Real-Life Management of Pancreatic Cysts: Simplified Review of Current Guidelines
Abstract
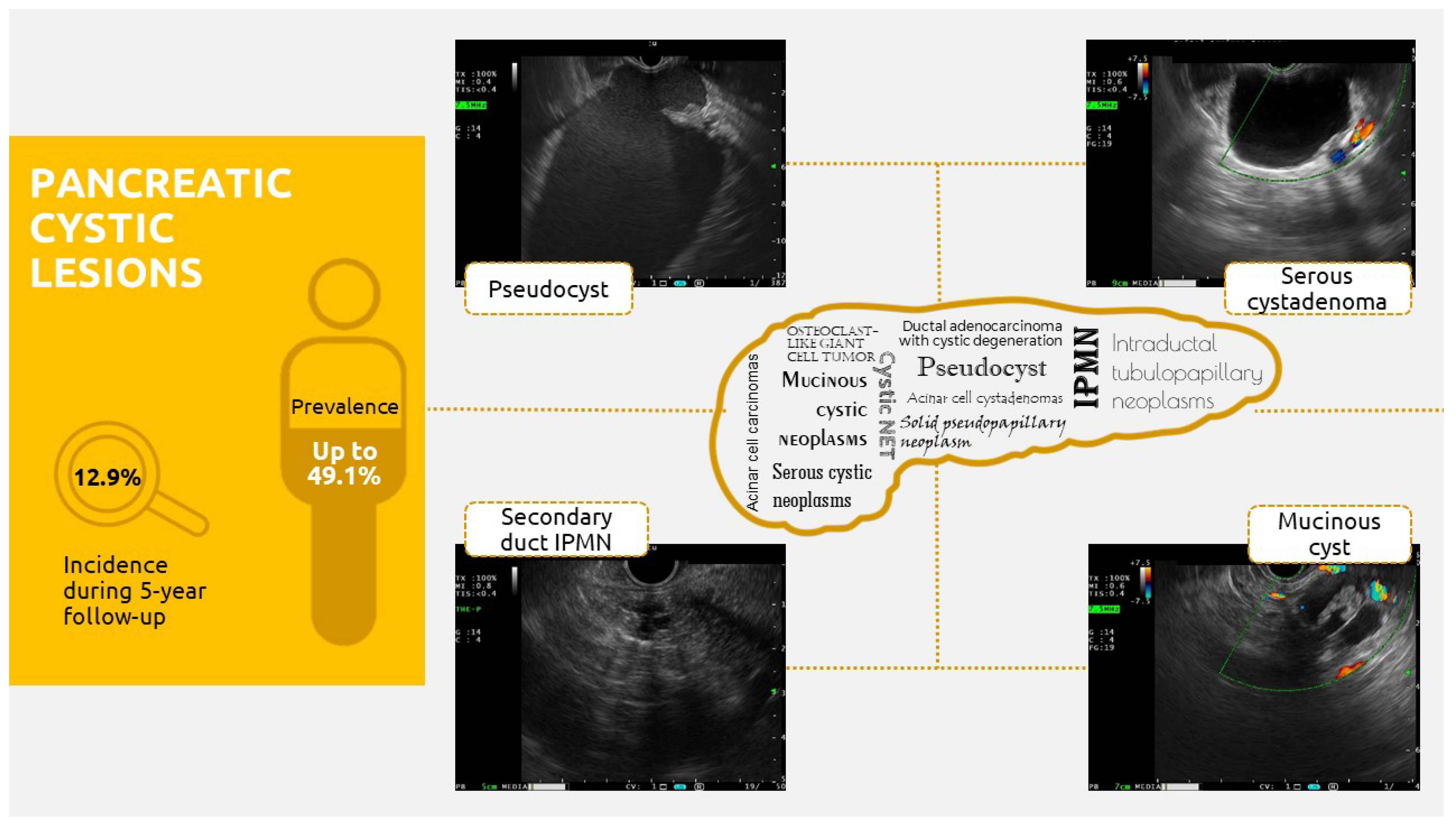
:1. Introduction
2. Where Do We Stand on Pancreatic Cystic Lesions?
- -
- Main-duct IPMNs (MD-IPMNs) (15–21% of IPMNs), which present segmental or diffuse dilatation of the Wirsung duct over 5 mm, without any other cause of obstruction, and are most often localized in the cephalic pancreas. The presence of this lesion is associated with the highest risk of developing malignant features (up to 81%); thus, the corresponding treatment is surgical. Up to 70% of patients are symptomatic [14].
- -
- Branch-duct IPMNs (BD-IPMNs) (41–64% of cases), which are characterized by round cystic lesions that communicate with the pancreatic duct, are most frequently encountered in the pancreatic head (uncinate process), and are often small cysts (5–20 mm) with a grape-like aspect. Indications for the use of a conservative treatment as a “golden standard” with respect to these lesions include multifocality (40% of cases), a high post-surgical recurrence rate (7–8%), and a lower risk of malignant progression (7–42%) [14].
- -
- Mixed-type IPMNs (MT-IPMNs), which present both the above features and are associated with malignant progression in 20–65% of cases. Thus, the corresponding treatment options are the same as those for MD-IPMNs [14].
3. Guidelines Conundrum on Pancreatic Cystic Lesions—How Can They Be Simplified?
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Jong, K.; Nio, C.Y.; Hermans, J.J.; Dijkgraaf, M.G.; Gouma, D.J.; van Eijck, C.H.J.; van Heel, E.; Klass, G.; Fockens, P.; Bruno, M.J. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin. Gastroenterol. Hepatol. 2010, 8, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Van Asselt, S.J.; de Vries, E.G.; van Dullemen, H.M.; Brouwers, A.H.; Walenkamp, A.M.; Giles, R.H.; Links, T.P. Pancreatic cyst development: Insights from von Hippel-Lindau disease. Cilia 2013, 2, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Blumenfeld, J.D.; Prince, M.R. MRI in autosomal dominant polycystic kidney disease. J. Magn. Reson. Imaging 2019, 280, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Kromrey, M.L.; Bulow, R.; Hubner, J.; Paperlein, C.; Lerc, M.M.; Ittermann, T.; Volzke, H.; Mayerle, J.; Kuhn, J.P. Prospective study on the incidence, prevalence and 5-year pancreatic-related mortality of pancreatic cysts in a population-based study. Gut 2018, 67, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Vege, S.S.; Ziring, B.; Jain, R.; Moayyedi, P.; Clinical Guidelines Committee, American Gastroenterology Association. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015, 148, 819–822. [Google Scholar] [CrossRef] [Green Version]
- Khalid, A.; McGrath, K. Classification of Pancreatic Cysts; Post, T.W., Ed.; UpToDate: Waltham, MA, USA, 2023. [Google Scholar]
- Cizginer, S.; Turner, B.; Bilge, A.R.; Karaca, C.; Pitman, M.B.; Brugge, W.R. Cyst fluid carcinoembryonic antigen is an accurate diagnostic marker of pancreatic mucinous cysts. Pancreas 2011, 40, 1024–1028. [Google Scholar] [CrossRef] [Green Version]
- Gaddam, S.; Ge, P.S.; Keach, J.W.; Mullady, D.; Fukami, N.; Edmundowicz, S.A.; Azar, R.R.; Shah, R.J.; Murad, F.M.; Kushnir, V.M.; et al. Suboptimal accuracy of carcinoembryonic antigen in differentiation of mucinous and nonmucinous pancreatic cysts: Results of a large multicenter study. Gastrointest. Endosc. 2015, 82, 1060–1069. [Google Scholar] [CrossRef]
- Feldman, M.; Lawrence, J.B. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease, 11th ed.; Elsevier Saunders: Philadelphia, PA, USA, 2021; Chapter 60; pp. 959–964. [Google Scholar]
- The European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018, 67, 789–804. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Fernandez-del Castillo, C.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.I. Revisions of international consuensus Fukoka guidelines for the management of IMN of the pancreas. Pancreatology 2017, 17, 738–753. [Google Scholar] [CrossRef]
- Elta, G.H.; Enestvedt, B.K.; Sauer, B.G.; Lennon, A.M. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am. J. Gastroenterol. 2018, 113, 464–479. [Google Scholar] [CrossRef]
- Basar, O.; Brugge, W.R. Pancreatic cyst guidelines: Which one to live by? Gastrointest. Endosc. 2017, 85, 1032–1035. [Google Scholar] [CrossRef]
- Levink, I.; Bruno, M.; Cahen, D. Management of Intraductal Papillary Mucinous Neoplasms: Controversies in Guidelines and Future Perspectives. Curr. Treat. Opt. Gastroenterol. 2018, 16, 316–332. [Google Scholar] [CrossRef] [Green Version]
- Tulla, K.A.; Maker, A.V. Can we better predict the biologic behavior of incidental IPMN? A comprehensive analysis of molecular diagnostics and biomarkers in intraductal papillary mucinous neoplasms of the pancreas. Langenbecks Arch. Surg. 2018, 403, 151–194. [Google Scholar] [CrossRef]
- Hao, S.; Takahashi, C.; Snyder, R.A.; Parikh, A.A. Stratifying Intraductal Papillary Mucinous Neoplasms by Cyst Fluid Analysis: Present and Future. Int. J. Mol. Sci. 2020, 21, 1147. [Google Scholar] [CrossRef] [Green Version]
- Kwak, H.A.; Liu, X.; Allende, D.S.; Pai, R.K.; Hart, J.; Xiao, S.Y. Interobserver variability in intraductal papillary mucinous neoplasm subtypes and application of their mucin immunoprofiles. Mod. Pathol. 2016, 29, 977–984. [Google Scholar] [CrossRef] [Green Version]
- Hirono, S.; Yamaue, H. Surgical strategy for intraductal papillary mucinous neoplasms of the pancreas. Surg. Today 2020, 50, 50–55. [Google Scholar] [CrossRef] [Green Version]
- McMillan, M.T.; Lewis, R.S.; Drebin, J.A.; Teitelbaum, U.R.; Lee, M.K.; Roses, R.E.; Fraker, D.L.; Vollmer, C.M. The efficacy of adjuvant therapy for pancreatic invasive intraductal papillary mucinous neoplasm (IPMN). Cancer 2016, 122, 521–533. [Google Scholar] [CrossRef] [Green Version]
- Podolsky, D.; Michael, C.; Anthony, K.; Fergus, S. Yamada’s Textbook of Gastroenterology, 6th ed.; Wiley Blackwell: Sussex, UK, 2016; pp. 1748–1760. [Google Scholar]
- Li-Geng, T.; Cai, G.; Aslanian, H.R. EUS diagnosis of cystic pancreatic neuroendocrine tumors. Videogie 2018, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Caglia, P.; Cannizzaro, M.T.; Tracia, A.; Amodeo, L.; Tracia, L.; Buffone, A.; Amodeo, C.; Cannizzaro, M.A. Cystic pancreatic neuroendocrine tumors: To date a diagnostic challenge. Int. J. Surg. 2015, 21, S44–S49. [Google Scholar] [CrossRef]
- Van Huijgevoort, N.C.M.; del Chiaro, M.; Wolfgang, C.L.; van Hooft, J.E.; Besselink, M.G. Diagnosis and management of pancreatic cystic neoplasms: Current evidence and guidelines. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 676–689. [Google Scholar] [CrossRef]
- Chen, A.L.; Misdraji, J.; Brugge, W.R.; Ferrone, C.R.; Pitman, M.B. Acinar cell cystadenoma: A challenging cytology diagnosis, facilitated by moray® micro-forceps biopsy. Diagn. Cytopathol. 2017, 45, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Sopha, S.C.; Terhune, J.H.; Hoover, L.; Uradomo, L.; Boutros, C.N. Acinar Cell Cystadenoma of the Pancreas: A Multidisciplinary and Contemporary Approach. J. Gastrointest. Surg. 2018, 22, 1797–1798. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Ji, L.; Qu, F.-Z.; Li, L.; Cao, C.-L.; Li, Z.-B.; Sun, B. Acinar cell cystadenoma of the pancreas: A retrospective analysis of ten-year experience from a single academic institution. Pancreatology 2016, 16, 625–631. [Google Scholar] [CrossRef]
- Sakorafas, G.H.; Smyrniotis, V.; Reid-Lombardo, K.M.; Sarr, M.G. Primary pancreatic cystic neoplasms of the pancreas revisited. Part IV: Rare cystic neoplasms. Surg. Oncol. 2012, 21, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ke, N.; Liu, X.; Gong, S. Mature cystic teratoma of the pancreas with 30 years of clinical course. Medicine 2018, 97, e0405. [Google Scholar] [CrossRef]
- Kim, H.; Koh, Y. Mature cystic teratoma of the pancreas: A rare cystic neoplasm. Open Med. 2019, 14, 872–874. [Google Scholar] [CrossRef]
- Kin, H.J.; Park, M.S.; Chung, T.; Kin, B.; Lee, J.H.; Kin, J.K. Multimodality imaging studies of intraductal tubulopapillary neoplasms of the pancreas. Diagn. Interv. Radiol. 2019, 25, 251–256. [Google Scholar] [CrossRef]
- Fritz, S.; Küper-Steffen, R.; Feilhauer, K.; Sommer, C.M.; Richter, G.M.; Bosse, A.; Köninger, J. Intraductal tubular papillary neoplasm (ITPN), a novel entity of pancreatic epithelial neoplasms and precursor of cancer: A case report and review of the literature. Int. J. Surg. Case Rep. 2019, 55, 187–191. [Google Scholar] [CrossRef]
- Fan, X.; Wang, W.; Li, C.F.; Tang, T.; Han, Y.; An, K. An osteoclast-like giant cell tumor embedded in the mural nodule of a pancreatic mucinous cystic neoplasm. A case report and literature review. Medicine 2019, 98, e15246. [Google Scholar] [CrossRef]
- Temesgen, W.M.; Wachtel, M.; Dissanaike, S. Osteoclastic giant cell tumor of the pancreas. Int. J. Surg. Case Rep. 2014, 5, 175–179. [Google Scholar] [CrossRef] [Green Version]
- Balaban, D.V.; Marin, F.S.; Manucu, G.; Zoican, A.; Ciochina, M.; Mina, V.; Patoni, C.; Vladut, C.; Bucurica, S.; Costache, R.S.; et al. Clinical characteristics and outcomes in carbohydrate antigen 19-9 negative pancreatic cancer. World J. Clin. Oncol. 2022, 13, 630–640. [Google Scholar] [CrossRef]
- Passerini, R.; Cassatella, M.C.; Boveri, S.; Salvatici, M.; Radice, D.; Zorzino, L. The pitfalls of CA 19-9: Routine testing and comparison of two automated immunoassays in a reference oncology center. Am. J. Clin. Pathol. 2012, 138, 281–287. [Google Scholar] [CrossRef] [Green Version]
- Keane, M.; Afgani, E. A review of the diagnosis and management of premalignant pancreatic cystic lesions. J. Clin. Med. 2021, 10, 1284. [Google Scholar] [CrossRef]
- Crippa, S.; Arcidiacono, P.G.; De Cobelli, F.; Falconi, M. Review of the diagnosis and management of intraductal papillary mucionou neoplasms. United Eur. J. 2020, 8, 249–255. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, A.; Yamaguchi, T.; Ohtsuka, M.; Ishihara, T.; Sudo, K.; Nakamura, K.; Hara, T.; Denda, T.; Miyazaki, M. Usefulness of multidetector computed tomography for detecting protruding lesions in intraductal papillary mucinous neoplasm of the pancreas in comparison with single-detector computed tomography and endoscopic ultrasonography. Pancreas 2009, 38, 131–136. [Google Scholar] [CrossRef]
- Suzuki, R.; Thosani, N.; Annangi, S.; Guha, S.; Bhutani, M.S. Diagnostic yield of EUS-FNA-based cytology distinguishing malignant and benign IPMNs: A systematic review and meta-analysis. Pancreatology 2014, 14, 380–384. [Google Scholar] [CrossRef]
- Megibow, A.J.; Baker, M.E.; Morgan, D.E.; Kamel, I.R.; Sahani, D.V.; Newman, E.; Brugge, W.R.; Berland, L.L.; Padharipande, P.V. Management of Incidental Pancreatic Cysts: A White Paper of the ACR Incidental Findings Committee. J. Am. Coll. Radiol. 2017, 14, 911–923. [Google Scholar] [CrossRef]
- ASGE Standards of Practice Committee; Khashab, M.A.; Chithadi, K.V.; Acosta, R.D.; Bruining, D.H.; Chandrasekhara, V.; Eloubeidi, M.A.; Fanelli, R.D.; Faulx, A.L.; Fonkalsrud, L.; et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest. Endosc. 2015, 81, 81–89. [Google Scholar] [CrossRef]
- Bratzler, D.W.; Dellinger, E.P.; Olsen, K.M.; Perl, T.M.; Auwaerter, P.G.; Bolon, M.K.; Fish, D.N.; Napolitano, L.M.; Sawyer, R.G.; Slain, D.; et al. Clinical Practice Guidelines for Antimicrobial Prophylaxis in Surgery. Am. J. Health Syst. Pharm. 2013, 70, 195–283. [Google Scholar] [CrossRef] [Green Version]
- Allison, M.C.; Sandoe, J.A.T.; Tighe, R.; A Simpson, I.; Hall, R.J.; Elliott, T.S.J. Antibiotic prophylaxis in gastrointestinal endoscopy. Gut 2009, 58, 868–880. [Google Scholar] [CrossRef]
- Perri, G.; Marchegiani, G.; Frigerio, I.; Dervenis, C.G.; Conlon, K.C.; Bassi, C.; Salvia, R. Management of Pancreatic Cystic Lesions. Dig. Surg. 2019, 37, 1–9. [Google Scholar] [CrossRef] [PubMed]

 Absolute surgical indication;
Absolute surgical indication;  Relative surgical indication;
Relative surgical indication;  EUS ± FNA indication.
EUS ± FNA indication.
 Absolute surgical indication;
Absolute surgical indication;  Relative surgical indication;
Relative surgical indication;  EUS ± FNA indication.
EUS ± FNA indication.| Guidelines | AGA 2015 | ACR 2017 | IAP 2017 | Revised Fukoka 2017 | ACG 2018 | EAP 2018 |
|---|---|---|---|---|---|---|
| SCA, SPN, NET, cystic ADK | SPN, NET, cystic ADK | SCA-symptomatic or >40 mm SPN, NET, cystic ADK | IPMN | SPN | ||
| Jaundice | Present | Present | Present | Present | Present | |
| Acute pancreatitis | Yes | Yes | Yes | |||
| New onset diabetes | Yes + MCN/IPMN | Yes | ||||
| CA 19-9 | Elevated | Elevated | Elevated | Elevated | ||
| Lymph nodes | Increased | Increased | ||||
| Size | >30 mm | >30 mm | >30 mm | ≥30 mm | >30 mm | >40 mm |
| Growth rate | Increasing | +20% longest axe | >5 mm/2 years | ≥5 mm/2 years | >3 mm/year | >5 mm/year |
| Cyst wall | Thickened | Thickened | Thickened | |||
| Nodule | Nodule + MPD dilation | Non enhancing | Enhancing, <5 mm | Enhancing, <5 mm | Yes, at EUS | Enhancing, <5 mm |
| Enhancing | Enhancing, >5 mm | Enhancing, ≥5 mm | Enhancing, > 5 mm Solid mass | |||
| MPD | Dilated | 7–9 mm | Abrupt change with distal atrophy; 5–9 mm | Abrupt change with distal atrophy; 5–9 mm | >5 mm obstructed | 5–9 mm |
| >10 mm | >10 mm | ≥10 mm | Involved at EUS | >10 mm | ||
| Dysplasia/neoplasia at EUS FNA cytology | Yes | Yes | Yes | Yes | Yes | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlăduţ, C.; Bilous, D.; Ciocîrlan, M. Real-Life Management of Pancreatic Cysts: Simplified Review of Current Guidelines. J. Clin. Med. 2023, 12, 4020. https://doi.org/10.3390/jcm12124020
Vlăduţ C, Bilous D, Ciocîrlan M. Real-Life Management of Pancreatic Cysts: Simplified Review of Current Guidelines. Journal of Clinical Medicine. 2023; 12(12):4020. https://doi.org/10.3390/jcm12124020
Chicago/Turabian StyleVlăduţ, Cătălina, Dana Bilous, and Mihai Ciocîrlan. 2023. "Real-Life Management of Pancreatic Cysts: Simplified Review of Current Guidelines" Journal of Clinical Medicine 12, no. 12: 4020. https://doi.org/10.3390/jcm12124020
APA StyleVlăduţ, C., Bilous, D., & Ciocîrlan, M. (2023). Real-Life Management of Pancreatic Cysts: Simplified Review of Current Guidelines. Journal of Clinical Medicine, 12(12), 4020. https://doi.org/10.3390/jcm12124020




