Urinary Lithiasis Risk Assessment after Bariatric Surgery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
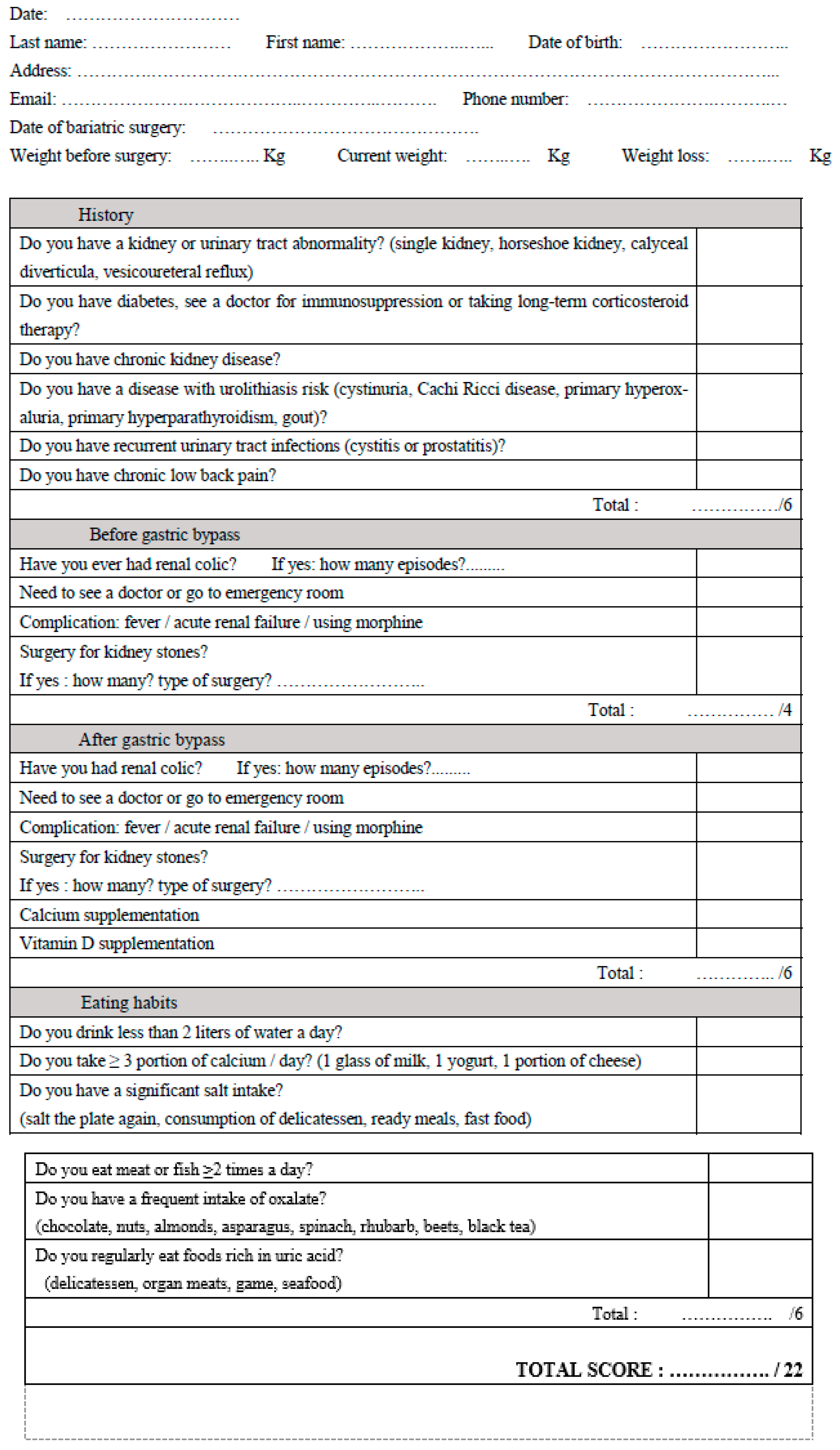
2.2. Questionnaire
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angrisani, L.; Santonicola, A.; Iovino, P.; Formisano, G.; Buchwald, H.; Scopinaro, N. Bariatric Surgery Worldwide 2013. Obes. Surg. 2015, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Lieske, J.C.; Mehta, R.A.; Milliner, D.S.; Rule, A.D.; Bergstralh, E.J.; Sarr, M.G. Kidney stones are common after bariatric surgery. Kidney Int. 2015, 87, 839–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monda, S.M.; Vetter, J.M.; Olsen, M.A.; Keller, M.R.; Eagon, J.C.; Chevinsky, M.S.; Markollari, V.; Venkatesh, R.; Desai, A.C. The Risks of Stone Diagnosis and Stone Removal Procedure After Different Bariatric Surgeries. J. Endourol. 2021, 35, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Matlaga, B.R.; Shore, A.D.; Magnuson, T.; Clark, J.M.; Johns, R.; Makary, M.A. Effect of Gastric Bypass Surgery on Kidney Stone Disease. J. Urol. 2009, 181, 2573–2577. [Google Scholar] [CrossRef] [PubMed]
- Haddad, N.; Scheffler, P.; Elkoushy, M.A.; Court, O.; Christou, N.V.; Andersen, R.E.; Andonian, S. Long-term incidence of urolithiasis post-bariatric surgery. Can. Urol. Assoc. J. 2014, 8, 688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadiyar, N.; Geraghty, R.M.; Premakumar, Y.; Somani, B.K. Changes in Urine Composition and Risk of Kidney Stone Disease Following Bariatric Surgery: A Systematic Review over Last 2 Decades. Curr. Urol. Rep. 2022, 23, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, B. Primary and secondary hyperoxaluria: Understanding the enigma. World J. Nephrol. 2015, 4, 235. [Google Scholar] [CrossRef] [PubMed]
- Premakumar, Y.; Gadiyar, N.; Hameed, B.M.Z.; Veneziano, D.; Somani, B.K. Association of Kidney Stone Disease (KSD) with Primary Gastrointestinal Surgery: A Systematic Review over Last 2 Decades. Curr. Urol. Rep. 2021, 22, 34. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, U.H.; Duffy, A.J.; Roberts, K.E.; Shariff, A.H. Nephrolithiasis after bariatric surgery: A review of pathophysiologic mechanisms and procedural risk. Int. J. Surg. Lond. Engl. 2016, 36, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.D.; Canales, B.K. Kidney stone risk following modern bariatric surgery. Curr. Urol. Rep. 2014, 15, 401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thongprayoon, C.; Cheungpasitporn, W.; Vijayvargiya, P.; Anthanont, P.; Erickson, S.B. The risk of kidney stones following bariatric surgery: A systematic review and meta-analysis. Ren. Fail. 2016, 38, 424–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daudon, M.; Traxer, O.; Lechevallier, E.; Saussine, C. Épidémiologie des lithiases urinaires. Prog. En Urol. 2008, 18, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Rieu, P. Lithiases d’infection. Ann. Urol. 2005, 39, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Tarplin, S.; Ganesan, V.; Monga, M. Stone formation and management after bariatric surgery. Nat. Rev. Urol. 2015, 12, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Daudon, M.; Bader, C.A.; Jungers, P. Urinary calculi: Review of classification methods and correlations with etiology. Scanning Microsc. 1993, 7, 1081–1104; discussion 1104–1106. [Google Scholar] [PubMed]
- American Educational Research Association. Report and Recommendations for the Reauthorization of the Institute of Education Sciences; American Educational Research Association: Washington, DC, USA, 2011; ISBN 978-0-935302-35-6. [Google Scholar]

| Male Gender n (%)/Female | 125 (87.4)/18 (12.6) |
|---|---|
| Mean age at time of study (years old) (±SD) | 49.06 (±10.78) |
| Mean age at time of surgery (years old) (±SD) | 44.28 (±10.76) |
| Mean time between surgery questionnaire (months) | 60.75 (±4.95) |
| Mean weight before gastric bypass (kg) | 122.22 (±19.35) |
| Mean current weight (kg) | 82.78 (±19.09) |
| Mean weight loss (kg) | 39.44 (±15.15) |
| Mean total score of questionnaire | 5.34 (±2.17) |
| Urolithiasis | Total | ||||
|---|---|---|---|---|---|
| No | Yes | ||||
| Questionnaire score | <6 | Patients | 88 | 2 | 90 |
| % Score | 97.8% | 2.2% | 100% | ||
| % Stone | 76.5% | 7.1% | 62.9% | ||
| Total (%) | 61.5% | 1.4% | 62.9% | ||
| ≥6 | Patients | 27 | 26 | 53 | |
| % Score | 50.9% | 49.1% | 100% | ||
| % Lithiasis | 23.5% | 92.9% | 37.1% | ||
| Total (%) | 18.9% | 18.2% | 37.1% | ||
| Total | Patients | 115 | 28 | 143 | |
| Score | 80.4% | 19.6% | 100% | ||
| Lithiasis | 100% | 100% | 100% | ||
| Total | 80.4% | 19.6% | 100% | ||
| Variable | Score | p | ||
|---|---|---|---|---|
| <6 | ≥6 | |||
| Gender | Female | 80 (55.9) | 45 (31.5) | 0.603 |
| Male | 10 (7.0) | 8 (5.6) | ||
| Urinary tract abnormality | no | 90 (100) | 53 (100) | - |
| yes | 0 | 0 | ||
| Immunosuppression | no | 86 (60.1) | 43 (30.1) | 0.008 |
| yes | 4 (2.8) | 10 (7.0) | ||
| Chronic renal failure | no | 90 (62.9) | 51 (35.7) | 0.136 |
| yes | 0 | 2 (1.4) | ||
| Stone risk disease | no | 89 (62.2) | 50 (35.0) | 0.144 |
| yes | 1 (0.7) | 3 (2.1) | ||
| Recurrent urinary tract infection | no | 81 (56.6) | 38 (26.6) | 0.006 |
| yes | 9 (6.3) | 15 (10.5) | ||
| Chronic low back pain | no | 69 (48.3) | 18 (12.6) | 0.001 |
| yes | 21 (14.7) | 35 (24.5) | ||
| Renal colic before GB | no | 89 (62.2) | 35 (24.5) | 0.001 |
| yes | 1 (0.7) | 18 (12.6) | ||
| Stone surgery before GB | no | 90 (62.9) | 50 (35.0) | 0.049 |
| yes | 0 | 3 (2.1) | ||
| Renal stone after GB | no | 89 (62.2) | 40 (28.0) | 0.001 |
| yes | 1 (0.7) | 13 (9.1) | ||
| Stone surgery after GB | no | 90 (62.9) | 49 (34.3) | 0.018 |
| yes | 0 | 4 (2.8) | ||
| Calcium supplementation | no | 62 (43.4) | 15 (10.5) | 0.001 |
| yes | 28 (19.6) | 38 (26.6) | ||
| Vitamin D supplementation | no | 30 (21.0) | 6 (4.2) | 0.001 |
| yes | 60 (42.0) | 47 (32.9) | ||
| Insufficient hydratation | no | 10 (7.0) | 4 (2.8) | 0.572 |
| yes | 80 (55.9) | 49 (34.3) | ||
| Excess calcium intake | no | 33 (23.2) | 26 (18.3) | 0.218 |
| yes | 56 (39.4) | 27 (19.0) | ||
| Excess salt intake | no | 79 (55.2) | 31 (21.7) | 0.001 |
| yes | 11 (7.7) | 22 (15.4) | ||
| Excess protein intake | no | 42 (29.4) | 21 (14.7) | 0.486 |
| yes | 48 (33.6) | 32 (22.4) | ||
| Excess oxalate intake | no | 61 (42.7) | 22 (15.4) | 0.003 |
| yes | 29 (20.3) | 31 (21.7) | ||
| Excess uric acid intake | no | 80 (55.9) | 40 (28.0) | 0.057 |
| yes | 10 (7.0) | 13 (9.1) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, M.; Ait Said, K.; Menahem, B.; Morello, R.; Tillou, X. Urinary Lithiasis Risk Assessment after Bariatric Surgery. J. Clin. Med. 2023, 12, 4040. https://doi.org/10.3390/jcm12124040
Tran M, Ait Said K, Menahem B, Morello R, Tillou X. Urinary Lithiasis Risk Assessment after Bariatric Surgery. Journal of Clinical Medicine. 2023; 12(12):4040. https://doi.org/10.3390/jcm12124040
Chicago/Turabian StyleTran, Marie, Khelifa Ait Said, Benjamin Menahem, Rémy Morello, and Xavier Tillou. 2023. "Urinary Lithiasis Risk Assessment after Bariatric Surgery" Journal of Clinical Medicine 12, no. 12: 4040. https://doi.org/10.3390/jcm12124040
APA StyleTran, M., Ait Said, K., Menahem, B., Morello, R., & Tillou, X. (2023). Urinary Lithiasis Risk Assessment after Bariatric Surgery. Journal of Clinical Medicine, 12(12), 4040. https://doi.org/10.3390/jcm12124040