. Case Rep. 2021, 2021, omab046. [Google Scholar] [CrossRef] [PubMed]
- Tomson, T.; Walczak, T.; Sillanpaa, M.; Sander, J.W. Sudden unexpected death in epilepsy: A review of incidence and risk factors. Epilepsia 2005, 46, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Dasheiff, R.M. Sudden unexpected death in epilepsy: A series from an epilepsy surgery program and speculation on the relationship to sudden cardiac death. J. Clin. Neurophysiol. 1991, 8, 216–222. [Google Scholar] [CrossRef]
- Tavernor, S.J.; Brown, S.W.; Tavernor, R.M.; Gifford, C. Electrocardiograph QT lengthening associated with epileptiform EEG discharges—A role in sudden unexplained death in epilepsy? Seizure 1996, 5, 79–83. [Google Scholar] [CrossRef] [Green Version]
- Kwon, S.; Lee, S.; Hyun, M.; Choe, B.H.; Kim, Y.; Park, W.; Cho, Y. The potential for QT prolongation by antiepileptic drugs in children. Pediatr. Neurol. 2004, 30, 99–101. [Google Scholar] [CrossRef]
- Tomson, T.; Nashef, L.; Ryvlin, P. Sudden unexpected death in epilepsy: Current knowledge and future directions. Lancet Neurol. 2008, 7, 1021–1031. [Google Scholar] [CrossRef]
- Espinosa, P.S.; Lee, J.W.; Tedrow, U.B.; Bromfield, E.B.; Dworetzky, B.A. Sudden unexpected near death in epilepsy: Malignant arrhythmia from a partial seizure. Neurology 2009, 72, 1702–1703. [Google Scholar] [CrossRef]
- Glasscock, E.; Yoo, J.W.; Chen, T.T.; Klassen, T.L.; Noebels, J.L. Kv1.1 potassium channel deficiency reveals brain-driven cardiac dysfunction as a candidate mechanism for sudden unexplained death in epilepsy. J. Neurosci. 2010, 30, 5167–5175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuele, S.U. Effects of seizures on cardiac function. J. Clin. Neurophysiol. 2009, 26, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.N.; Hofman, N.; Haglund, C.M.; Cascino, G.D.; Wilde, A.A.; Ackerman, M.J. Identification of a possible pathogenic link between congenital long QT syndrome and epilepsy. Neurology 2009, 72, 224–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Surges, R.; Adjei, P.; Kallis, C.; Erhuero, J.; Scott, C.A.; Bell, G.S.; Sander, J.W.; Walker, M.C. Pathologic cardiac repolarization in pharmacoresistant epilepsy and its potential role in sudden unexpected death in epilepsy: A case-control study. Epilepsia 2010, 51, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Lerche, H.; Shah, M.; Beck, H.; Noebels, J.; Johnston, D.; Vincent, A. Ion channels in genetic and acquired forms of epilepsy. J. Physiol. 2013, 591, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Koopmann, T.T.; Le Scouarnec, S.; Yang, T.; Ingram, C.R.; Schott, J.J.; Demolombe, S.; Probst, V.; Anselme, F.; Escande, D.; et al. Sodium channel β1 subunit mutations associated with Brugada syndrome and cardiac conduction disease in humans. J. Clin. Investig. 2008, 118, 2260–2268. [Google Scholar] [CrossRef] [Green Version]
- Tu, E.; Bagnall, R.D.; Duflou, J.; Semsarian, C. Post-mortem review and genetic analysis of sudden unexpected death in epilepsy (SUDEP) cases. Brain Pathol. 2011, 21, 201–208. [Google Scholar] [CrossRef]
- Aurlien, D.; Leren, T.P.; Taubøll, E.; Gjerstad, L. New SCN5A mutation in a SUDEP victim with idiopathic epilepsy. Seizure 2009, 18, 158–160. [Google Scholar] [CrossRef] [Green Version]
- Obeyesekere, M.N.; Klein, G.J.; Modi, S.; Leong-Sit, P.; Gula, L.J.; Yee, R.; Skanes, A.C.; Krahn, A.D. How to Perform and Interpret Provocative Testing for the Diagnosis of Brugada Syndrome, Long-QT Syndrome, and Catecholaminergic Polymorphic Ventricular Tachycardia. Circ. Arrhythmia Electrophysiol. 2011, 4, 958–964. [Google Scholar] [CrossRef] [Green Version]
- Corrado, D.; van Tintelen, P.J.; McKenna, W.J.; Hauer, R.N.W.; Anastastakis, A.; Asimaki, A.; Basso, C.; Bauce, B.; Brunckhorst, C.; Bucciarelli-Ducci, C.; et al. Arrhythmogenic right ventricular cardiomyopathy: Evaluation of the current diagnostic criteria and differential diagnosis. Eur. Heart J. 2019, 41, 1414–1429. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Falgueras, A.; Sarquella-Brugada, G.; Brugada, J.; Brugada, R.; Campuzano, O. Cardiac Channelopathies and Sudden Death: Recent Clinical and Genetic Advances. Biology 2017, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, A.; Clough, P.; Cooper, P.; Scheepers, B.; Fitzpatrick, A.P. Misdiagnosis of epilepsy: Many seizure-like attacks have a cardiovascular cause. J. Am. Coll. Cardiol. 2000, 36, 181–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandecasteele, K.; De Cooman, T.; Chatzichristos, C.; Cleeren, E.; Swinnen, L.; Macea Ortiz, J.; Van Huffel, S.; Dümpelmann, M.; Schulze-Bonhage, A.; De Vos, M.; et al. The power of ECG in multimodal patient-specific seizure monitoring: Added value to an EEG-based detector using limited channels. Epilepsia 2021, 62, 2333–2343. [Google Scholar] [CrossRef] [PubMed]
- Qaraqe, M.; Ismail, M.; Serpedin, E.; Zulfi, H. Epileptic seizure onset detection based on EEG and ECG data fusion. Epilepsy Behav. 2016, 58, 48–60. [Google Scholar] [CrossRef]
- Billeci, L.; Marino, D.; Insana, L.; Vatti, G.; Varanini, M. Patient-specific seizure prediction based on heart rate variability and recurrence quantification analysis. PLoS ONE 2018, 13, e0204339. [Google Scholar] [CrossRef] [Green Version]
- Lamberts, R.J.; Blom, M.T.; Novy, J.; Belluzzo, M.; Seldenrijk, A.; Penninx, B.W.; Sander, J.W.; Tan, H.L.; Thijs, R.D. Increased prevalence of ECG markers for sudden cardiac arrest in refractory epilepsy. J. Neurol. Neurosurg. Psychiatry 2015, 86, 309–313. [Google Scholar] [CrossRef] [Green Version]
- Abdelghani, M.S.; Chapra, A.; Asaad, N.; Hayat, S.A. Epilepsy and Brugada Syndrome: Association or Uncommon Presentation? Heart Views 2020, 21, 114–117. [Google Scholar] [CrossRef]
- Haugaa, K.H.; Vestervik, T.T.; Andersson, S.; Amlie, J.P.; Jørum, E.; Gjerstad, L.; Taubøll, E. Abnormal electroencephalograms in patients with long QT syndrome. Heart Rhythm. 2013, 10, 1877–1883. [Google Scholar] [CrossRef]


| Variable | Total |
|---|---|
| No. of patients | 117 |
| Age, median y (±IQR) | 48 ± 12 |
| Male (%) | 72 (62%) |
| Neurological characteristics | |
| Etiology of epilepsy (%) | |
| Idiopathic | 74 (63%) |
| Genetic | 1 (1%) |
| Structural | 42 (36%) |
| Neoplastic | 14 (12%) |
| Vascular | 23 (20%) |
| Dysplasia | 4 (4%) |
| Type of seizure (%) | |
| Generalized | 65 (56%) |
| Focal | 19 (16%) |
| Unknown | 33 (28%) |
| Seizure during sleep (%) | |
| Yes | 32 (27%) |
| No | 86 (74%) |
| EEG at time of admission (%) | |
| Not IEDs | 81 (69%) |
| IEDs | 36 (31%) |
| Ongoing AEDs | |
| Single | 40 (34%) |
| Multiple | 10 (9%) |
| None | 67 (57%) |
| Cardiological characteristics | |
| Hypertension | 33 (28%) |
| Diabetes Mellitus | 18 (15%) |
| CKD | 7 (6%) |
| CAD | 16 (14%) |
| SCD family history | 1 (1%) |
| Previous arrhythmias | 5 (4%) |
| Structural heart disease | 7 (6%) |
| Variables | Total (%) |
|---|---|
| Post-ictal ECG abnormalities | 52 (44%) |
| Rhythm disturbances | |
| Tachyarrhythmias | 3 (3%) |
| Bradyarrhythmias | 3 (3%) |
| Brugada pattern type I | 2 (2%) |
| Brugada pattern type II–III | 6 (4%) |
| QTc modifications | |
| QTc prolongation (>470 ms) | 20 (17%) |
| QTc prolongation (>500 ms) | 5 (4%) |
| QTc shortening (<340 ms) | 3 (3%) |
| ERP | 4 (3%) |
| Right precordial abnormalities | 5 (4%) |
| Basal ECG abnormalities | 28 (24%) |
| Rhythm disturbances | |
| Tachyarrhythmias | 2 (2%) |
| Bradyarrhythmias | 0 |
| Brugada pattern type I | 0 |
| Brugada pattern type II–III | 2 |
| QTc modifications | |
| QTc prolongation (>470 ms) | 14 (12%) |
| QTc prolongation (>500 ms) | 2 (2%) |
| QTc shortening (<340 ms) | 1 (1%) |
| ERP | 4 (3%) |
| Right precordial abnormalities | 3 (3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gigli, L.; Sala, S.; Preda, A.; Okubo, K.; Peretto, G.; Frontera, A.; Varrenti, M.; Baroni, M.; Carbonaro, M.; Vargiu, S.; et al. Electrocardiogram Changes in the Postictal Phase of Epileptic Seizure: Results from a Prospective Study. J. Clin. Med. 2023, 12, 4098. https://doi.org/10.3390/jcm12124098
Gigli L, Sala S, Preda A, Okubo K, Peretto G, Frontera A, Varrenti M, Baroni M, Carbonaro M, Vargiu S, et al. Electrocardiogram Changes in the Postictal Phase of Epileptic Seizure: Results from a Prospective Study. Journal of Clinical Medicine. 2023; 12(12):4098. https://doi.org/10.3390/jcm12124098
Chicago/Turabian StyleGigli, Lorenzo, Simone Sala, Alberto Preda, Kenji Okubo, Giovanni Peretto, Antonio Frontera, Marisa Varrenti, Matteo Baroni, Marco Carbonaro, Sara Vargiu, and et al. 2023. "Electrocardiogram Changes in the Postictal Phase of Epileptic Seizure: Results from a Prospective Study" Journal of Clinical Medicine 12, no. 12: 4098. https://doi.org/10.3390/jcm12124098
APA StyleGigli, L., Sala, S., Preda, A., Okubo, K., Peretto, G., Frontera, A., Varrenti, M., Baroni, M., Carbonaro, M., Vargiu, S., Di Resta, C., Striano, P., Mazzone, P., & Della Bella, P. (2023). Electrocardiogram Changes in the Postictal Phase of Epileptic Seizure: Results from a Prospective Study. Journal of Clinical Medicine, 12(12), 4098. https://doi.org/10.3390/jcm12124098









