1. Introduction
Currently, the observed increase in the incidence of obesity is caused by global development and progress in all areas of civilization, in particular economic, industrial, infrastructure, social and cultural. The prevalence of obesity in the last twenty years has reached the level of a global pandemic, has made it one of the main issues and directions of the activities of all world public health organizations and is treated as a civilization disease, with huge socioeconomic and psychosocial importance [
1]. Many years of research on obesity revealed its etiological and pathogenetic connection with many common diseases, such as cardiovascular disease, including atherosclerosis, hypertension, insulin-dependent diabetes, dyslipidemia, obstructive sleep apnea, bone and joint disease, depression, some types of cancer, reproductive system diseases and others [
2,
3].
The understanding of the relationship between obesity and other frequent metabolic co-morbidities has led to the definition of a new disease entity—metabolic syndrome. Currently applicable criteria for recognizing metabolic syndrome adopted by the International Diabetes Federation (IDF) and American Heart Association/National Heart, Lung and Blood Institute (AHA//NHLBI) in 2009 include abnormal waist circumference (depending on the population); abnormal triglyceride concentration (>150 mg/dL or the use of hypolipular treatment; abnormal HDL cholesterol fraction (<40 mg/dL (M), <50 mg/dL (K) or hypolipemic treatment used); abnormal arterial pressure (≥130/85 mm Hg or hypotensive treatment used); fasting glycemia (≥100 mg/dL) or use of hypoglycemic treatment. To recognize metabolic syndrome, the presence of three out of the five criteria [
4] should be determined.
Analysis of the issues of pathological obesity and metabolic syndrome in terms of co-morbidity leads to the conclusion that they are a significant factor promoting the development of cardiovascular diseases, increasing the risk of the occurrence of myocardial infarction 2.5-fold, causing a 1.5-fold increase in total mortality and 2-fold increase in the frequency of all cardiovascular incidents, including brain stroke [
5]. The proven fact is that the prevalence of this syndrome in the world population is increasing and reaches levels that allow us to treat it as a problem and disease threatening public health and civilization [
6]. Metabolic syndrome occurs in a world population with a frequency of about 20 to even 40%, depending on the analyzed region and/or ethnic group and the age of patients. The occurrence of MS has a tendency to grow over time and gives disturbing prognoses regarding the frequency of its occurrence in the future; it is more common in women than in men, and its frequency increases significantly with age [
7].
Lipid balance disorders are one of the main diseases coexisting with obesity and metabolic syndrome. Patients with abdominal obesity are more highly exposed to atherogenic dyslipidemia, which is associated with an increased risk of cardiovascular diseases with atherosclerotic disease and an increased risk of morbidity and mortality due to cardiovascular disease [
8,
9]. Research and treatment focus on improving lipid profiles in the pursuit of a potential reduction in diseases related to the cardiovascular system. To diagnose dyslipidemia in metabolic syndrome, practitioners are required to find disorder in triglyceride and lipoprotein concentrations in patient plasma: a TG concentration of ≥150 mg/dL and/or HDL concentration of <40 mg/dL (m) and <50 mg/dL (k). However, in the course of obesity and metabolic syndrome, deviations in the concentration and functions of other lipids are also observed, such as an increase in the concentration of very low lipoproteins of VLDL and chylomikrone, as well as of LDL.
The pathomechanism of the development of atherogenic dyslipidemia in metabolic syndrome is closely associated with insulin resistance and an excess of free fatty acids in the bloodstream. The accumulation of free fatty acids is the result of, among others, an intensified course of insulin resistance, lipolysis and their lowered uptake at the level of adipocytes. The excess of free fatty acids results in their accumulation in the liver, re-estrification into triglycerides and the intensified synthesis of TG-rich lipoprotein molecules with very-low-density VLDL [
10]. At the same time, the influx of lipids supplied through food is higher in the case of insulin resistance than that in healthy subjects, which results in the phenomenon of postprandial hyperlipidemia. Exogenous lipids enter the bloodstream in the form of chylomikrone molecules, produced in enterocytes. Postable hyperlipidemia is also closely related to changes in the course of insulin resistance, because chylomikrons and VLDL are used in the same metabolic pathways [
11].
The third factor increasing triglyceridemia is hepatic lipogenesis de novo, which is not inhibited in the case of insulin resistance and results in TG [
12] increases. Hypertriglyceridemia associated with a high concentration of VLDL-1 stimulates adverse, biochemical changes in HDL and LDL lipoproteins. The process of replacing cholesterol esters with HDL and LDL for triglycerides from VLDL takes place through the cholesteryl ester transfer protein (CETP). As a result, the amount of cholesterol esters increases in VLDL, and the number of triglycerides increases in HDL and LDL. Furthermore, in the liver through the hepatic lipase, “small and dense” HDL and LDL [
13] are created from these overloaded TG lipoproteins. Structurally abnormal HDL loses the possibility of cholesterol return transport from tissues (including blood vessels) to the liver, and also has a high plasma clearance, which is why it is quickly removed from the bloodstream and its concentration decreases. Small and dense LDL (SDLDL), on the other hand, has less affinity for receptors on hepatocytes than does normal LDL, which causes its longer maintenance in plasma [
10].
2. Material and Methods
The aim of the study was to assess the effectiveness of LSG in the treatment of obesity, weight loss based on BMI, %TWL, %EWL, %EBMI examined after 1, 3, 6 and 12 months of observation and the assessment of LSG effectiveness in the treatment of dyslipidemia based on a change in total cholesterol, LDL, HDL, non-HDL and triglyceride concentrations.
Demographic, biometric and clinical data on patients from the study group were obtained prospectively on the day of surgery and as part of outpatient postoperative visits, which patients attended 1 month, 3 months, 6 months and 1 year after LSG. During these visits, blood samples were also taken in order to analyze laboratory and biochemical parameters.
The study group included 196 patients who underwent laparoscopic sleeve gastrectomy in our Department in 2016–2020 with complete data gathered during 12-month postoperative observation. A total of 107 men (54.6%) and 89 women (45.4%) were included in the study. The average age of patients in the study group was 44.9 years; the youngest patient was 21 years old, and the oldest was 66 years old. The median BMI value on the day of the operation was 47.7 kg/m2. A total of 30 (15.3%) patients reported hypercholesterolaemia as a comorbidity upon preoperative examination. In total, 43 (21.9%) patients were taking hypolipidemic drugs before surgery.
In terms of the statistical analysis, collected data were analyzed using STATA 13.0 software. Measurable variables observed in the study group for individual parameters subjected to statistical analysis are presented as averages with standard deviations (SD) or as a medians with interquartile intervals, according to each case. Statistical comparisons of measurable variables were conducted using repetitive measurements with a Wilcoxon test with multiple comparisons of post hoc variables. Pearson’s coefficient was used to evaluate the correlation of variables. Analyzed variables were considered statistically significant at the level of significance of p ≤ 0.05.
3. Results
The weight reduction rate as a result of LSG, analyzed on the basis of changes in bariatric parameters in the intervals of postoperative observation, is presented in
Table 1. The results of body weight reduction analysis observed after laparoscopic sleeve gastrectomy are also reported. At the end of the 1-year follow up, the average BMI in the study group was 33.7 kg/m
2.
The dynamics of weight loss in the group were similar in the observation intervals between 0 and 1 month, 1 and 3 months and 3 and 6 months, while in the interval between 6 and 12 months it slightly decelerated. Throughout the postoperative observation, a positive trend was maintained for body weight reduction, which was expressed as a negative trend of mean BMI values and positive trends of increases in the values of %TWL, %EWL and %EBL. Operated patients within 1 year after the procedure lost an average of about 1/3 of their total, initial body weight, which accounted for about 66% of their excess BMI resulting from obesity.
Furthermore, the plasma lipidogram was analyzed. For this purpose, repetitive determinations were conducted on total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL-C), low-density lipoprotein (LDL-C) and the level of all lipoproteins associated with AP-B (non-HDL = TC-HDL-C) during the follow-up visits of patients in the 1st, 3rd, 6th and 12th month after laparoscopic sleeve surgery.
Before operation, only 30 (15.3%) patients reported dyslipidemia as a comorbidity but 86 (43.9%) patients had a HDL concentration of < 50 mg/dL (M) or a HDL concentration of < 40 mg/dL (K) and 112 (57.1%) had a triglyceride concentration of >150 mg/dL.
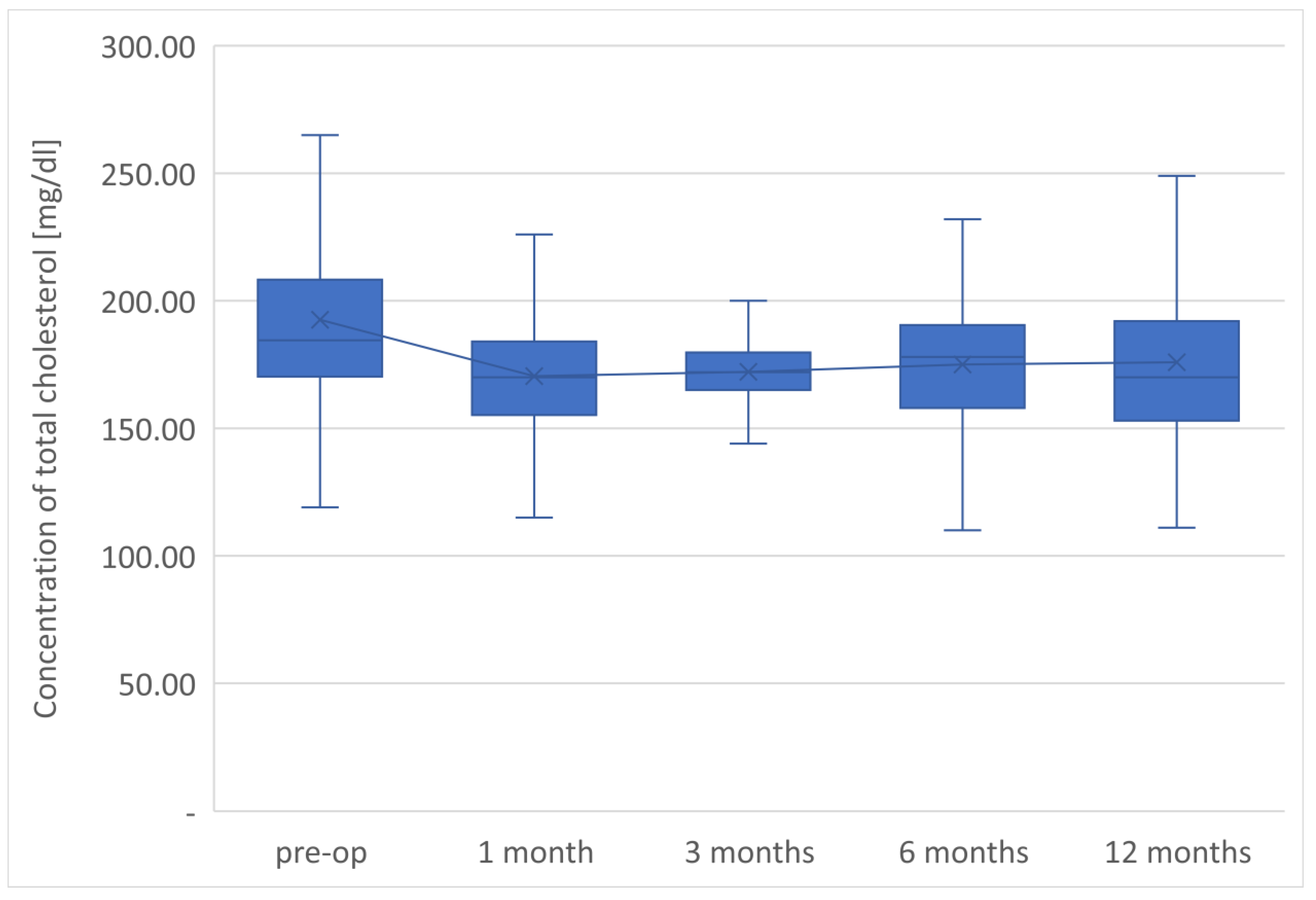
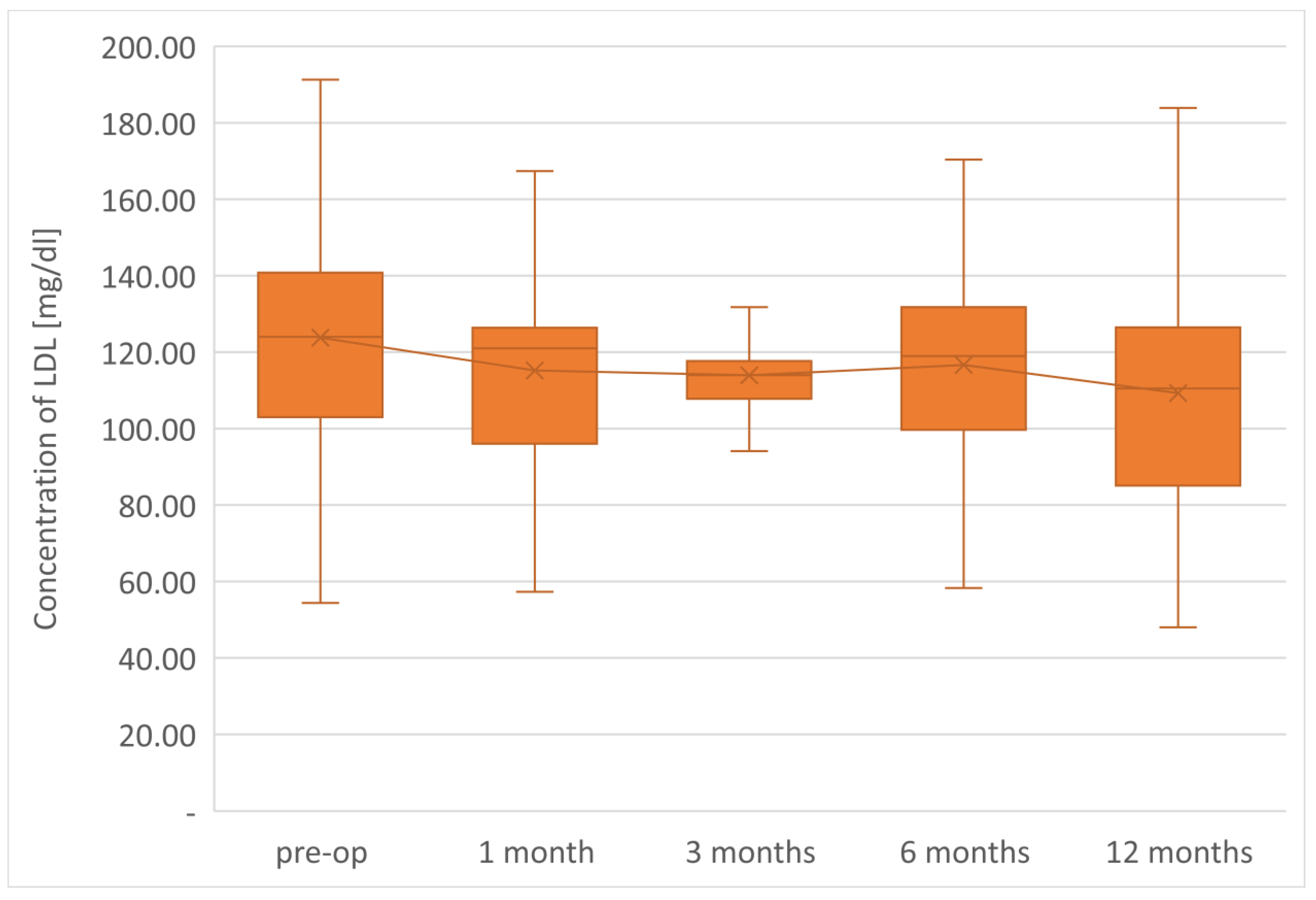
The final determinations of the plasma lipid profile of patients undergoing LSG surgery after 1 year from the procedure showed an average TC concentration of 175.9 mg/dL, so it decreased by 16.6 mg/dL in relation to the preoperative value. The level of the LDL-C fraction finally reached an average of 109.3 mg/dL, i.e., it was 14.5 mg/dL lower than the preoperative value.
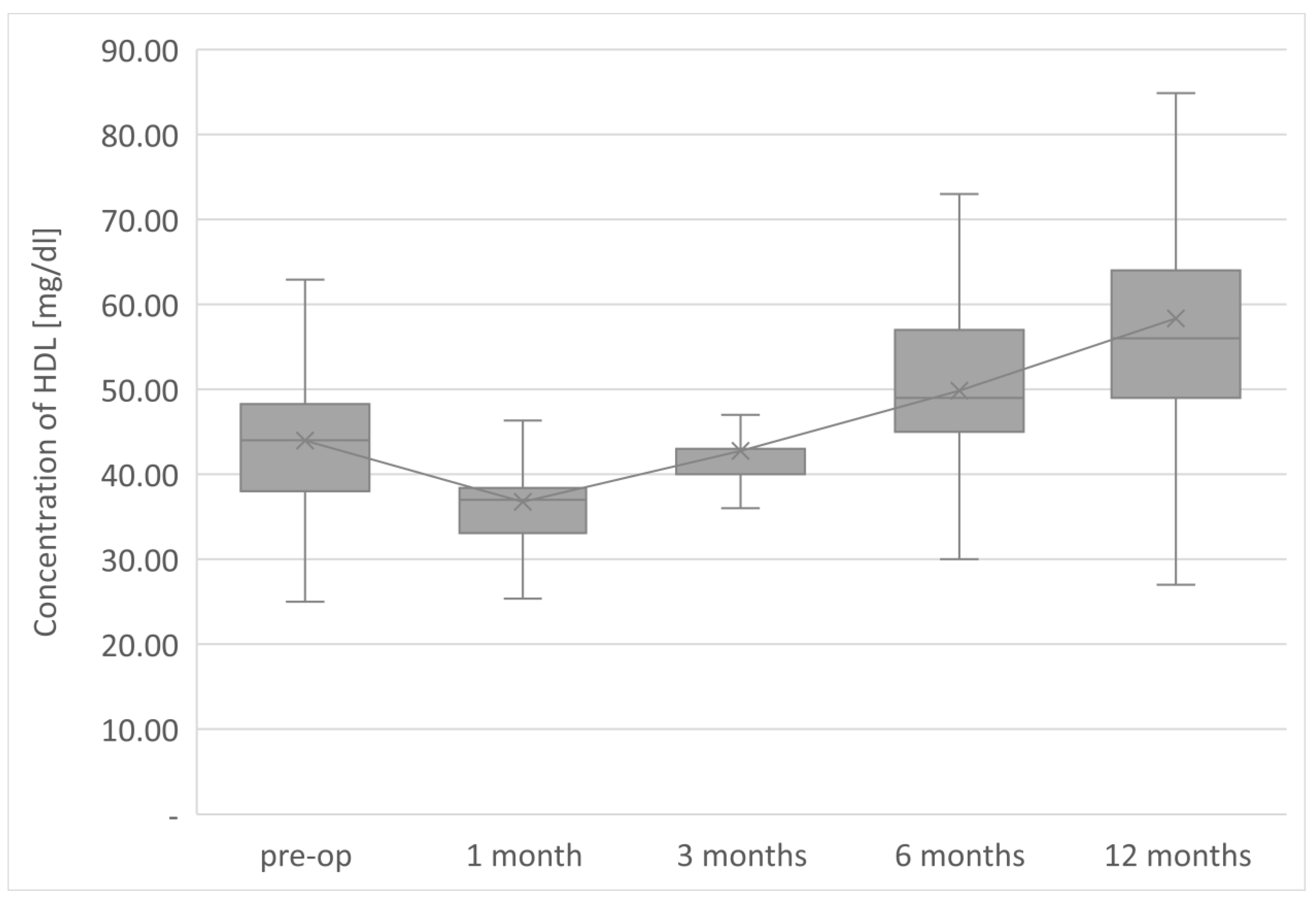
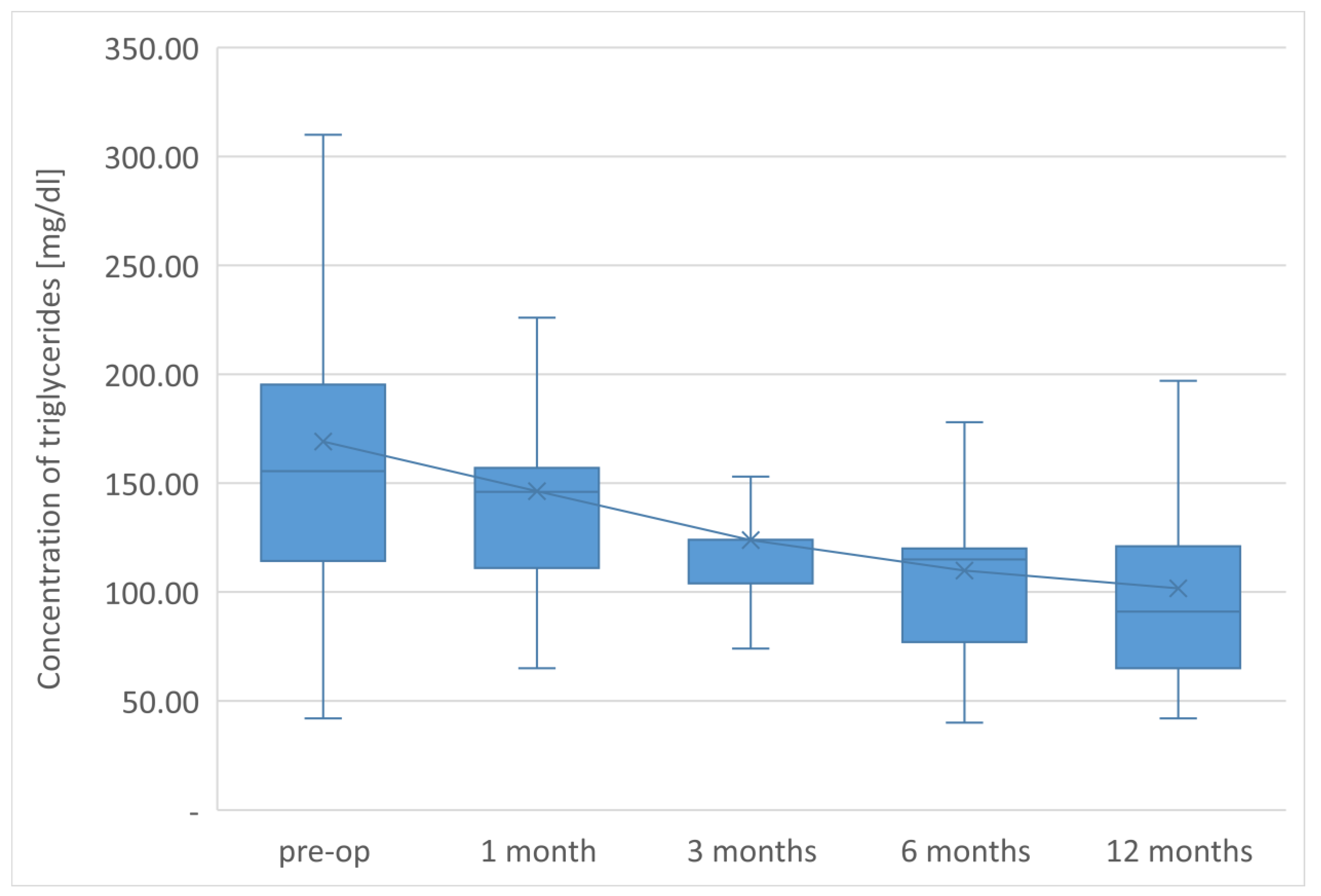
The average TG concentration in plasma significantly decreased from 148.6 mg/dL to 117.5 mg/dL. However, the average concentration of high-density lipoprotein fraction increased to 58.3 mg/dL, and at the end of the observation it was 14.3 mg/dL higher than that before LSG. The level of non-HDL lipoproteins in the plasma of examined patients at the end of the observation was 117.5 mg/dL and it was lower than the initial value by 31.1 mg/dL. All changes observed in this group of patients in the lipid balance parameters after 12 months were statistically significant (
p < 0.05). The aforementioned changes in the lipid profile of patients after LSG are presented in
Table 2 and
Figure 1,
Figure 2,
Figure 3,
Figure 4 and
Figure 5.
The correlation between changes in concentrations of total cholesterol and LDL, HDL, non-HDL and triglycerides with the bariatric results obtained after 12 months after the surgery, in terms of BMI, %EWL, %EBL, %EBL, %TWL, was examined (
Table 3). No correlation was observed between the bariatric results and the change in total cholesterol, low-density lipoproteins (LDL), non-HDL, or triglycerides. A correlation between BMI, %EWL and %EBL 12 months after surgery and a change in high-density lipoprotein concentration were demonstrated.
p < 0.05 was recognized as the level of statistical significance.
4. Discussion
Obesity is undoubtedly one of the largest problems of modern health care. According to the World Obesity Federation, a population of 1 billion will live with obesity in 2030 [
14]. The scale of the problem forces us to look for effective methods of preventing and treating obesity and coexisting diseases to reduce the morbidity and mortality associated with obesity.
The presented results of the 1-year observation of patients after sleeve gastrectomy confirm the effectiveness of surgery in the treatment of obesity and weight reduction. Considering the confirmed close relationship of body mass and obesity with comorbidity and mortality, statistically significant decreases in body weight and BMI after surgery lead to a conclusion of the indisputable health benefits after laparoscopic sleeve gastrectomy [
14,
15]. The results of available review and research publications analyzing the subject of postoperative weight loss, BMI, %TWL, %EWL, and %EBL after LSG were in line with the results obtained in our study [
16,
17]. Sleeve gastectomy is considered an effective and safe method of treating obesity [
18,
19].
Dyslipidemia is an asymptomatic disease. Patients are unaware of the disease. Dyslipidemia can be diagnosed only via laboratory tests, and a large part of the population does not report it. This delays proper treatment. Dyslipidemia should be treated in many ways, including through diet, physical activity, weight reduction and pharmacological agents. Patients applying for bariatric surgery due to obesity often do not report that they suffer from dyslipidemia, and it is diagnosed only after preoperative examinations. Only 15.3% of patients knew about the disease before surgery, only 21.9% were taking lipid-lowering drugs and more than 50% should have been diagnosed and treated dyslipidemia.
The parameters of the assessment of changes in a patient’s lipid profile after surgery were observed at the control points to be TC, TG, HDL, LDL and non-HDL lipid fractions. Analysis of changes in the above lipidogram parameters among patients undergoing laparoscopic sleeve gastrectomy after 1, 3, 6 and 12 months of postoperative control showed different, non-linear variations of the average concentrations of individual fractions in the group; however, the final effect of the procedure obtained after 1 year of observation was a reduction in the average atherogenic levels of lipids, i.e., TC, TG, LDL-C and non-HDL, which was accompanied by a simultaneous increase in the level of anti-atherosclerotic HDL-C lipoprotein.
Such clinically beneficial characteristics of changes in the lipid profile occurring after bariatric procedures is also described by many other authors studying the issue [
20,
21,
22]. Regarding the average concentration of HDL-C lipid fractions and triglycerides in the plasma of analyzed patients obtained at the end of the observation and the criteria for diagnosing metabolic syndrome, it should be noticed that a decrease in the TG level below the threshold value of 150 mg/dL was obtained; however, the concentration of HDL-C increased above the desired amount of 50 mg/dL, so this procedure proved to be effective in the treatment of dyslipidemia defined by the criteria for metabolic syndrome. Our study proves the effectiveness of laparoscopic sleeve gastrectomy not only in weight reduction, but also in improving lipid metabolism.
An alternative non-invasive bariatric method is endoscopic sleeve gastroplasty. With endoscopic access, the volume of the stomach is reduced, and the mechanism of gastric emptying is also impaired. It leads to an earlier feeling of satiety, reduced food intake and weight loss. Its advantage over laparoscopic sleeve gastrectomy is the lack of scars and the possibility of reversing anatomical changes. The procedure can be performed under intravenous anesthesia, which is an alternative for patients who are not eligible for surgical treatment. Studies confirm the effectiveness of endoscopic sleeve gastroplasty in the treatment of metabolic disorders and weight reduction in obese patients [
23].
In the assessment of cardiovascular risk, researchers and clinical practitioners take into account the treatment of atherogenic dyslipidemia, based mainly on the reduction in the low-density fraction of LDL cholesterol. Scientific research confirms that a reduction in the LDL fraction correlates most closely with a reduction in cardiovascular risk [
24]. Scientific data are available that indicate the role of non-HDL as an alternative therapeutic determinant in the assessment of CVD risk, covering all fractions containing apoB with a stronger correlation with CDV risk than LDL concentration in patients with a high TG concentration who were not fasting at the time of examination. Non-HDL may be considered an alternative therapeutic goal in the treatment of dyslipidemia and prevention of cardiovascular diseases, but it is not widely used [
25]. In connection with the above, a beneficial effect of bariatric operations may be observed in CVD risk reduction based on changes in the lipid profile in the form of a decrease in LDL.
The atherogenic properties of lipoproteins rich in TG are widely proven [
26]. Some authors indicate the significant importance of hypertriglyceridemia and low HDL levels in the increase in cardiovascular risk. Although genetic evidence indicated triglycerides (TG) in plasma as an independent risk factor of CVD, no consensus has been reached as to the targeting of the elevated level of these lipoproteins to prevent CVD [
26].
5. Conclusions
Laparoscopic sleeve gastrectomy is an effective method of morbid obesity treatment.
Sleeve gastrectomy improves lipid profiles independently of weight loss, reducing the concentration of total cholesterol, LDL, non-HDL, and triglycerides and increasing HDL concentration. The therapeutic effect is observed in lipid fractions, the concentrations of which are taken into account in the diagnosis of MS.
The correlation of bariatric effects with a change in high-density lipoproteins was proven. No such correlations were observed in other lipid fractions.
Author Contributions
Conceptualization, P.P., P.A.W. and H.R.H.; validation, P.P., J.R.Ł. and H.R.H.; resources, P.P., P.A.W. and A.Ł.; data curation, P.P., P.A.W., A.Ł., J.C. and H.R.H.; writing—original draft preparation, P.P. and P.A.W.; writing—review and editing, P.P. and H.R.H.; supervision, K.N. and H.R.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Medical University of Białystok (the reference number of the consent: R-I-002/248/2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hady, R.H.; Zbucki, R.L.; Luba, M.E.; Gołaszewski, P.; Gołaszewski, P.; Ladny, J.; Dada, J.W. Obesity as social disease and the influence of environmental factors on BMI in own material. Adv. Clin. Exp. Med. 2010, 19, 369–378. [Google Scholar]
- Pantalone, K.M.; Hobbs, T.M.; Chagin, K.M.; Kong, S.X.; Wells, B.J.; Kattan, M.W.; Bouchard, J.; Sakurada, B.; Milinovich, A.; Weng, W.; et al. Prevalence and recognition of obesity and its associated comorbidities: Cross-sectional analysis of electronic health record data from a large US integrated health system. BMJ Open 2017, 7, e017583. [Google Scholar] [CrossRef]
- Diemieszczyk, I.; Głuszyńska, P.; Wojciak, P.A.; Ładny, J.R.; Razak Hady, H. Metabolic syndrome. Etiology and pathogenesis. Wiad. Lek. 2021, 74, 2510–2515. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed] [Green Version]
- Hu, G.; Qiao, Q.; Tuomilehto, J.; Balkau, B.; Borch-Johnsen, K.; Pyorala, K. Prevalence of the metabolic syndrome and its relation to all-cause and cardiovascular mortality in non-diabetic European men and women. Arch. Intern. Med. 2004, 164, 1066–1076. [Google Scholar] [CrossRef]
- Kassi, E.; Pervanidou, P.; Kaltsas, G.; Chrousos, G. Metabolic syndrome: Definitions and controversies. BMC Med. 2011, 9, 48. [Google Scholar] [CrossRef] [Green Version]
- Kalinowski, P.; Mianowana, M. Zespół Metaboliczny cz. II: Epidemiologia zespołu metabolicznego w Polsce i na świecie. J. Educ. Health Sport 2016, 6, 466–480. [Google Scholar]
- Grundy, S.M. Atherogenic dyslipidemia: Lipoprotein abnormalities and implications for therapy. Am. J. Cardiol. 1995, 75, 45B–52B. [Google Scholar] [CrossRef]
- Rader, D.J. Effect of Insulin Resistance, Dyslipidemia, and Intra-abdominal Adiposity on the Development of Cardiovascular Disease and Diabetes Mellitus. Am. J. Med. 2007, 120, S12–S18. [Google Scholar] [CrossRef]
- Madhu, K.; Manjunath, C.N.; Rawal, J.R.; Irani, P.M. Atherogenic dyslipidemia. Indian J. Endocrinol. Metab. 2013, 17, 969. [Google Scholar] [CrossRef]
- Lewis, G.F.; Carpentier, A.; Adeli, K.; Giacca, A. Disordered Fat Storage and Mobilization in the Pathogenesis of Insulin Resistance and Type 2 Diabetes. Endocr. Rev. 2002, 23, 201–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Viscarra, J.; Kim, S.-J.; Sul, H.S. Transcriptional regulation of hepatic lipogenesis. Nat. Rev. Mol. Cell Biol. 2015, 16, 678–689, Erratum in Nat. Rev. Mol. Cell Biol. 2016, 17, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barter, P. The realities of dyslipidaemia in metabolic syndrome and diabetes. Br. J. Diabetes Vasc. Dis. 2005, 5, S7–S11. [Google Scholar] [CrossRef]
- Lobstein, T.; Brinsden, H.; Neveux, M. World Obesity Atlas 2022. World Obesity, 4 March 2022. [Google Scholar]
- Wojciak, P.A.; Pawłuszewicz, P.; Diemieszczyk, I.; Komorowska-Wojtunik, E.; Czerniawski, M.; Krętowski, A.; Błachnio-Zabielska, A.; Dadan, J.; Ładny, J.R.; Hady, H.R. Laparoscopic sleeve gastrectomy: A study of efficiency in treatment of metabolic syndrome components, comorbidities and influence on certain biochemical markers. Videosurgery Other Miniinvasive Tech. 2020, 15, 136–147. [Google Scholar] [CrossRef] [PubMed]
- O’brien, P.E.; Hindle, A.; Brennan, L.; Skinner, S.; Burton, P.; Smith, A.; Crosthwaite, G.; Brown, W. Long-Term Outcomes after Bariatric Surgery: A Systematic Review and Meta-analysis of Weight Loss at 10 or More Years for All Bariatric Procedures and a Single-Centre Review of 20-Year Outcomes after Adjustable Gastric Banding. Obes. Surg. 2018, 29, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, H.J.; Nedrebø, B.G.; Fosså, A.; Andersen, J.R.; Assmus, J.; Dagsland, V.H.; Dankel, S.N.; Gudbrandsen, O.A.; Fernø, J.; Hjellestad, I.; et al. Seven-year trajectories of body weight, quality of life and comorbidities following Roux-en-Y gastric bypass and sleeve gastrectomy. Int. J. Obes. 2022, 46, 739–749. [Google Scholar] [CrossRef]
- Głuszyńska, P.; Diemieszczyk, I.; Szczerbiński, Ł.; Krętowski, A.; Major, P.; Hady, H.R. Risk Factors for Early and Late Complications after Laparoscopic Sleeve Gastrectomy in One-Year Observation. J. Clin. Med. 2022, 11, 436. [Google Scholar] [CrossRef]
- Woźniewska, P.; Diemieszczyk, I.; Hady, H. Complications associated with laparoscopic sleeve gastrectomy—A review. Gastroenterol. Rev. 2021, 16, 5–9. [Google Scholar] [CrossRef]
- Garay, L.A.; García, M.I.N.; Martínez, R.G.-C.; Pérez, N.M.T.; Rojas, J.L.V. Medium/long term evaluation of lipid profile after bariatric surgery (gastric bypass versus sleeve gastrectomy). Endocrinol. Diabetes Nutr. 2021, 68, 372–380. [Google Scholar] [CrossRef]
- Al Khalifa, K.; Al Ansari, A.; Alsayed, A.R.; Violato, C. The Impact of Sleeve Gastrectomy on Hyperlipidemia: A Systematic Review. J. Obes. 2013, 2013, 643530. [Google Scholar] [CrossRef]
- Sharma, C.; Platat, C.; Gariballa, S.; Al Muhairi, S.J.; Al Aidaros, A.; Mannaerts, G.H.H.; Al Afari, H.S.; Yasin, J.; Al-Dirbashi, O.Y.; Alkaabi, J. Metabolomic Profiling of Lipids and Fatty Acids: 3 Years Postoperative Laparoscopic Sleeve Gastrectomy. Biology 2021, 10, 298. [Google Scholar] [CrossRef]
- Alexandre, F.; Lapergola, A.; Vannucci, M.; Pizzicannella, M.; D’urso, A.; Saviano, A.; Mutter, D.; Vix, M.; Perretta, S. Endoscopic management of obesity: Impact of endoscopic sleeve gastroplasty on weight loss and co-morbidities at six months and one year. J. Visc. Surg. 2023, 160, S38–S46. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boekholdt, S.M.; Arsenault, B.J.; Mora, S.; Pedersen, T.R.; LaRosa, J.C.; Nestel, P.J.; Simes, R.J.; Durrington, P.; Hitman, G.A.; Welch, K.M.A.; et al. Association of LDL cho-lesterol, non-HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: A metaanalysis. JAMA 2012, 307, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, H.N.; Packard, C.J.; Chapman, M.J.; Borén, J.; Aguilar-Salinas, C.A.; Averna, M.; Ference, B.A.; Gaudet, D.; Hegele, R.A.; Kersten, S.; et al. Triglyceride-rich lipoproteins and their remnants: Metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies—A consensus statement from the European Atherosclerosis Society. Eur. Heart J. 2021, 42, 4791–4806. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).