Effect of Turmeric–Boswellia–Sesame Formulation in Menstrual Cramp Pain Associated with Primary Dysmenorrhea—A Double-Blind, Randomized, Placebo-Controlled Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participant Selection
2.3. Study Intervention
2.4. Randomization Procedures
2.5. Pain Assessment
2.5.1. Categorical Pain Intensity (0–3) (at Screening)
2.5.2. Numerical Rating Scale for Pain (NRS) (0–10)
2.5.3. Categorical Pain Relief Scale (0–4) (PRS)
2.5.4. Global Evaluation Assessment
2.6. Study Outcome Measures
2.7. Evaluation of Safety
2.8. Study Procedure
2.8.1. Screening Phase Visit 1 (Day-21 to -1):
2.8.2. Treatment Phase
2.9. Statistical Analysis
3. Results
4. Discussion
Limitations and Future Direction of Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ju, H.; Jones, M.; Mishra, G. The Prevalence and Risk Factors of Dysmenorrhea. Epidemiol. Rev. 2014, 36, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Lobo, R.A.; Gershenson, D.M.; Lentz, G.M.; Valea, F.A. Comprehensive Gynecology E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2016; ISBN 0323430031. [Google Scholar]
- Abd-El-Maeboud, K.H.I.; Kortam, M.A.M.F.; Ali, M.S.; Ibrahim, M.I.; Mohamed, R.M.M.Z. A Preliminary Pilot Randomized Crossover Study of Uzara (Xysmalobium undulatum) versus Ibuprofen in the Treatment of Primary Dysmenorrhea. PLoS ONE 2014, 9, e104473. [Google Scholar] [CrossRef] [Green Version]
- Patel, V.; Tanksale, V.; Sahasrabhojanee, M.; Gupte, S.; Nevrekar, P. The Burden and Determinants of Dysmenorrhoea: A Population-based Survey of 2262 Women in Goa, India. BJOG Int. J. Obstet. Gynaecol. 2006, 113, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Harlow, S.D.; Campbell, O.M.R. Epidemiology of Menstrual Disorders in Developing Countries: A Systematic Review. BJOG Int. J. Obstet. Gynaecol. 2004, 111, 6–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weissman, A.M.; Hartz, A.J.; Hansen, M.D.; Johnson, S.R. The Natural History of Primary Dysmenorrhoea: A Longitudinal Study. BJOG Int. J. Obstet. Gynaecol. 2004, 111, 345–352. [Google Scholar] [CrossRef]
- Berkley, K.J.; McAllister, S.L. Don’t Dismiss Dysmenorrhea! Pain 2011, 152, 1940–1941. [Google Scholar] [CrossRef]
- Chantler, I.; Mitchell, D.; Fuller, A. Actigraphy Quantifies Reduced Voluntary Physical Activity in Women with Primary Dysmenorrhea. J. Pain 2009, 10, 38–46. [Google Scholar] [CrossRef]
- Iacovides, S.; Avidon, I.; Baker, F.C. What We Know about Primary Dysmenorrhea Today: A Critical Review. Hum. Reprod. Update 2015, 21, 762–778. [Google Scholar] [CrossRef] [Green Version]
- Dawood, M.Y. Primary Dysmenorrhea: Advances in Pathogenesis and Management. Obstet. Gynecol. 2006, 108, 428–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, C.L.; Farquhar, C.; Roberts, H.; Proctor, M. Oral Contraceptive Pill as Treatment for Primary Dysmenorrhoea. Cochrane Database Syst. Rev. 2009.
- Lefebvre, G.; Pinsonneault, O.; Antao, V.; Black, A.; Burnett, M.; Feldman, K.; Lea, R.; Robert, M. Primary Dysmenorrhea Consensus Guideline. J. Obstet. Gynaecol. Can 2005, 27, 1117–1146. [Google Scholar] [PubMed]
- Mahvash, N.; Eidy, A.; Mehdi, K.; Zahra, M.T.; Mani, M.; Shahla, H. The Effect of Physical Activity on Primary Dysmenorrhea of Female University Students. World Appl. Sci. J. 2012, 17, 1246–1252. [Google Scholar]
- Bernardi, M.; Lazzeri, L.; Perelli, F.; Reis, F.M.; Petraglia, F. Dysmenorrhea and Related Disorders. F1000Res 2017, 6, 1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harel, Z. Dysmenorrhea in Adolescents and Young Adults: An Update on Pharmacological Treatments and Management Strategies. Expert. Opin. Pharmacother. 2012, 13, 2157–2170. [Google Scholar] [CrossRef] [PubMed]
- Oladosu, F.A.; Tu, F.F.; Hellman, K.M. Nonsteroidal Antiinflammatory Drug Resistance in Dysmenorrhea: Epidemiology, Causes, and Treatment. Am. J. Obstet. Gynecol. 2018, 218, 390–400. [Google Scholar] [CrossRef]
- Burnett, M.; Lemyre, M. No. 345-Primary Dysmenorrhea Consensus Guideline. J. Obstet. Gynaecol. Can. 2017, 39, 585–595. [Google Scholar] [CrossRef]
- Zahradnik, H.-P.; Hanjalic-Beck, A.; Groth, K. Nonsteroidal Anti-Inflammatory Drugs and Hormonal Contraceptives for Pain Relief from Dysmenorrhea: A Review. Contraception 2010, 81, 185–196. [Google Scholar] [CrossRef]
- Mirabi, P.; Alamolhoda, S.H.; Esmaeilzadeh, S.; Mojab, F. Effect of Medicinal Herbs on Primary Dysmenorrhoea-a Systematic Review. Iran. J. Pharm. Res. 2014, 13, 757. [Google Scholar]
- Lantz, R.C.; Chen, G.J.; Solyom, A.M.; Jolad, S.D.; Timmermann, B.N. The Effect of Turmeric Extracts on Inflammatory Mediator Production. Phytomedicine 2005, 12, 445–452. [Google Scholar] [CrossRef]
- Bahrami, A.; Zarban, A.; Rezapour, H.; Agha Amini Fashami, A.; Ferns, G.A. Effects of Curcumin on Menstrual Pattern, Premenstrual Syndrome, and Dysmenorrhea: A Triple-blind, Placebo-controlled Clinical Trial. Phytother. Res. 2021, 35, 6954–6962. [Google Scholar] [CrossRef]
- Singh, G.B.; Atal, C.K. Pharmacology of an Extract of Salai Guggal Ex-Boswellia Serrata, a New Non-Steroidal Anti-Inflammatory Agent. Agents Actions 1986, 18, 407–412. [Google Scholar] [CrossRef]
- Basch, E.; Boon, H.; Heerema, T.D.; Foppo, I.; Hashmi, S.; Hasskarl, J.; Sollars, D.; Ulbricht, C. Boswellia: An Evidence-Based Systematic Review by the Natural Standard Research Collaboration. J. Herb. Pharmacother. 2004, 4, 63–83. [Google Scholar] [CrossRef]
- Singletary, K.W. Sesame: Potential Health Benefits. Nutr. Today 2022, 57, 271–287. [Google Scholar] [CrossRef]
- Smith, R.P. The Role of Prostaglandins in Dysmenorrhea and Menorrhagia. In Dysmenorrhea and Menorrhagia; Springer: Berlin/Heidelberg, Germany, 2018; pp. 75–88. [Google Scholar]
- Agresti, A. Analysis of Ordinal Categorical Data; John Wiley & Sons: Hoboken, NJ, USA, 2010; Volume 656, ISBN 0470082895. [Google Scholar]
- Lee, L.K.; Chen, P.C.Y.; Lee, K.K.; Kaur, J. Menstruation among Adolescent Girls in Malaysia: A Cross-Sectional School Survey. Singap. Med. J. 2006, 47, 869. [Google Scholar]
- Dawood, M.Y.; Khan-Dawood, F.S. Clinical Efficacy and Differential Inhibition of Menstrual Fluid Prostaglandin F2α in a Randomized, Double-Blind, Crossover Treatment with Placebo, Acetaminophen, and Ibuprofen in Primary Dysmenorrhea. Am. J. Obstet. Gynecol. 2007, 196, 35-e1. [Google Scholar] [CrossRef] [PubMed]
- Harel, Z. Dysmenorrhea in Adolescents and Young Adults: Etiology and Management. J. Pediatr. Adolesc. Gynecol. 2006, 19, 363–371. [Google Scholar] [CrossRef]
- Mueller, A.; Siemer, J.; Schreiner, S.; Koesztner, H.; Hoffmann, I.; Binder, H.; Beckmann, M.W.; Dittrich, R. Role of Estrogen and Progesterone in the Regulation of Uterine Peristalsis: Results from Perfused Non-Pregnant Swine Uteri. Human. Reprod. 2006, 21, 1863–1868. [Google Scholar] [CrossRef] [Green Version]
- Ham, E.A.; Cirillo, V.J.; Zanetti, M.E.; Kuehl, F.A., Jr. Estrogen-Directed Synthesis of Specific Prostaglandins in Uterus. Proc. Natl. Acad. Sci. USA 1975, 72, 1420–1424. [Google Scholar] [CrossRef] [Green Version]
- Maybin, J.A.; Critchley, H.O.D.; Jabbour, H.N. Inflammatory Pathways in Endometrial Disorders. Mol. Cell. Endocrinol. 2011, 335, 42–51. [Google Scholar] [CrossRef]
- Utami, R.B.; Damayanti, D.F.; Rodiah, D. The Effectiveness of Curcuma Longa Drink in Decreasing the Intensity of Dysmenorrhea. Biomed. Pharmacol. J. 2020, 13, 2055–2060. [Google Scholar] [CrossRef]
- Bahmani, M.; Eftekhari, Z.; Jelodari, M.; Saki, K.; Abdollahi, R.; Majlesi, M.; Rafieian Kopaei, M.; Rasouli, S. Effect of Iranian Herbal Medicines in Dysmenorrhea Phytotherapy. J. Chem. Pharm. Res. 2015, 7, 519–526. [Google Scholar]
- Okuyan, E.; Günakan, E.; Halit, A.; Çakmak, Y. The Effect of Turmeric on Primary Dysmenorrhea: Prospective Case-Control Study. J. Surg. Med. 2021, 5, 715–717. [Google Scholar] [CrossRef]
- Mohammadian, F.; Ghoreishi, A.; Sheikh Fathollali, M.; Manshoori, A.; Hajizadeh, M.; Khoshdel, A.; Shahriari, G.; Mahmoodi, M. The Effect of Consuming Sesame (Sesamum Indicum) on Primary Dysmenorrhea in Students at Rafsanjan University of Medical Sciences (RUMS). Community Health J. 2017, 8, 1–9. [Google Scholar]
- Yavari, M.; Rouholamin, S.; Tansaz, M.; Bioos, S.; Esmaeili, S. Sesame a Treatment of Menstrual Bleeding Cessation in Iranian Traditional Medicine: Results from a Pilot Study. Shiraz E. Med. J. 2014, 15, e21893. [Google Scholar] [CrossRef]
- Su, S.; Hua, Y.; Wang, Y.; Gu, W.; Zhou, W.; Duan, J.; Jiang, H.; Chen, T.; Tang, Y. Evaluation of the Anti-Inflammatory and Analgesic Properties of Individual and Combined Extracts from Commiphora Myrrha, and Boswellia Carterii. J. Ethnopharmacol. 2012, 139, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cao, H.; Yu, Z.; Peng, H.-Y.; Zhang, C. Curcumin Inhibits Endometriosis Endometrial Cells by Reducing Estradiol Production. Iran. J. Reprod. Med. 2013, 11, 415. [Google Scholar] [PubMed]
- Bachmeier, B.E.; Mirisola, V.; Romeo, F.; Generoso, L.; Esposito, A.; Dell’Eva, R.; Blengio, F.; Killian, P.H.; Albini, A.; Pfeffer, U. Reference Profile Correlation Reveals Estrogen-like Trancriptional Activity of Curcumin. Cell. Physiol. Biochem. 2010, 26, 471–482. [Google Scholar] [CrossRef] [Green Version]
- Dassen, H.; Punyadeera, C.; Kamps, R.; Delvoux, B.; van Langendonckt, A.; Donnez, J.; Husen, B.; Thole, H.; Dunselman, G.; Groothuis, P. Estrogen Metabolizing Enzymes in Endometrium and Endometriosis. Human. Reprod. 2007, 22, 3148–3158. [Google Scholar] [CrossRef] [Green Version]
- Thaina, P.; Tungcharoen, P.; Wongnawa, M.; Reanmongkol, W.; Subhadhirasakul, S. Uterine Relaxant Effects of Curcuma Aeruginosa Roxb. Rhizome Extracts. J. Ethnopharmacol. 2009, 121, 433–443. [Google Scholar] [CrossRef]
- Khayat, S.; Fanaei, H.; Kheirkhah, M.; Moghadam, Z.B.; Kasaeian, A.; Javadimehr, M. Curcumin Attenuates Severity of Premenstrual Syndrome Symptoms: A Randomized, Double-Blind, Placebo-Controlled Trial. Complement. Ther. Med. 2015, 23, 318–324. [Google Scholar] [CrossRef]
- Penalvo, J.L.; Heinonen, S.-M.; Aura, A.-M.; Adlercreutz, H. Dietary Sesamin Is Converted to Enterolactone in Humans. J. Nutr. 2005, 135, 1056–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namiki, M. The Chemistry and Physiological Functions of Sesame. Food Rev. Int. 1995, 11, 281–329. [Google Scholar] [CrossRef]
- Kurzer, M.S.; Xu, X. Dietary Phytoestrogens. Annu. Rev. Nutr. 1997, 17, 353. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Z.; Ma, X.-Q.; Yang, D.-H.; Guo, Z.-R.; Liu, G.-R.; Zhao, G.-X.; Tang, J.; Zhang, Y.-N.; Ma, M.; Cai, S.-Q.; et al. Production of Enterodiol from Defatted Flaxseeds through Biotransformation by Human Intestinal Bacteria. BMC Microbiol. 2010, 10, 115. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.-H.; Kang, Y.-P.; Wang, N.-H.; Jou, H.-J.; Wang, T.-A. Sesame Ingestion Affects Sex Hormones, Antioxidant Status, and Blood Lipids in Postmenopausal Women. J. Nutr. 2006, 136, 1270–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Parameters | Placebo | Treatment | ||||
|---|---|---|---|---|---|---|
| Mean | SD | SE | Mean | SD | SE | |
| Age (years) | 26.40 | 4.55 | 0.83 | 26.67 | 4.26 | 0.78 |
| Height (cm) | 156.31 | 5.17 | 0.94 | 155.30 | 4.34 | 0.79 |
| Weight (kg) | 54.07 | 4.95 | 0.90 | 54.10 | 5.27 | 0.96 |
| Duration of menstrual cycle (days) | 28.50 | 2.71 | 0.50 | 29.27 | 2.49 | 0.45 |
| Variable | Count | Mean | SD | SE | 95% LCL (Mean) | 95% UCL (Mean) | p-Value |
|---|---|---|---|---|---|---|---|
| Placebo | 30 | 1.5 | 2.11 | 0.39 | 0.71 | 2.29 | p < 0.001 * |
| Treatment | 30 | 18.9 | 3.08 | 0.56 | 17.75 | 20.05 |
| Category (% Max TOTPAR) | Placebo | Treatment | Placebo (%) | Treatment (%) |
|---|---|---|---|---|
| <30 | 20 | 0 | 66.67 | 0 |
| 30–49 | 4 | 0 | 13.33 | 0 |
| 50–69 | 1 | 6 | 3.33 | 20 |
| ≥70 | 5 | 24 | 16.67 | 80 |
| SPID (0–6 h) | Treatment (n = 30) | Placebo (n = 30) |
|---|---|---|
| Mean | 34.317 | 1.7 |
| SD | 7.7454 | 3.0727 |
| 95% CI LCL (Mean) | 31.4245 | 0.5526 |
| 95% CI UCL (Mean) | 37.2088 | 2.8474 |
| Mean Difference (Placebo treatment) | −32.6 | |
| SE (Mean Diff) | 1.52132 | |
| 95% CI LCL (Mean Difference) | −35.66192 | |
| 95% CI UCL (Mean Difference) | −29.57141 | |
| p 2-sided * | <0.0001 | |
| Time | Placebo | Treatment | LSM Difference (P-T) | * p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LSM | SE (LSM) | 95% CI | p-Value | LSM | SE (LSM) | 95% CI | p-Value | |||||
| LL | UL | LL | UL | |||||||||
| 0.5 | 0.2333 | 0.2079 | −0.1789 | 0.6455 | 1.0000 | 2.7 | 0.2079 | 2.2878 | 3.1122 | <0.0001 | −2.4667 | <0.0001 |
| 1 | 0.3333 | 0.2079 | −0.0789 | 0.7455 | 1.0000 | 3.7667 | 0.2079 | 3.3545 | 4.1789 | <0.0001 | −3.4333 | <0.0001 |
| 1.5 | 0.2333 | 0.2079 | −0.1789 | 0.6455 | 1.0000 | 4.6 | 0.2079 | 4.1878 | 5.0122 | <0.0001 | −4.3667 | <0.0001 |
| 0.2 | 0.2079 | −0.2122 | 0.6122 | 1.0000 | 5.2667 | 0.2079 | 4.8545 | 5.6789 | <0.0001 | −5.0667 | <0.0001 | |
| 2.5 | 0.2667 | 0.2079 | −0.1455 | 0.6789 | 1.0000 | 5.7667 | 0.2079 | 5.3545 | 6.1789 | <0.0001 | −5.5 | <0.0001 |
| 3 | 0.2 | 0.2079 | −0.2122 | 0.6122 | 1.0000 | 6.1667 | 0.2079 | 5.7545 | 6.5789 | <0.0001 | −5.9667 | <0.0001 |
| 3.5 | 0.3333 | 0.2079 | −0.0789 | 0.7455 | 1.0000 | 6.4667 | 0.2079 | 6.0545 | 6.8789 | <0.0001 | −6.1333 | <0.0001 |
| 4 | 0.3333 | 0.2079 | −0.0789 | 0.7455 | 1.0000 | 6.6333 | 0.2079 | 6.2211 | 7.0455 | <0.0001 | −6.3 | <0.0001 |
| 4.5 | 0.3333 | 0.2079 | −0.0789 | 0.7455 | 1.0000 | 6.7333 | 0.2079 | 6.3211 | 7.1455 | <0.0001 | −6.4 | <0.0001 |
| 5 | 0.3 | 0.2079 | −0.1122 | 0.7122 | 1.0000 | 6.7667 | 0.2079 | 6.3545 | 7.1789 | <0.0001 | −6.4667 | <0.0001 |
| 5.5 | 0.3667 | 0.2079 | −0.0455 | 0.7789 | 1.0000 | 6.8667 | 0.2079 | 6.4545 | 7.2789 | <0.0001 | −6.5 | <0.0001 |
| 6 | 0.2667 | 0.2079 | −0.1455 | 0.6789 | 1.0000 | 6.9 | 0.2079 | 6.4878 | 7.3122 | <0.0001 | −6.6333 | <0.0001 |
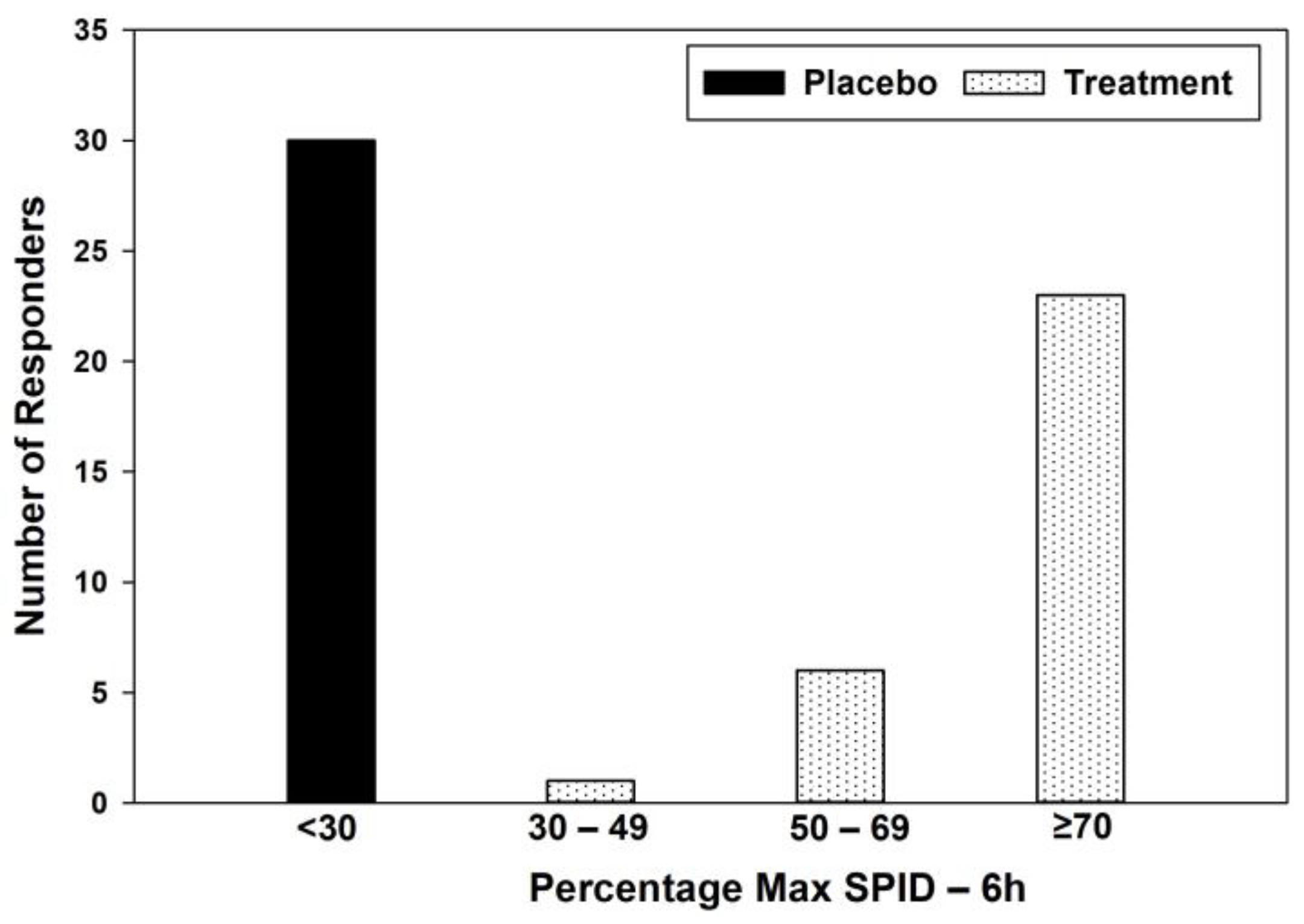
| Category | Placebo | Treatment | Placebo (%) | Treatment (%) |
|---|---|---|---|---|
| <30 | 30 | 0 | 100 | 0 |
| 30–49 | 0 | 1 | 0 | 3.33 |
| 50–69 | 0 | 6 | 0 | 20 |
| ≥70 | 0 | 23 | 0 | 76.67 |
| Poor (=0) | Fair (=1) | Good (=2) | Very Good (=3) | Excellent (=4) | Mean ± SD | Mean Difference ± SE | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Treatment, n (%) | 0 | 0 | 0 | 8 (26.7%) | 22 (73.3%) | 3.73 ± 0.45 | −3.57 ± 0.11 | <0.0001 |
| Placebo, n (%) | 25 (83.3%) | 5 (16.7%) | 0 | 0 | 0 | 0.17 ± 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agarwal, D.; Chaudhary, P. Effect of Turmeric–Boswellia–Sesame Formulation in Menstrual Cramp Pain Associated with Primary Dysmenorrhea—A Double-Blind, Randomized, Placebo-Controlled Study. J. Clin. Med. 2023, 12, 3968. https://doi.org/10.3390/jcm12123968
Agarwal D, Chaudhary P. Effect of Turmeric–Boswellia–Sesame Formulation in Menstrual Cramp Pain Associated with Primary Dysmenorrhea—A Double-Blind, Randomized, Placebo-Controlled Study. Journal of Clinical Medicine. 2023; 12(12):3968. https://doi.org/10.3390/jcm12123968
Chicago/Turabian StyleAgarwal, Divya, and Priyanka Chaudhary. 2023. "Effect of Turmeric–Boswellia–Sesame Formulation in Menstrual Cramp Pain Associated with Primary Dysmenorrhea—A Double-Blind, Randomized, Placebo-Controlled Study" Journal of Clinical Medicine 12, no. 12: 3968. https://doi.org/10.3390/jcm12123968
APA StyleAgarwal, D., & Chaudhary, P. (2023). Effect of Turmeric–Boswellia–Sesame Formulation in Menstrual Cramp Pain Associated with Primary Dysmenorrhea—A Double-Blind, Randomized, Placebo-Controlled Study. Journal of Clinical Medicine, 12(12), 3968. https://doi.org/10.3390/jcm12123968






