Remimazolam as an Adjunct to General Anesthesia in Children: Adverse Events and Outcomes in a Large Cohort of 418 Cases
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Antonik, L.J.; Goldwater, D.R.; Kilpatrick, G.J.; Tilbrook, G.S.; Borkett, K.M. A Placebo- and Midazolam-Controlled Phase I Single Ascending-Dose Study Evaluating the Safety, Pharmacokinetics, and Pharmacodynamics of Remimazolam. Anesth. Analg. 2012, 115, 274–283. [Google Scholar] [CrossRef]
- Ko, C.C.; Hung, K.C.; Illias, A.M.; Chiu, C.C.; Yu, C.H.; Lin, C.M.; Chen, I.W.; Sun, C.K. The use of remimazolam versus propofol for induction and maintenance of general anesthesia: A systematic review and meta-analysis. Front. Pharmacol. 2023, 14, 1101728. [Google Scholar] [CrossRef] [PubMed]
- Investigation of Remimazolam in Children Undergoing Sedation for Medical Procedures. Available online: https://clinicaltrials.gov/ct2/show/NCT04851717 (accessed on 23 February 2023).
- Kiyokawa, M.; Saito, J.; Nakai, K.; Hirota, K.A. A Pract. A Remimazolam and Remifentanil Anesthetic for a Pediatric Patient With a Medium-Chain Acyl-CoA Dehydrogenase Deficiency: A Case Report. A Practice 2022, 16, e01646. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, Y.; Kuratani, N.; Tateno, K.; Hoshijima, H.; Nakamura, T.; Mieda, T.; Doi, K.; Nagasaka, H. Anesthetic management with remimazolam for a pediatric patient with Duchenne muscular dystrophy. Medicine 2021, 100, e28209. [Google Scholar] [CrossRef] [PubMed]
- Arashiro, A.; Shinzato, H.; Kamizato, K.; Kakinohana, M. Spinal fusion with motor evoked potential monitoring using remimazolam in Alström syndrome: A case report. Medicine 2021, 24, 100. [Google Scholar] [CrossRef]
- Yamadori, Y.; Yamagami, Y.; Matsumoto, Y.; Koizumi, M.; Nakamura, A.; Mizuta, D.; Yasuda, K.; Shirakami, G. General anesthesia with remimazolam for a pediatric patient with MELAS and recurrent epilepsy: A case report. JA Clin. Rep. 2022, 16, 75. [Google Scholar] [CrossRef]
- Habre, W.; Disma, N.; Virag, K.; Becke, K.; Hansen, T.G.; Jöhr, M.; Leva, B.; Morton, N.S.; Vermeulen, P.M.; Zielinska, M.; et al. Incidence of severe critical events in paediatric anaesthesia (APRICOT): A prospective multicentre observational study in 261 hospitals in Europe Walid Habre. Lancet Respir. Med. 2017, 5, 412–425. [Google Scholar] [CrossRef]
- Disma, N.; Veyckemans, F.; Virag, K.; Hansen, T.G.; Becke, K.; Harlet, P.; Vutskits, L.; Walker, S.M.; de Graaff, J.C.; Zielinska, M.; et al. Morbidity and mortality after anaesthesia in early life: Results of the European prospective multicentre observational study, neonate and children audit of anaesthesia practice in Europe (NECTARINE). Br. J. Anaesth. 2021, 126, 1157–1172. [Google Scholar] [CrossRef]
- Shioji, N.; Everett, T.; Suzuki, Y.; Aoyama, K. Pediatric sedation using dexmedetomidine and remimazolam for magnetic resonance imaging. J. Anesth. 2022, 36, 1–4. [Google Scholar] [CrossRef]
- Mason, K.P.; Zurakowski, D.; Zgleszewski, S.E.; Robson, C.D.; Carrier, M.; Hickey, P.R.; Dinardo, J.A. High dose dexmedetomidine as the sole sedative for pediatric MRI. Paediatr. Anaesth. 2008, 18, 403–411. [Google Scholar] [CrossRef]
- Mason, K.P.; Zurakowski, D.; Zgleszewski, S.; Prescilla, R.; Fontaine, P.J.; Dinardo, J.A. Incidence and predictors of hypertension during high-dose dexmedetomidine sedation for pediatric MRI. Paediatr. Anaesth. 2010, 20, 516–523. [Google Scholar] [CrossRef]
- Van Berkel Patel, M.; Bolton, S.; Hamilton, C. Standard- versus High-Dose Dexmedetomidine for Sedation in the Intensive Care Unit. Hosp. Pharm. 2022, 57, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Fu, Y.; Deng, F.; Shao, Y.; Lu, Y.G.; Song, J.C. Median Effective Dose of Dexmedetomidine Inducing Bradycardia in Elderly Patients Determined by Up-and-Down Sequential Allocation Method. Int. J. Med. Sci. 2022, 19, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.; Perez, R.; Musial, B.; Lukens, C.; Adjepong, Y.A.; Manthous, C.A. Outcomes of Patients with Alcohol Withdrawal Syndrome Treated with High-Dose Sedatives and Deferred Intubation. Ann. Am. Thorac. Soc. 2016, 13, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lin, C.; Chen, S.; Huang, Y.; Cheng, Q.; Yao, Y. Remimazolam for the Prevention of Emergence Delirium in Children Following Tonsillectomy and Adenoidectomy Under Sevoflurane Anesthesia: A Randomized Controlled Study. Drug Des. Dev. Ther. 2022, 16, 3413–3420. [Google Scholar] [CrossRef]
- Schüttler, J.; Eisenried, A.; Lerch, M.; Fechner, J.; Jeleazcov, C.; Ihmsen, H. Pharmacokinetics and Pharmacodynamics of Remimazolam (CNS 7056) after Continuous Infusion in Healthy Male Volunteers: Part I. Pharmacokinetics and Clinical Pharmacodynamics. Anesthesiology 2020, 132, 636–651. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kawashima, S.; Makino, H.; Doi, M.; Nakajima, Y. Comparison of postoperative nausea and vomiting between remimazolam and propofol: A propensity score-matched, retrospective, observational, single-center cohort study. Korean J. Anesthesiol. 2023, 76, 143–151. [Google Scholar] [CrossRef]
- Doi, M.; Morita, K.; Takeda, J.; Sakamoto, A.; Yamakage, M.; Suzuki, T. Efficacy and safety of remimazolam versus propofol for general anesthesia: A multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J. Anesth. 2020, 34, 543–553. [Google Scholar] [CrossRef]
- Hasegawa, G.; Hirata, N.; Yoshikawa, Y.; Yamakage, M. Differential effects of remimazolam and propofol on heart rate variability during anesthesia induction. J. Anesth. 2022, 36, 239–245. [Google Scholar] [CrossRef]
- Doi, M.; Hirata, N.; Suzuki, T.; Morisaki, H.; Morimatsu, H.; Sakamoto, A. Safety and efficacy of remimazolam in induction and maintenance of general anesthesia in high-risk surgical patients (ASA Class III): Results of a multicenter, randomized, double-blind, parallel-group comparative trial. J. Anesth. 2020, 34, 491–501. [Google Scholar] [CrossRef]
- Hu, B.; Zhang, M.; Wu, Z.; Zhang, X.; Zou, X.; Tan, L.; Song, T.; Li, X. Comparison of Remimazolam Tosilate and Etomidate on Hemodynamics in Cardiac Surgery: A Randomised Controlled Trial. Drug Des. Dev. Ther. 2023, 17, 381–388. [Google Scholar] [CrossRef] [PubMed]
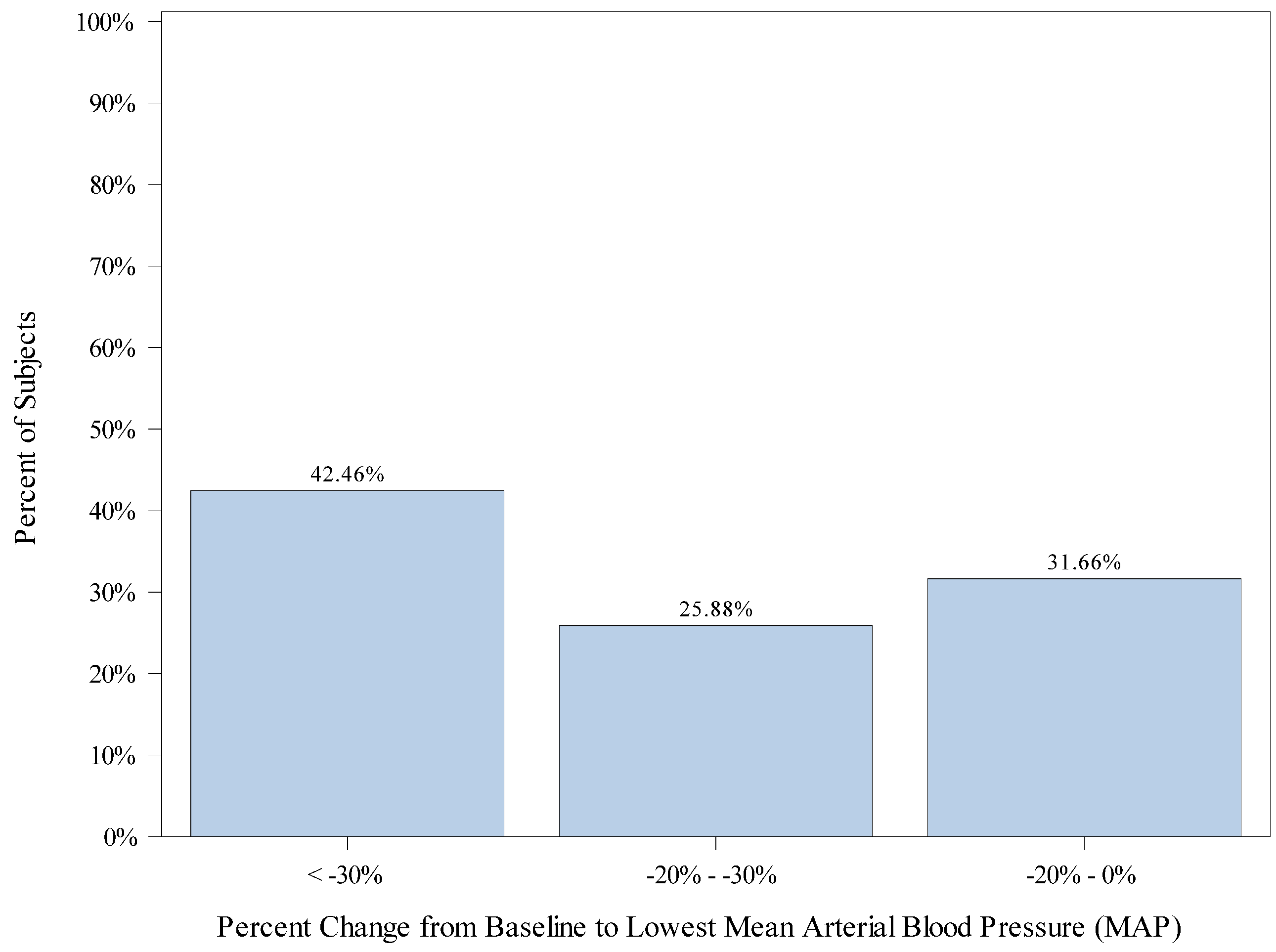


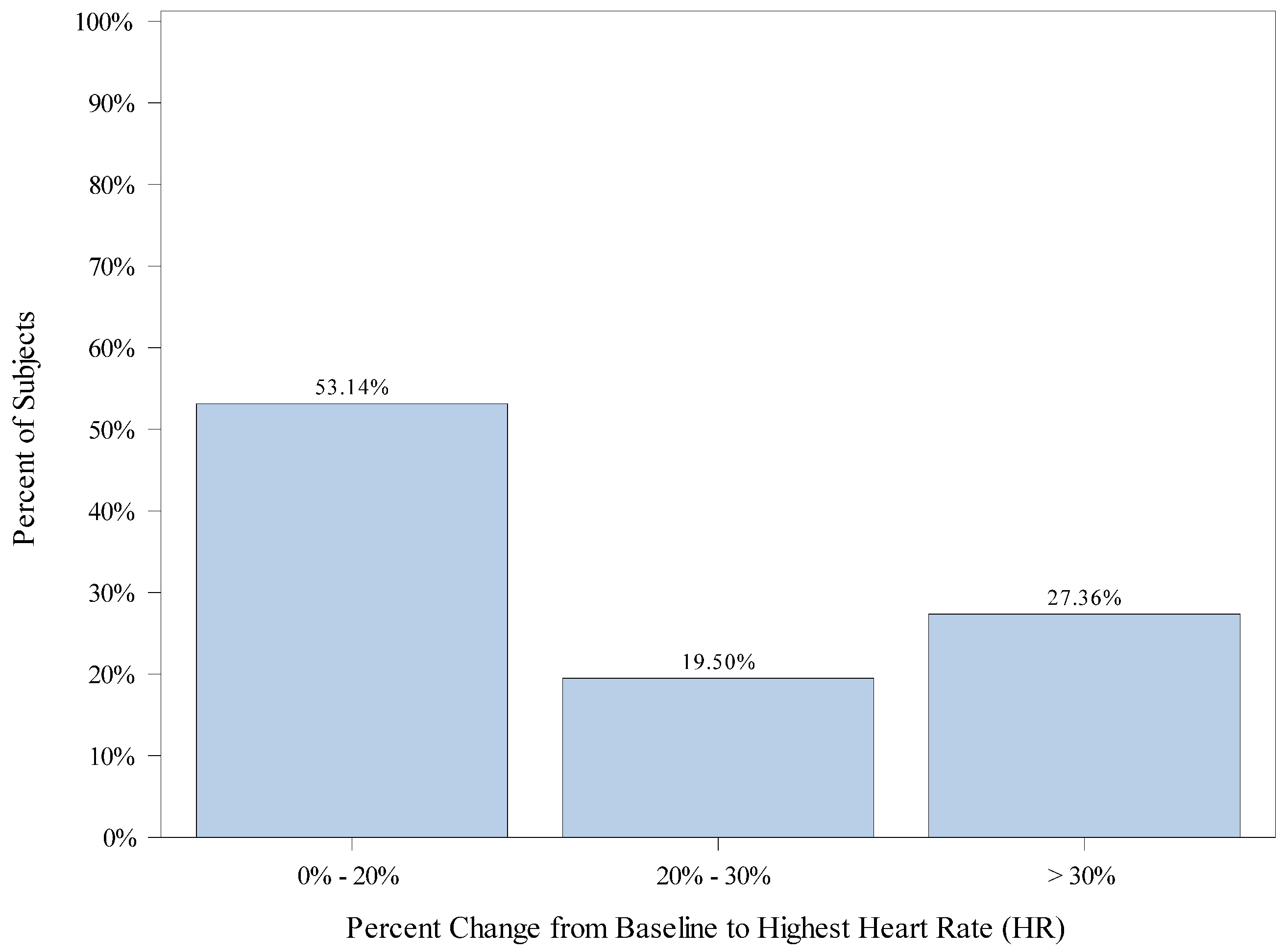
| Variable | Statistics (n = 418) | |
|---|---|---|
| Age (yrs) | Mean (SD) | 4.6 (4.52) |
| Median | 3.0 | |
| Min, Max | 0.0, 17.9 | |
| Weight (kg) | Mean (SD) | 16.5 (13.50) |
| Median | 12.1 | |
| Min, Max | 2.1, 75.9 | |
| BMI | Mean (SD) | 16.2 (2.92) |
| Median | 16.1 | |
| Min, Max | 6.9, 29.0 | |
| ASA Score | Mean (SD) | 1.9 (0.77) |
| Median | 2.0 | |
| Min, Max | 1.0, 4.0 | |
| ASA Score, n (%) | 1 | 127 (30.4) |
| 1E | 21 (5.0) | |
| 2 | 160 (38.3) | |
| 2E | 16 (3.8) | |
| 3 | 78 (18.7) | |
| 3E | 13 (3.1) | |
| 4 | 1 (0.2) | |
| 4E | 2 (0.5) | |
| Baseline Heart Rate (bpm) | Mean (SD) | 107.5 (20.68) |
| Median | 108.0 | |
| Min, Max | 50.0, 169.0 | |
| Baseline MAP (mmHg) | Mean (SD) | 65.0 (9.25) |
| Median | 64.0 | |
| Types of Procedure (%) | Central venous catheter insertion | 22 (5.3) |
| Dental surgery | 1 (0.2) | |
| Gastrointestinal endoscopy | 65 (15.6) | |
| General surgery | 83 (19.9) | |
| Neurosurgery | 17 (4.1) | |
| Ophthalmic surgery | 10 (2.4) | |
| Orthopedic surgery | 40 (9.6) | |
| Otorhinolaryngological surgery | 21 (5.0) | |
| Percutaneous biopsy | 15 (3.6) | |
| Plastic surgery | 62 (14.8) | |
| Spine surgery | 11 (2.6) | |
| Thoracic surgery | 4 (1.0) | |
| Urologic surgery | 39 (9.3) | |
| Others | 28 (6.7) | |
| Anesthesia Induction Method, n (%) | Already intubated | 12 (2.9) |
| Intravenous (IV) | 187 (44.8) | |
| Rapid Sequence (IV) Induction | 13 (3.1) | |
| Inhalation | 206 (49.3) | |
| Variable | Statistics | |
|---|---|---|
| Duration of Procedure (mins) | Mean (SD) | 81.2 (75.58) |
| Median | 55.0 | |
| Min./Max. | 3.0/490.0 | |
| Remimazolam administered (mg/kg/total time that reimimazolam administered) | Mean (SD) | 0.48 (0.601) |
| Median | 0.29 | |
| Min./Max. | 0.01/4.09 | |
| Time from last dose of remimazolam to awakening * (mins) | Mean (SD) | 34.3 (15.80) |
| Median | 31.0 | |
| Min./Max. | 12.0/108.0 | |
| PACU time ** (mins) | Mean (SD) | 13.8 (7.08) |
| Median | 13.0 | |
| Min./Max. | 1.0/40.0 | |
| Variable | Statistics (mg) | |
|---|---|---|
| Total Amount Propofol (n = 45) | Mean (SD) | 79.1 (78.21) |
| Median | 50.0 | |
| Total Amount Sevoflurane (n = 230) | ||
| Total Amount Fentanyl (n = 364) | Mean (SD) | 0.1 (0.12) |
| Median | 0.1 | |
| Total Amount Remifentanil (n = 395) | Mean (SD) | 1.5 (1.64) |
| Median | 1.0 | |
| Total Amount Morphine (n = 71) | Mean (SD) | 3.0 (1.66) |
| Median | 3.0 | |
| Total Amount Ketamine (n = 12) | Mean (SD) | 14.8 (9.53) |
| Median | 10.0 | |
| Total Amount Flumazenil (n = 55) | Mean (SD) | 0.2 (0.16) |
| Median | 0.2 | |
| Total Amount Ephedrine (n = 21) | Mean (SD) | 7.6 (5.14) |
| Median | 8.0 | |
| Total Amount Dobutamine (n = 1) | Mean (SD) | 2.3 (NA) |
| Total Amount Dopamine (n = 1) | Mean (SD) | 5.4 (NA) |
| Spontaneous eye opening in response to verbal stimulation or mild tactile stimulation |
| Absent, minimal, or mild pain/discomfort |
| Spontaneous movements of extremities to verbal request or in response to mild stimulation |
| Absent or mild nausea |
| Oxygen saturation ≥ 97% |
| Normal respiratory pattern |
| No extreme tachycardia or bradycardia |
| No extreme hypertension or hypotension |
| Absence of shivering |
| Surgical wounds and conditions acceptable per surgical service |
| Temperature ≥ 36 °C but afebrile |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimoto, Y.; Hirano, T.; Kuratani, N.; Cavanaugh, D.; Mason, K.P. Remimazolam as an Adjunct to General Anesthesia in Children: Adverse Events and Outcomes in a Large Cohort of 418 Cases. J. Clin. Med. 2023, 12, 3930. https://doi.org/10.3390/jcm12123930
Kimoto Y, Hirano T, Kuratani N, Cavanaugh D, Mason KP. Remimazolam as an Adjunct to General Anesthesia in Children: Adverse Events and Outcomes in a Large Cohort of 418 Cases. Journal of Clinical Medicine. 2023; 12(12):3930. https://doi.org/10.3390/jcm12123930
Chicago/Turabian StyleKimoto, Yoshitaka, Tatsuya Hirano, Norifumi Kuratani, David Cavanaugh, and Keira P. Mason. 2023. "Remimazolam as an Adjunct to General Anesthesia in Children: Adverse Events and Outcomes in a Large Cohort of 418 Cases" Journal of Clinical Medicine 12, no. 12: 3930. https://doi.org/10.3390/jcm12123930
APA StyleKimoto, Y., Hirano, T., Kuratani, N., Cavanaugh, D., & Mason, K. P. (2023). Remimazolam as an Adjunct to General Anesthesia in Children: Adverse Events and Outcomes in a Large Cohort of 418 Cases. Journal of Clinical Medicine, 12(12), 3930. https://doi.org/10.3390/jcm12123930


.png)


