A Personalized Approach to Determining the Caloric Needs of Children with Prader–Willi Syndrome Treated with Growth Hormone
Abstract
1. Introduction
2. Methods
2.1. Study Design and Population
2.2. Variables
2.3. Measurements
2.3.1. Auxological Variables
2.3.2. Daily Caloric Intake
2.3.3. Physical Activity
2.3.4. Total Energy Expenditure
2.4. Total Energy Expenditure = Henry BMR × Activity Factor Ethics
2.5. Statistical Analysis
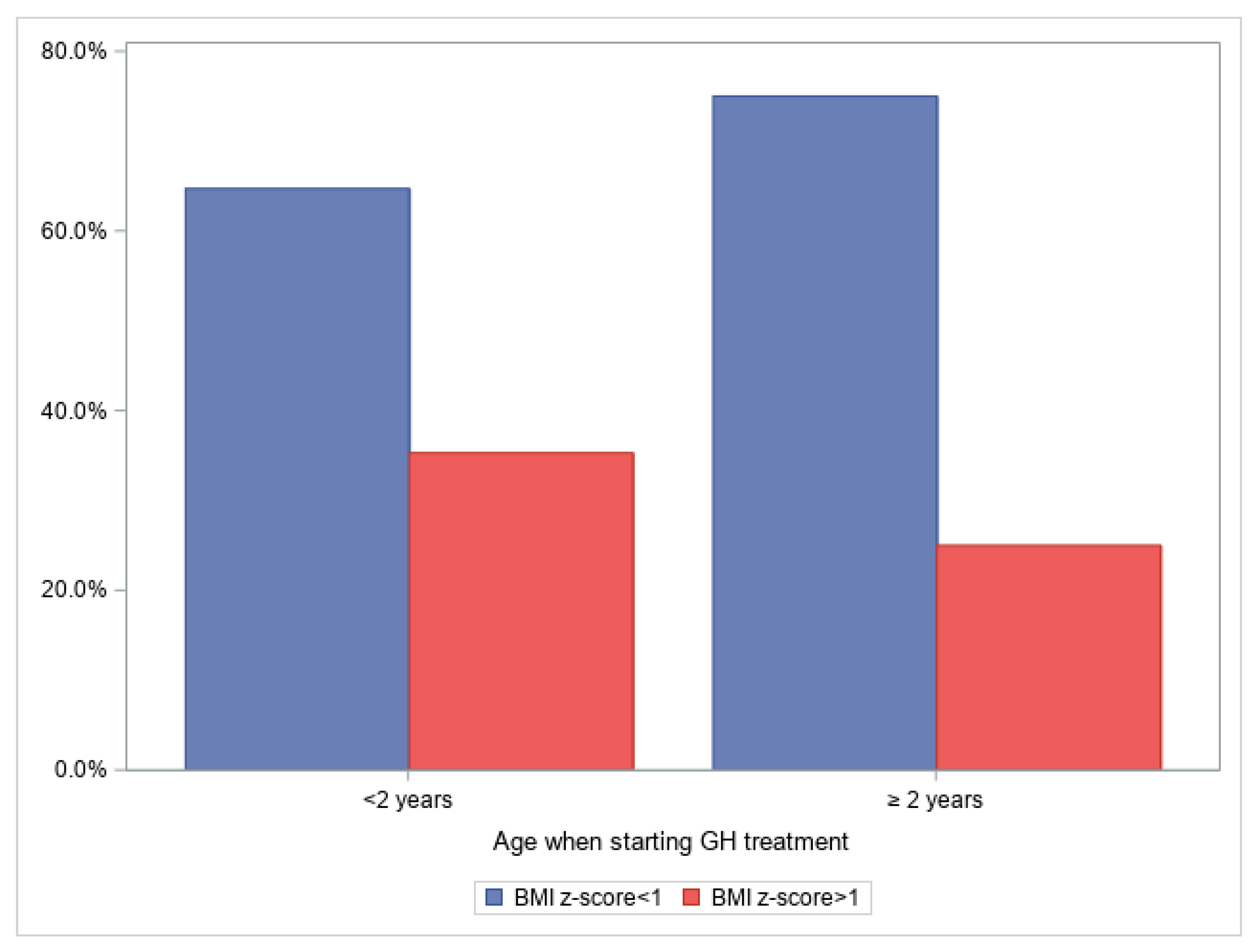
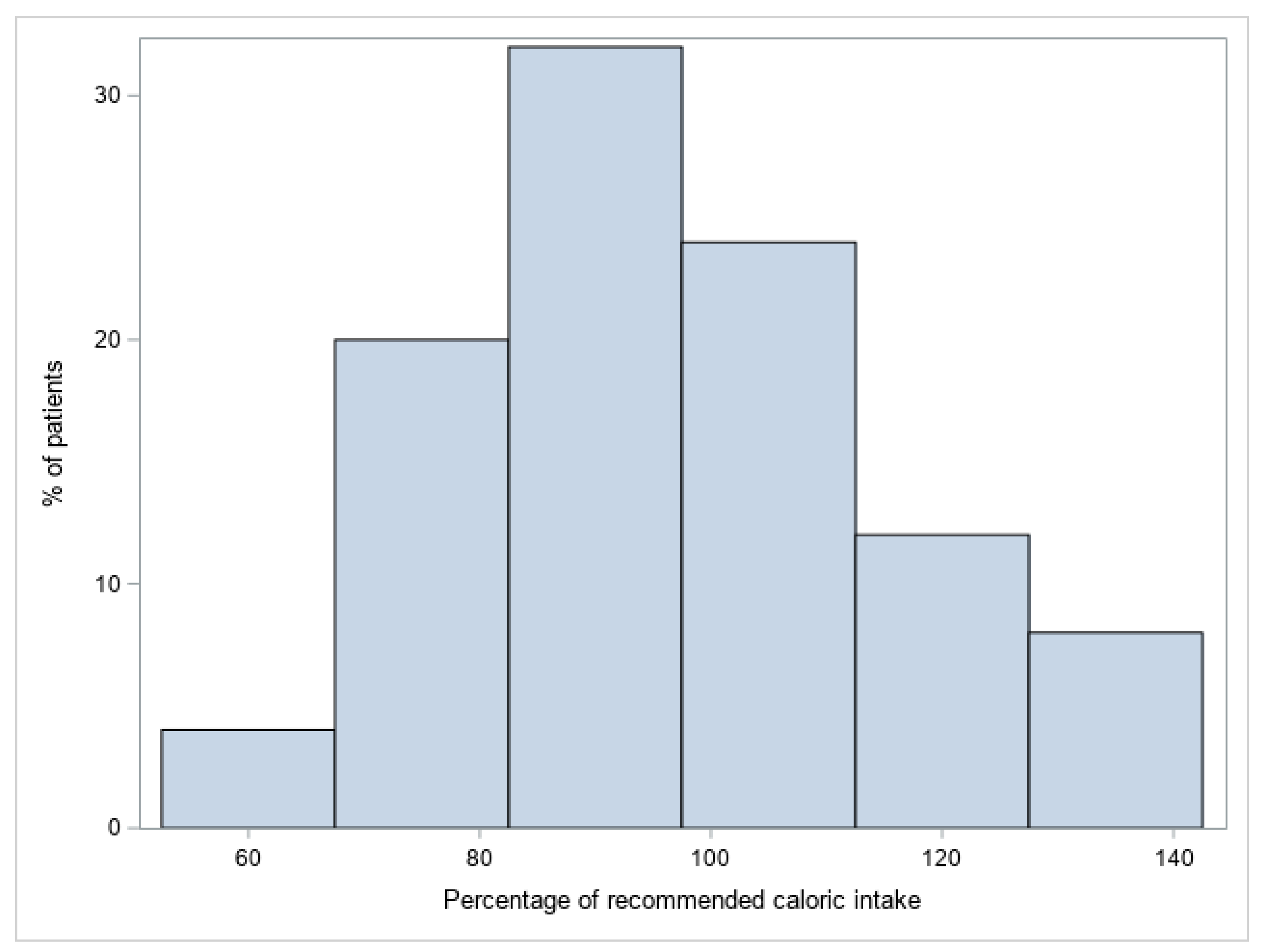
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Butler, M.G.; Manzardo, A.M.; Forster, J.L. Prader-Willi Syndrome: Clinical Genetics and Diagnostic Aspects with Treatment Approaches. Curr. Pediatr. Rev. 2016, 12, 136–166. [Google Scholar] [CrossRef]
- Butler, M.G.; Lee, J.; Cox, D.M.; Manzardo, A.M.; Gold, J.A.; Miller, J.L.; Roof, E.; Dykens, E.; Kimonis, V.; Driscoll, D.J. Growth Charts for Prader-Willi Syndrome during Growth Hormone Treatment. Clin. Pediatr. 2016, 55, 957–974. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Faggiano, F.; Maiorino, M.I.; Parrillo, M.; Pugliese, G.; Ruggeri, R.M.; Scarano, E.; Savastano, S.; Colao, A.; et al. Obesity in Prader–Willi syndrome: Physiopathological mechanisms, nutritional and pharmacological approaches. J. Endocrinol. Investig. 2021, 44, 2057–2070. [Google Scholar] [CrossRef]
- Cassidy, S.B.; Schwartz, S.; Miller, J.L.; Driscoll, D.J. Prader-Willi syndrome. Genet. Med. 2012, 14, 10–26. [Google Scholar] [CrossRef]
- Bekx, M.T.; Carrel, A.L.; Shriver, T.C.; Li, Z.; Allen, D.B. Decreased energy expenditure is caused by abnormal body composition in infants with Prader-Willi Syndrome. J. Pediatr. 2003, 143, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Schoeller, D.A.; Levitsky, L.L.; Bandini, L.G.; Dietz, W.W.; Walczak, A. Energy expenditure and body composition in Prader-Willi syndrome. Metabolism 1988, 37, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Alsaif, M.; Elliot, S.A.; MacKenzie, M.L.; Prado, C.M.; Field, C.J.; Haqq, A.M. Energy Metabolism Profile in Individuals with Prader-Willi Syndrome and Implications for Clinical Management: A Systematic Review. Adv. Nutr. 2017, 8, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.G. Management of obesity in Prader-Willi syndrome. Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 592–593. [Google Scholar] [CrossRef]
- Holm, V.A.; Pipes, P.L. Food and children with prader-willi syndrome. Am. J. Dis. Child. 1976, 130, 1063–1067. [Google Scholar] [CrossRef]
- Butler, M.G.; Miller, J.L.; Forster, J.L. Prader-Willi Syndrome—Clinical Genetics, Diagnosis and Treatment Approaches: An Update. Curr. Pediatr. Rev. 2019, 15, 207–244. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Orsso, C.E.; Deehan, E.C.; Triador, L.; Field, C.J.; Tun, H.M.; Han, J.C.; Müller, T.D.; Haqq, A.M. Current and emerging therapies for managing hyperphagia and obesity in Prader-Willi syndrome: A narrative review. Obes. Rev. 2020, 21, e12992. [Google Scholar] [CrossRef]
- Fermin Gutierrez, M.A.; Mendez, M.D. Prader-Willi Syndrome. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Bakker, N.E.; Kuppens, R.J.; Siemensma, E.P.; Tummers-de Lind Van Wijngaarden, R.F.; Festen, D.A.; Bindels-de Heus, G.C.; Bocca, G.; Haring, D.A.J.P.; Hoorweg-Nijman, J.J.G.; Houdijk, E.C.A.M.; et al. Eight years of growth hormone treatment in children with prader-willi syndrome: Maintaining the positive effects. J. Clin. Endocrinol. Metab. 2013, 98, 4013–4022. [Google Scholar] [CrossRef]
- Carrel, A.L.; Myers, S.E.; Whitman, B.Y.; Eickhoff, J.; Allen, D.B. Long-term growth hormone therapy changes the natural history of body composition and motor function in children with prader-willi syndrome. J. Clin. Endocrinol. Metab. 2010, 95, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Fernández, C.; Lorenzo, H.; Vrotsou, K.; Aresti, U.; Rica, I.; Sánchez, E. Estudio de Crecimiento de Bilbao. Curvas y Tablas de Crecimiento. Estudio Transversal; Fundación Fasustino Orbegozo: Bilbao, Spain, 2011; ISBN 978-84-615-7707-1. [Google Scholar]
- Walker, J.L.; Ardouin, S.; Burrows, T. The validity of dietary assessment methods to accurately measure energy intake in children and adolescents who are overweight or obese: A systematic review. Eur. J. Clin. Nutr. 2018, 72, 185–197. [Google Scholar] [CrossRef]
- National Academy (Institute) of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; National Academy of Science: Washington, DC, USA, 2005. [Google Scholar]
- Henry, C. Basal metabolic rate studies in humans: Measurement and development of new equations. Public Health Nutr. 2005, 8, 1133–1152. [Google Scholar] [CrossRef]
- Lindmark, M.; Trygg, K.; Giltvedt, K.; Kolset, S.O. Nutritient intake of young children with Prader–Willi syndrome. Food Nutr. Res. 2010, 54, 2112. [Google Scholar] [CrossRef]
- Meade, C.; Martin, R.; McCrann, A.; Lyons, J.; Roche, E. Dietary intake and growth in children with Prader–Willi syndrome. J. Hum. Nutr. Diet. 2021, 34, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Bakker, N.E.; Siemensma, E.P.; Koopman, C.; Hokken-Koelega, A.C. Dietary Energy Intake, Body Composition and Resting Energy Expenditure in Prepubertal Children with Prader-Willi Syndrome before and during Growth Hormone Treatment: A Randomized Controlled Trial. Horm. Res. Paediatr. 2015, 83, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, M.L.; Triador, L.; Gill, J.K.; Pakseresht, M.; Mager, D.; Field, C.J.; Haqq, A.M. Dietary intake in youth with prader-willi syndrome. Am. J. Med. Genet. Part A 2018, 176, 2309–2317. [Google Scholar] [CrossRef]
- Rubin, D.A.; Nowak, J.; McLaren, E.; Patiño, M.; Castner, D.M.; Dumont-Driscoll, M.C. Nutritional intakes in children with Prader–Willi syndrome and non-congenital obesity. Food Nutr. Res. 2015, 59, 29427. [Google Scholar] [CrossRef]
- Garcia-Ribera, S.; Amat-Bou, M.; Climent, E.; Llobet, M.; Chenoll, E.; Corripio, R.; Ibáñez, L.; Ramon-Krauel, M.; Lerin, C. Specific Dietary Components and Gut Microbiota Composition are Associated with Obesity in Children and Adolescents with Prader–Willi Syndrome. Nutrients 2020, 12, 1063. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.L.; Lynn, C.H.; Shuster, J.J.; Driscoll, D.J. A reduced-energy intake, well-balanced diet improves weight control in children with Prader-Willi syndrome. J. Hum. Nutr. Diet. 2013, 26, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, É.; Molnár, D. Prader–Willi Syndrome: Possibilities of Weight Gain Prevention and Treatment. Nutrients 2022, 14, 1950. [Google Scholar] [CrossRef] [PubMed]
- Goldman, V.E.; Naguib, M.N.; Vidmar, A.P. Anti-Obesity Medication Use in Children and Adolescents with Prader–Willi Syndrome: Case Review and Literature Search. J. Clin. Med. 2021, 10, 4540. [Google Scholar] [CrossRef] [PubMed]
| Sedentary (PAL * 1.0–1.39) | Low Active (PAL * 1.4–1.59) | Active (PAL * 1.6–1.89) | Very Active (PAL * 1.9–2.5) | |
|---|---|---|---|---|
| Typical daily living activities (e.g., household tasks, walking to the bus) | Typical daily living activities PLUS 30–60 min of daily moderate activity (e.g., walking at 5–7 km/h) | Typical daily living activities PLUS at least 60 min of daily moderate activity | Typical daily living activities PLUS at least 60 min of daily moderate activity PLUS an additional 60 min of vigorous activity or 120 min of moderate activity | |
| Boys 3–18 y | 1.00 | 1.13 | 1.26 | 1.42 |
| Girls 3–18 y | 1.00 | 1.16 | 1.31 | 1.56 |
| Men 19 y+ | 1.00 | 1.11 | 1.25 | 1.48 |
| Women 19 y+ | 1.00 | 1.12 | 1.27 | 1.45 |
| Variable | Statistics |
|---|---|
| Age (years) | 6.72 ± 2.81 |
| Age at start of GH (years) * | 1.40 (0.78; 2.29) |
| Weight (kg) * | 24.6 (16.6; 33.7) |
| Height or length (cm) * | 122.0 (105.0; 137.0) |
| BMI (kg/m2) * | 16.8 (15.1; 20.4) |
| BMI z-score * | −0.10 (−0.75; 1.50) |
| Caloric intake (kcal/d) | 1208 ± 186 |
| Grams protein/kg/d | 2.44 ± 0.92 |
| %Proteins | 19.1 ± 2.90 |
| %Lipids | 34.1 ± 6.55 |
| %Carbohydrates | 46.8 ± 7.47 |
| BMR (kcal/d) | 1290 ± 317 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Couto-Rosende, Y.; Garcia-Tirado, D.; Palacio-Marco, M.; Caixàs, A.; Corripio, R. A Personalized Approach to Determining the Caloric Needs of Children with Prader–Willi Syndrome Treated with Growth Hormone. J. Clin. Med. 2023, 12, 3967. https://doi.org/10.3390/jcm12123967
Couto-Rosende Y, Garcia-Tirado D, Palacio-Marco M, Caixàs A, Corripio R. A Personalized Approach to Determining the Caloric Needs of Children with Prader–Willi Syndrome Treated with Growth Hormone. Journal of Clinical Medicine. 2023; 12(12):3967. https://doi.org/10.3390/jcm12123967
Chicago/Turabian StyleCouto-Rosende, Yolanda, Diana Garcia-Tirado, Mónica Palacio-Marco, Assumpta Caixàs, and Raquel Corripio. 2023. "A Personalized Approach to Determining the Caloric Needs of Children with Prader–Willi Syndrome Treated with Growth Hormone" Journal of Clinical Medicine 12, no. 12: 3967. https://doi.org/10.3390/jcm12123967
APA StyleCouto-Rosende, Y., Garcia-Tirado, D., Palacio-Marco, M., Caixàs, A., & Corripio, R. (2023). A Personalized Approach to Determining the Caloric Needs of Children with Prader–Willi Syndrome Treated with Growth Hormone. Journal of Clinical Medicine, 12(12), 3967. https://doi.org/10.3390/jcm12123967





