Venous Thromboembolism (VTE) in Post-Prostatectomy Patients: Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Material and Methods
2.1. Protocol and Registration
2.2. Evidence Acquisition
- Population: male patients, prostatectomy for prostate cancer;
- Intervention: pharmacological/combined prophylaxis (PP) for VTE;
- Comparator/control: no prophylaxis or mechanical prophylaxis for VTE.
- Population: prostatectomy for non-prostate cancer or part of other surgery, such as cystoprostatectomy.
- Intervention: if the interventions are ill-defined or structural methods are inadequate;
- Comparator/control: studies that lacked proper grouping into control, and intervention;
- Study design: studies that did not fulfill the above criterion and lacked any defined outcomes.
2.3. Outcome Measures
- Primary outcomes: VTE occurrence with
- Overall incidence of VTE in post-RP Patients;
- Surgical approach: open, minimally invasive (laparoscopic or robot-assisted laparoscopic prostatectomy);
- Pelvic lymph node dissection (PLND);
- Prophylaxis (no prophylaxis, mechanical only, pharmacological only, combined).
- Secondary outcomes:
2.4. Search Methods
2.5. Study Selection
2.6. Data Extraction
2.7. Quality Assessment
2.8. Statistical Analysis
- a
- Statistical evaluation of overall VTE occurrence.
- b
- Statistical evaluation of VTE occurrence depending on the type of surgical procedure (Open/Minimally Invasive Surgery (MIS)) and whether PLND was performed or not.
- MIS procedures vs. open procedures;
- Procedures using PLND vs. procedures without PLND.
- c
- Statistical Evaluation of VTE occurrence depending on the method of prophylaxis used (mechanical or combined).
3. Results
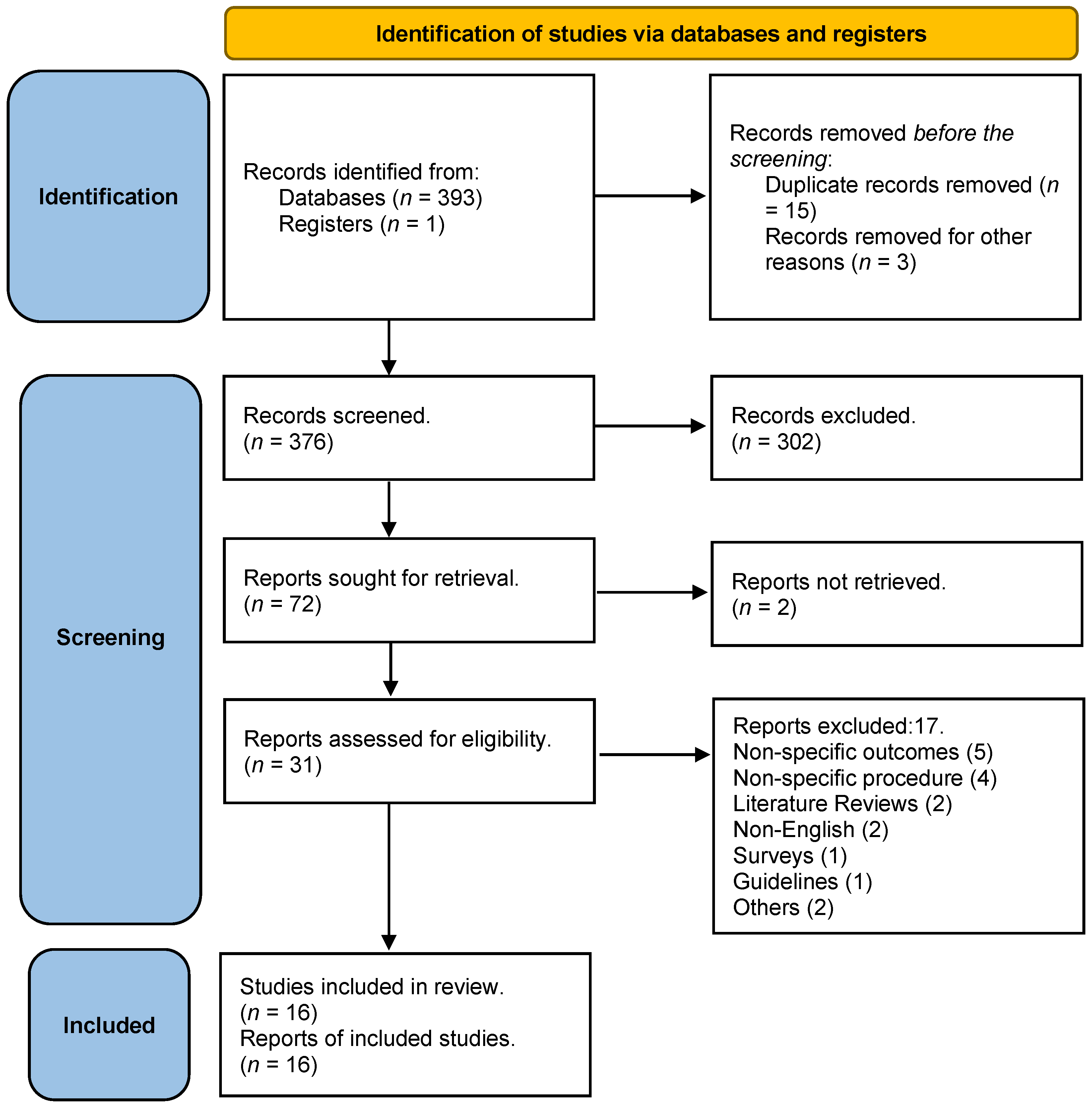
3.1. Study Selection Results
3.2. Quality Assessment Results
3.3. Study Characteristics
3.4. Clinical-Pathological Results
3.5. Demographics and VTE Risk Factors
3.6. Surgical Procedure Results
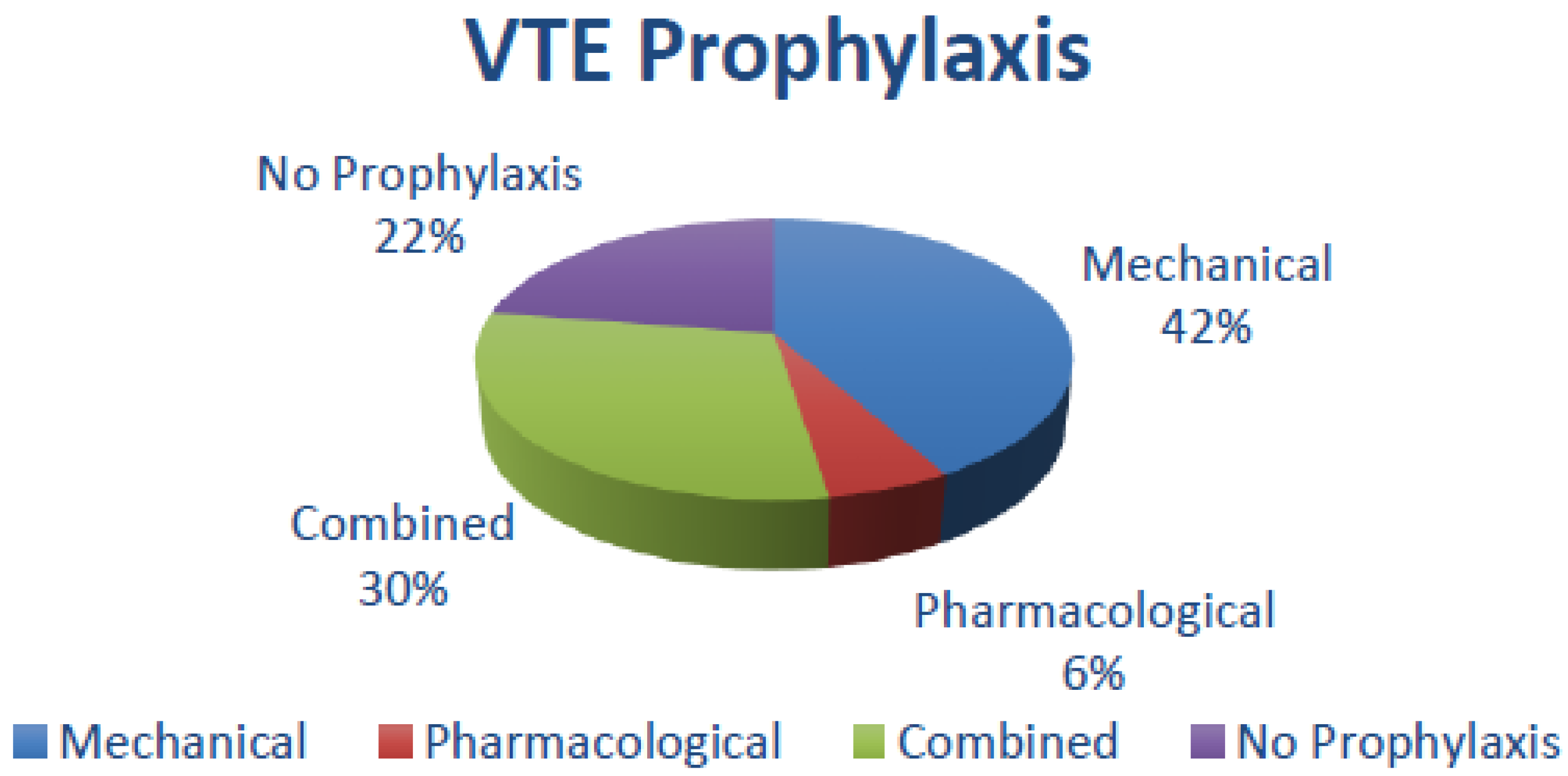
3.7. Thromboprophylaxis and VTE Episodes
3.8. Surgical Approaches and VTE Episodes
3.9. Duration and Timing of Thromboprophylaxis
3.10. Statistical Results
- a
- Statistical outcome of overall VTE occurrence
- b
- Statistical outcome of VTE occurrence depending on the type of surgical procedure (Open/MIS) and whether PLND was performed or not.
- c
- Statistical outcome of VTE occurrence depending on the method of prophylaxis used (mechanical or combined).
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guyatt, G.H.; Eikelboom, J.W.; Gould, M.K.; Garcia, D.A.; Crowther, M.; Murad, M.H.; Kahn, S.R.; Falck-Ytter, Y.; Francis, C.W.; Lansberg, M.G.; et al. Approach to Outcome Measurement in the Prevention of Thrombosis in Surgical and Medical Patients. Chest 2012, 141, e185S–e194S. [Google Scholar] [CrossRef]
- Kibel, A.S.; Loughlin, K.R. Pathogenesis and Prophylaxis of Postoperative Thromboembolic Disease in Urological Pelvic Surgery. J. Urol. 1995, 153, 1763–1774. [Google Scholar] [CrossRef]
- Geerts, W.H.; Heit, J.A.; Clagett, G.P.; Pineo, G.F.; Colwell, C.W.; Anderson, F.A.; Wheeler, H.B. Prevention of Venous Thromboembolism. Chest 2001, 119, 132S–175S. [Google Scholar] [CrossRef]
- Geerts, W.H.; Pineo, G.F.; Heit, J.A.; Bergqvist, D.; Lassen, M.R.; Colwell, C.W.; Ray, J.G. Prevention of Venous Thromboembolism. Chest 2004, 126, 338S–400S. [Google Scholar] [CrossRef]
- Zhan, C.; Miller, M.R. Excess Length of Stay, Charges, and Mortality Attributable to Medical Injuries During Hospitalization. JAMA 2003, 290, 1868–1874. [Google Scholar] [CrossRef]
- Rossignol, G.; Léandri, P.; Gautier, J.; Quintens, H.; Gabay-Torbiero, L.; Tap, G. Radical Retropubic Prostatectomy: Complications and Quality of Life (429 Cases, 1983–1989). Eur. Urol. 1991, 19, 186–191. [Google Scholar] [CrossRef]
- Brenner, D.W.; Fogle, M.A.; Schellhammer, P.F. Venous Thromboembolism. J. Urol. 1989, 142, 1403–1411. [Google Scholar] [CrossRef]
- De Martino, R.R.; Goodney, P.P.; Spangler, E.L.; Wallaert, J.B.; Corriere, M.A.; Rzucidlo, E.M.; Walsh, D.B.; Stone, D.H. Variation in thromboembolic complications among patients undergoing commonly performed cancer operations. J. Vasc. Surg. 2012, 55, 1035–1040.e4. [Google Scholar] [CrossRef] [PubMed]
- Beyer, J.; Wessela, S.; Hakenberg, O.; Wirth, M.; Schellong, S. Incidence and Disease Pattern of Venous Thrombembolism after Radical Prostatectomy. Blood 2005, 106, 1627. [Google Scholar] [CrossRef]
- O’Donnell, M.; I Weitz, J. Thromboprophylaxis in surgical patients. Can. J. Surg. 2003, 46, 129–135. [Google Scholar] [PubMed]
- Pridgeon, S.; Allchorne, P.; Turner, B.; Peters, J.; Green, J. Venous thromboembolism (VTE) prophylaxis and urological pelvic cancer surgery: A UK national audit. BJU Int. 2014, 115, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Tikkinen, K.A.; Craigie, S.; Agarwal, A.; Violette, P.D.; Novara, G.; Cartwright, R.; Naspro, R.; Siemieniuk, R.A.; Ali, B.; Eryuzlu, L.; et al. Procedure-specific Risks of Thrombosis and Bleeding in Urological Cancer Surgery: Systematic Review and Meta-analysis. Eur. Urol. 2017, 73, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Violette, P.D.; Cartwright, R.; Briel, M.; Tikkinen, K.A.; Guyatt, G.H. Guideline of guidelines: Thromboprophylaxis for urological surgery. BJU Int. 2016, 118, 351–358. [Google Scholar] [CrossRef]
- Weinberg, A.; Wright, J.; Deibert, C.; Lu, Y.-S.; Hershman, D.; Neugut, A.; Spencer, B. Nationwide practice patterns for the use of venous thromboembolism prophylaxis among men undergoing radical prostatectomy. World J. Urol. 2013, 32, 1313–1321. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. 2013. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 22 December 2022).
- Patel, H.D.; Faisal, F.A.; Trock, B.J.; Joice, G.A.; Schwen, Z.R.; Pierorazio, P.M.; Johnson, M.H.; Bivalacqua, T.J.; Han, M.; Gorin, M.A.; et al. Effect of Pharmacologic Prophylaxis on Venous Thromboembolism after Radical Prostatectomy: The PREVENTER Randomized Clinical Trial. Eur. Urol. 2020, 78, 360–368. [Google Scholar] [CrossRef]
- Valverde-Martinez, S.; Gonzalez-Rayo, L.-A.; Padilla-Fernandez, B.; Pereira-Bruno, J.; Coelho, H.; Montesino-Semper, M.; Müller-Arteaga, C.; Alvarez-Ossorio-Fernandez, J.-L.; Migliorini, F.; Garcia-Cenador, M.-B.; et al. Profilaxis farmacológica de la tromboembolia venosa en la prostatectomía radical. Sci. Direct 2020, 154, 113–118. [Google Scholar] [CrossRef]
- Tollefson, M.K.; Karnes, R.J.; Rangel, L.; Carlson, R.; Boorjian, S.A. Blood Type, Lymphadenectomy and Blood Transfusion Predict Venous Thromboembolic Events Following Radical Prostatectomy with Pelvic Lymphadenectomy. J. Urol. 2014, 191, 646–651. [Google Scholar] [CrossRef]
- Chan, S.Y.S.; Leung, V.F.Y.; Yee, C.H.; Chan, E.S.Y.; Hou, S.S.M.; Chu, W.; Ng, C.F. Incidence of postoperative deep vein thrombosis after robotic-assisted laparoscopic prostatectomy: A prospective study in Chinese patients. Int. Urol. Nephrol. 2014, 46, 2139–2142. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, D.J.; Scarpato, K.R.; Staff, I.; Champagne, A.; Tortora, J.; Wagner, J.R.; Kesler, S.S. Does Heparin Prophylaxis Reduce the Risk of Venous Thromboembolism in Patients Undergoing Robot-Assisted Prostatectomy? J. Endourol. 2013, 27, 800–803. [Google Scholar] [CrossRef]
- Abel, E.J.; Wong, K.; Sado, M.; Leverson, G.E.; Patel, S.R.; Downs, T.M.; Jarrard, D.F. Surgical Operative Time Increases the Risk of Deep Venous Thrombosis and Pulmonary Embolism in Robotic Prostatectomy. JSLS J. Soc. Laparosc. Robot. Surg. 2014, 18, 282–287. [Google Scholar] [CrossRef]
- Dyer, J.; Wyke, S.; Lynch, C. Hospital Episode Statistics data analysis of postoperative venous thromboembolus in patients undergoing urological surgery: A review of 126,891 cases. Ind. Mark. Manag. 2013, 95, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Van Hemelrijck, M.; Garmo, H.; Holmberg, L.; Bill-Axelson, A.; Carlsson, S.; Akre, O.; Stattin, P.; Adolfsson, J. Thromboembolic Events following Surgery for Prostate Cancer. Eur. Urol. 2012, 63, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Eifler, J.; Levinson, A.; Hyndman, M.; Trock, B.; Pavlovich, C. Pelvic Lymph Node Dissection is Associated with Symptomatic Venous Thromboembolism Risk during Laparoscopic Radical Prostatectomy. J. Urol. 2011, 185, 1661–1666. [Google Scholar] [CrossRef]
- Beyer, J.; Wessela, S.; Hakenberg, O.W.; Kuhlisch, E.; Halbritter, K.; Froehner, M.; Wirth, M.P.; Schellong, S.M. Incidence, risk profile and morphological pattern of venous thromboembolism after prostate cancer surgery. J. Thromb. Haemost. 2009, 7, 597–604. [Google Scholar] [CrossRef]
- Grasso, M.; Confalonieri, S.; Blanco, S.; Grasso, A.; Angelo, S. Preoperative blood donation program and postoperative Low Molecular Weight Heparine (LMWH) prophylaxis in patients undergoing radical prostatectomy. Archivos Españoles de Urología 2009, 62, 161–166. [Google Scholar] [CrossRef]
- Cindolo, L.; Salzano, L.; Mirone, V.; Imbimbo, C.; Longo, N.; Kakkos, S.K.; Reddy, D.J. Thromboprophylaxis in Radical Retropubic Prostatectomy: Efficacy and Patient Compliance of a Dual Modality. Urol. Int. 2009, 83, 12–18. [Google Scholar] [CrossRef]
- Nakamura, K.; Kasraeian, A.; Yacoub, S.; Pendleton, J.; Anai, S.; Rosser, C.J. The use of enoxaparin to prevent venous thromboembolism in patients undergoing radical retropubic prostatectomy: Feasibility and utility. Int. Braz J. 2007, 33, 347–354. [Google Scholar] [CrossRef]
- Koya, M.P.; Manoharan, M.; Kim, S.S.; Soloway, M.S. Venous thromboembolism in radical prostatectomy: Is heparinoid prophylaxis warranted? BJU Int. 2005, 96, 1019–1021. [Google Scholar] [CrossRef]
- Cisek, L.J.; Walsh, P.C. Thromboembolic complications following radical retropubic prostatectomy Influence of external sequential pneumatic compression devices. Urology 1993, 42, 406–408. [Google Scholar] [CrossRef]
- Salous, A.K.; Reyad, A.; Sweeney, K.; Mavanur, A. A significant proportion of venous thromboembolism events in general surgical patients occurs after discharge: Analysis of the ACS-NSQIP Essentials database. Perioper. Med. 2019, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, A.; Skrudlik, P.; Kowalski, F.; Lipowski, P.; Ostrowska, M.; Adamczyk, P.; Adamowicz, J.; Drewa, T.; Juszczak, K. Current Thromboprophylaxis in Urological Cancer Patients during COVID Pandemic. Cent. Eur. J. Urol. 2022, 75, 128–134. [Google Scholar] [CrossRef]
- EAU Guidelines. Edn. Presented at the EAU Annual Congress Amsterdam 2022. ISBN 978-94-92671-16-5. Available online: https://uroweb.org/eau-guidelines/citing-usage-republication (accessed on 4 June 2023).
- NICE. Recommendations | Venous Thromboembolism in over 16s: Reducing the Risk of Hospital-Acquired Deep Vein Thrombosis or Pulmonary Embolism | Guidance | NICE. Nice.org.uk. Available online: https://www.nice.org.uk/guidance/ng89/chapter/Recommendations#interventions-for-people-having-abdominal-thoracic-or-head-and-neck-surgery (accessed on 8 February 2023).
- Forrest, J.B.; Clemens, J.Q.; Finamore, P.; Leveillee, R.; Lippert, M.; Pisters, L.; Touijer, K.; Whitmore, K. AUA Best Practice Statement for the Prevention of Deep Vein Thrombosis in Patients Undergoing Urologic Surgery. J. Urol. 2009, 181, 1170–1177. [Google Scholar] [CrossRef]
- Barber, E.L.; Gehrig, P.A.; Clarke-Pearson, D.L. Venous Thromboembolism in Minimally Invasive Compared with Open Hysterectomy for Endometrial Cancer. Obstet. Gynecol. 2016, 128, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Muntz, J. Duration of deep vein thrombosis prophylaxis in the surgical patient and its relation to quality issues. Am. J. Surg. 2010, 200, 413–421. [Google Scholar] [CrossRef]
| Author | Number of Awarded Stars in Each Domain | ||
|---|---|---|---|
| Selection | Comparability | Outcome | |
| (1) Patel, H. D. [17] | **** | ** | ** |
| (2) Valverde-Martinez, S. [18] | *** | * | ** |
| (3) Weinberg, A. [14] | *** | ** | |
| (4) Tollefson, M. K. [19] | *** | ** | |
| (5) Chan, S. [20] | ** | ** | |
| (6) Chalmers, D. J. [21] | *** | ** | |
| (7) Abel, E. [22] | *** | ** | |
| (8) Dyer, J. [23] | ** | * | |
| (9) Van Hemelrijck, M. [24] | ** | ** | |
| (10) Eifler, J. B. [25] | ** | ** | |
| (11) Beyer, J. [26] | *** | ** | |
| (12) Grasso, M. [27] | ** | * | |
| (13) Cindolo, L. [28] | ** | * | |
| (14) Nakamura, K. [29] | ** | * | |
| (15)Koya, M. [30] | *** | * | |
| (16) Cisek, L. [31] | ** | * | |
| Study | Study Type/ Time | Study Characteristics | Conclusion |
|---|---|---|---|
| (1) Patel, H. D. [17] | RCT (2017–2018) |
|
|
| (2) Valverde-Martinez, S. [18] | Retrospective (2013–2014) |
|
|
| (3) Weinberg, A. [14] | Observational (2000–2010) |
|
|
| (4) Tollefson, M. K. [19] | Retrospective (1987–2010) |
|
|
| (5) Chan, S.Y. [20] | Prospective (2007–2010) |
|
|
| (6) Chalmers, D. J. [21] | Prospective (2007–2011) |
|
|
| (7) Abel, E. [22] | Retrospective (2007–2011) |
|
|
| (8) Dyer, J. [23] | Retrospective (2009–2010) |
|
|
| (9) Van Hemelrijck, M. [24] | Retrospective (2002–2010) |
|
|
| (10) Eifler, J. B. [25] | Retrospective (2001–2009) |
|
|
| (11) Beyer, J. [26] | Prospective (2001–2003) |
|
|
| (12) Grasso, M. [27] | Retrospective (1999–2006) |
|
|
| (13) Cindolo, L. [28] | Prospective (2004–2006) |
|
|
| (14) Nakamura, K. [29] | Prospective (2003–2005) |
|
|
| (15) Koya, M. [30] | Prospective (1992–2004) |
|
|
| (16) Cisek, L. [31] | Prospective (1982–1993) |
|
|
| Study | Total Patients | Mean Age | Mean BMI kg/m2 | Family History (%) | VTE Background (%) | Smoking | Overall Risk Assessment (in %) | Caprini Score | Remarks in Relation to VTE Risk Factors/Scores | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | Int | High | |||||||||
| (1) Patel, H. D. [17] | 501 | 62 | 27.4 | - | - | - | - | - | - | 6 | The study concluded that most patients with prostate cancer undergoing RP are relatively healthy. Our study suggests that PP may be deferred based on surgeon preference up to a Caprini score of 7; PP may be justified for higher-risk patients with scores of 8. |
| (2) Valverde-Martinez, S. [18] | 610 | 64.1 | 28.03 | - | - | - | 94.8 | 4.1 | 1.1 | - | This study concluded that with respect to the PP used in different thromboembolic risk groups, there were differences in the low-risk group, but not in the intermediate and high-risk groups; this was probably due to the fact that this group covered 95% of the cases in the series. |
| (3) Weinberg, A. [14] | 94,709 | - | - | - | - | - | - | - | - | - | - |
| (4) Tollefson, M. K. [19] | 18,472 | 63 | 27.7 | - | - | - | - | - | - | - | They concluded that patients with VTE were significantly older than those not diagnosed with VTE (median age 65 vs. 63 years, p < 0.001). |
| (5) Chan, S.Y. [20] | 109 | 65.7 | <23 (33) >23 (67) | - | - | 18.1 | - | - | - | - | This study concluded that there was no difference in the incidence of DVT between patients with a history of smoking or diabetes or a high body mass (BMI) index and those without. |
| (6) Chalmers, D. J. [21] | 1486 | 59.9 | 28.1 | - | - | - | - | - | - | - | In this study, BMI was not found to be associated with VTE. |
| (7) Abel, E. [22] | 549 | 59.8 | - | - | 1.6 | 43.8 | - | A 5-point increase in body mass index was associated with an increased risk of VTEs (odds ratios of 2.0). | |||
| (8)Dyer, J. [23] | 3213 | 72.5 | - | - | - | - | - | - | - | - | - |
| (9) Van Hemelrijck, M. [24] | 16,304 | - | - | - | 0.6 | - | - | - | - | - | A previous history of VTE is a risk factor in patients undergoing RP. |
| (10)Eifler, J. B. [25] | 773 | 57.8 | 27.3 | - | - | - | - | - | - | - | A high incidence of VTE was found in patients with BMI in the top quartile who concomitantly underwent PLND. |
| (11) Beyer, J. [26] | 411 | 65.0 | 27.0 | 4.0 | 4.8 | - | - | - | - | A statistically higher risk was found in patients with a personal history of VTE; however, family history was not found with increased risk. | |
| (12) Grasso, M. [27] | 500 | 65.0 | - | - | - | - | - | - | - | - | - |
| (13) Cindolo, L. [28] | 184 | 69.0 | >25 (30%) | - | - | 28 | - | - | - | - | - |
| (14) Nakamura, K. [29] | 47 | 64.0 | - | - | - | - | - | - | - | - | - |
| (15)Koya, M. [30] | 1364 | 60.8 | - | - | - | - | - | - | - | - | - |
| (16) Cisek, L. [31] | 1300 | - | - | - | - | - | - | - | - | - | - |
| Study | Total Procedures | Open | Laparoscopic | Robotic | Unknown | PLND (%) |
|---|---|---|---|---|---|---|
| (1) Patel, H. D. [17] | 501 | 124 | - | 377 | - | 83.5 (419) |
| (2) Valverde-Martinez, S. [18] | 610 | 268 | 311 | 31 | - | - |
| (3) Weinberg, A. [14] | 94,709 | 68,244 | - | 26,465 | - | - |
| (4) Tollefson, M. K. [19] | 18,472 | 16,374 | - | 2098 | - | 100 |
| (5) Chan, S. [20] | 109 | - | - | 109 | - | 33.94 (37) |
| (6) Chalmers, D. J. [21] | 1486 | - | - | 1486 | - | 55 |
| (7) Abel, E. [22] | 549 | - | - | 549 | - | 12.9 (71/549) |
| (8) Dyer, J. [23] | 3213 | - | - | - | 3213 | - |
| (9) Van Hemelrijck, M. [24] | 16,304 | 11,137 | - | 5167 | - | 21.6 (3258/16,304) |
| (10) Eifler, J. B. [25] | 770 | - | 770 | - | - | 60.8 (468/770) |
| (11) Beyer, J. [26] | 411 | 411 | - | - | - | 100 |
| (12) Grasso, M. [27] | 500 | 500 | - | - | - | - |
| (13) Cindolo, L. [28] | 184 | 184 | - | - | - | 100 |
| (14) Nakamura, K. [29] | 47 | 47 | - | - | - | 87 (41/47) |
| (15)Koya, M. [30] | 1373 | 1373 | - | - | - | 67 (920/1373) |
| (16) Cisek, L. [31] | 1300 | 1300 | - | - | - | - |
| Total | 140,541 | 100,088 (71.21%) | 1084 (0.77%) | 36,156 (25.72%) | 3213 (2.28%) | 33.82% (6229/18,417) |
| Thromboprophylaxis | VTE Symptomatic Episodes (in %) | |||||||
|---|---|---|---|---|---|---|---|---|
| N | M | P | C | N | M | P | C | |
| (1) Patel, H. D. [17] | - | 250 | - | 251 | - | 2.0 | - | 0.8 |
| (2) Valverde-Martinez, S. [18] | 94 | 25 | 516 | 21 | 2.5 | |||
| (3) Weinberg, A. [14] | 20,438 | 35,591 | 4945 | 7720 | 0.25 | |||
| (4) Tollefson, M. K. [19] | - | - | - | 18,472 | 1.47 | |||
| (5) Chan, S. [20] | - | 109 | - | - | 0.09 | |||
| (6) Chalmers, D. J. [21] | - | 564 | - | 922 | - | 1.0 | - | 0.7 |
| (7) Abel, E. [22] | - | 540 | - | 9 | 1.8 | |||
| (8) Dyer, J. [23] | - | - | - | - | 1.0 | |||
| (9) Van Hemelrijck, M. [24] | - | - | - | - | 1.2 | |||
| (10) Eifler, J. B. [25] | - | 770 | - | - | 1.5 | |||
| (11) Beyer, J. [26] | - | - | - | 411 | 1.9 | |||
| (12) Grasso, M. [27] | - | - | - | 500 | 0.2 | |||
| (13) Cindolo, L. [28] | - | - | - | 184 | 0 | |||
| (14) Nakamura, K. [29] | - | - | - | 47 | 4 | |||
| (15)Koya, M. [30] | - | 1373 | - | - | 0.21 | |||
| (16) Cisek, L. [31] | 784 | 516 | - | - | 2.3 | |||
| Study | VTE Incidence Procedure Specific | DVT Incidence (in %) | PE Incidence (in %) | PLND (VTE) | Post-Op Bleeding Episodes (in %) | |||
|---|---|---|---|---|---|---|---|---|
| O | MIS | O | MIS | O | MIS | |||
| (1) Patel, H. D. [17] | 2.4 | 1.1 | - | - | 1.7 | 1.1 | ||
| (2) Valverde-Martinez, S. [18] | 2.5 | - | 1.4 | - | - | |||
| (3) Weinberg, A. [14] | 0.3 | 0.2 | - | 0.1 | 0.1 | - | - | |
| (4) Tollefson, M. K. [19] | 1.5 | 1.0 | - | 1.8 | 1.47 | - | ||
| (5) Chan, S.Y [20] | - | 0.9 | - | 0.0 | - | - | ||
| (6) Chalmers, D. J. [21] | - | 0.9 | - | - | 1.2 | - | ||
| (7) Abel, E. [22] | - | 1.8 | - | - | 0.5 | - | - | |
| (8) Dyer, J. [23] | 1.0 | - | - | - | - | |||
| (9) Van Hemelrijck, M. [24] | 1.5 | 0.8 | 0.9 | 0.6 | 0.6 | 0.2 | - | - |
| (10)Eifler, J. B. [25] | - | - | 0 | 1.5 | - | - | ||
| (11) Beyer, J. [26] | 1.9 | - | 0.9 | 0.9 | - | - | ||
| (12) Grasso, M. [27] | 0.2 | - | 0 | 0.2 | - | - | ||
| (13) Cindolo, L. [28] | 0 | - | 0 | 0 | - | - | ||
| (14) Nakamura, K. [29] | 4 | - | 0 | 4 | - | 2.1 | ||
| (15)Koya, M. [30] | 0.21 | - | 0.21 | 0 | - | - | ||
| (16) Cisek, L. [31] | 2.3 | - | 0.45 | 1.3 | - | - | ||
| Outcome | Method | Number | Heterogeneity | Outcome Occurrence | |
|---|---|---|---|---|---|
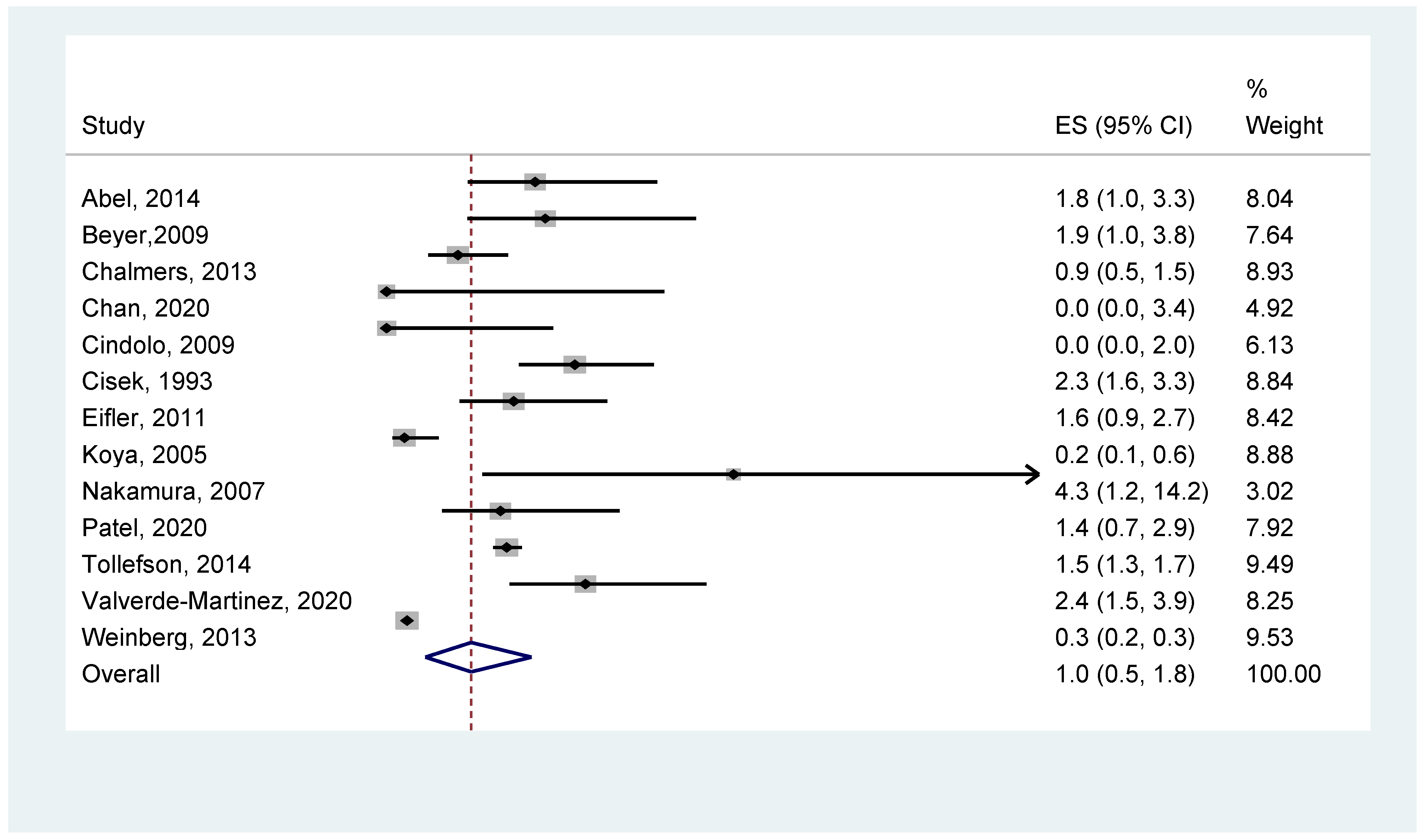
| Studies | p-Value | I2 | % (95% CI) | ||
| VTE | All combined | 16 | <0.001 | 97% | 1.0 (0.5, 1.5) |
| Comparison | Number | Heterogeneity | Group Difference | ||
|---|---|---|---|---|---|
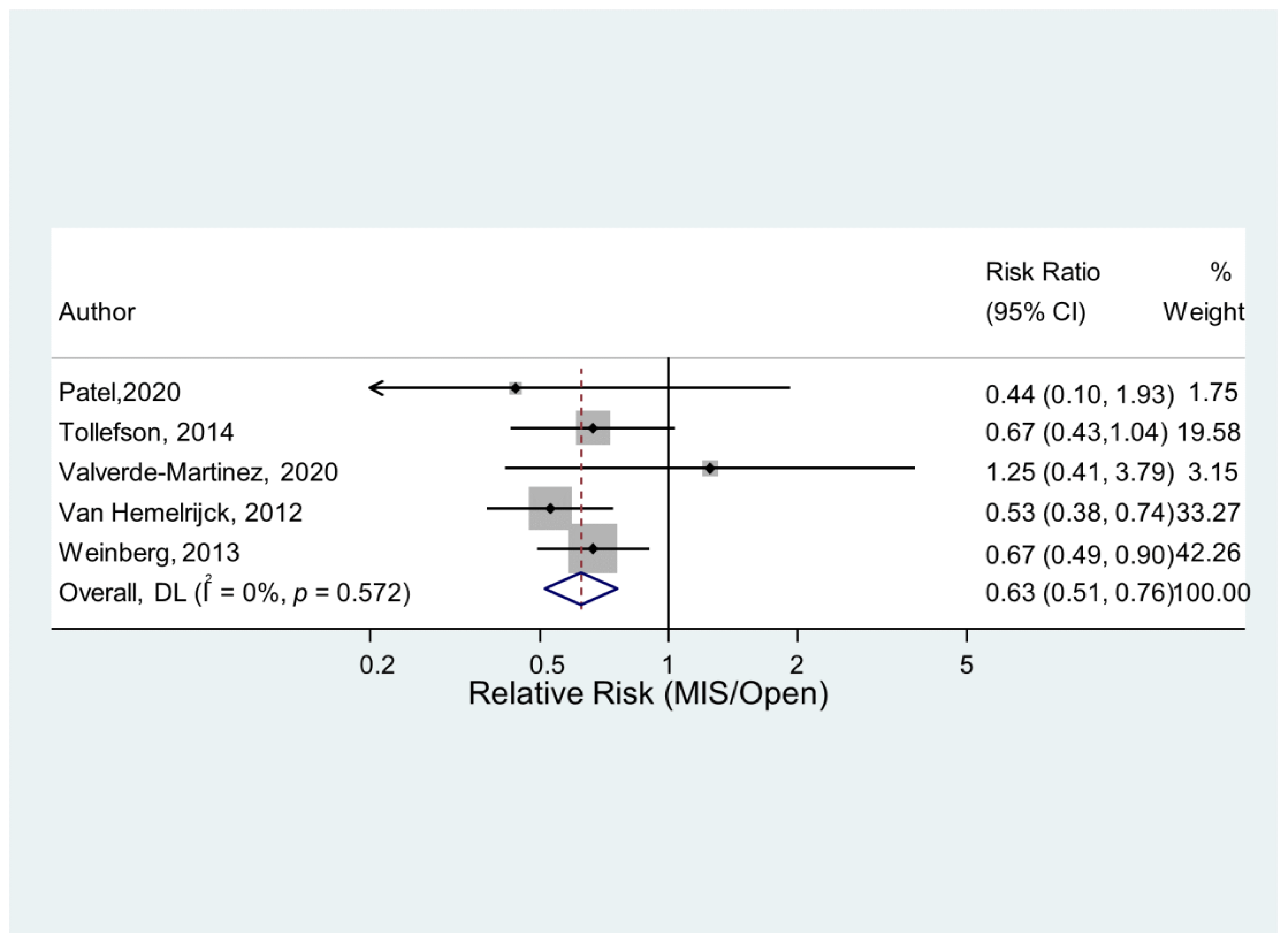
| Studies | p-Value | I2 | RR (95% CI) (*) | p-Value | |
| MIS/Open | 5 | 0.55 | 0% | 0.63 (0.52, 0.77) | <0.001 |
| PLND/no PLND | 2 | 0.96 | 0% | 2.79 (0.86, 8.94) | 0.09 |
| Prophylaxis | Number | Heterogeneity | VTE Occurrence | Method Diff. | |
|---|---|---|---|---|---|
| Method | Studies | p-Value | I2 | % (95% CI) | p-Value |
| Mechanical | 5 | 0.002 | 76% | 0.7 (0.1, 1.6) | 0.42 |
| Combined | 6 | 0.07 | 51% | 1.0 (0.5, 1.6) | |
| Open Radical Prostatectomy (+/− PLND) | |||
| Pharmacological# | Low Risk | Suggests | weak, moderate-quality evidence |
| Medium/High Risk | Recommends | strong, moderate- or high-quality evidence | |
| Mechanical* | All patients | Suggested | weak, low-quality evidence |
| Open radical prostatectomy with extended PLND | |||
| Pharmacological# | All patients | Recommends | strong, moderate, or high-quality evidence |
| Mechanical* | All patients | Suggests | weak, low-quality evidence |
| Laparoscopic Radical prostatectomy (Without PLND) | |||
| Pharmacological# | Low Risk | Recommends (Against) | strong, moderate-quality evidence |
| Medium and high risk | Suggests (Against) | weak, moderate- or high-quality evidence | |
| Mechanical* | Low risk | Suggests (Against) | weak, low-quality evidence |
| Medium and high risk | Suggests | weak, low-quality evidence | |
| Laparoscopic Radical prostatectomy (With Standard PLND) | |||
| Pharmacological# | Low Risk | Recommends (Against) | strong, moderate-quality evidence |
| Medium Risk | Suggests (Against) | weak, moderate- or high-quality evidence | |
| High Risk | Recommends | strong, high-quality evidence | |
| Mechanical* | All patients | Suggests | weak, low-quality evidence |
| Laparoscopic Radical prostatectomy (With Extended PLND) | |||
| Pharmacological# | Low Risk | Suggests (Against) | weak, moderate-quality evidence |
| Medium Risk | Suggests | weak, high-quality evidence | |
| High Risk | Recommends | strong, high-quality evidence | |
| Mechanical* | All patients | Suggested | weak, low-quality evidence |
| Robotic Radical prostatectomy (Without PLND) | |||
| Pharmacological# | Low Risk | Recommends (Against) | strong, moderate-quality evidence |
| Medium and High Risk | Suggests (Against) | weak, moderate-quality evidence | |
| Mechanical* | Low Risk | Suggests (Against) | weak, low-quality evidence |
| Medium and High Risk | Suggests | weak, low-quality evidence | |
| Robotic Radical prostatectomy (With Standard PLND) | |||
| Pharmacological# | Low Risk | Recommends (Against) | strong, moderate-quality evidence |
| Medium Risk | Suggests | weak, moderate-quality evidence | |
| High Risk | Suggests | weak, moderate-quality evidence | |
| Mechanical* | All patients | Suggests | weak, low-quality evidence |
| Robotic Radical prostatectomy (With Extended PLND) | |||
| Pharmacological# | Low Risk | Suggests (Against) | weak, moderate-quality evidence |
| Medium Risk | Suggests | weak, moderate-quality evidence | |
| High Risk | Recommends | strong, moderate-quality evidence | |
| Mechanical* | All patients | Suggests | weak, low-quality evidence |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wani, M.; Al-Mitwalli, A.; Mukherjee, S.; Nabi, G.; Somani, B.K.; Abbaraju, J.; Madaan, S. Venous Thromboembolism (VTE) in Post-Prostatectomy Patients: Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 3979. https://doi.org/10.3390/jcm12123979
Wani M, Al-Mitwalli A, Mukherjee S, Nabi G, Somani BK, Abbaraju J, Madaan S. Venous Thromboembolism (VTE) in Post-Prostatectomy Patients: Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(12):3979. https://doi.org/10.3390/jcm12123979
Chicago/Turabian StyleWani, Mudassir, Abdullah Al-Mitwalli, Subhabrata Mukherjee, Ghulam Nabi, Bhaskar K. Somani, Jayasimha Abbaraju, and Sanjeev Madaan. 2023. "Venous Thromboembolism (VTE) in Post-Prostatectomy Patients: Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 12: 3979. https://doi.org/10.3390/jcm12123979
APA StyleWani, M., Al-Mitwalli, A., Mukherjee, S., Nabi, G., Somani, B. K., Abbaraju, J., & Madaan, S. (2023). Venous Thromboembolism (VTE) in Post-Prostatectomy Patients: Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 12(12), 3979. https://doi.org/10.3390/jcm12123979