Clinical-Epidemiological Characteristics of Hidradenitis Suppurativa: A Retrospective Cohort Study from a Tertiary Care Centre in Northern Israel
Abstract
:1. Introduction
2. Materials and Methods
- (1)
- Complete remission: improvement of 100%.
- (2)
- Significant response: improvement of 50% or more (but not 100%).
- (3)
- Mild response: improvement of less than 50 % (but not 0%).
- (4)
- No response: improvement of 0%.
Statistical Analysis
3. Results
3.1. Associated Diseases
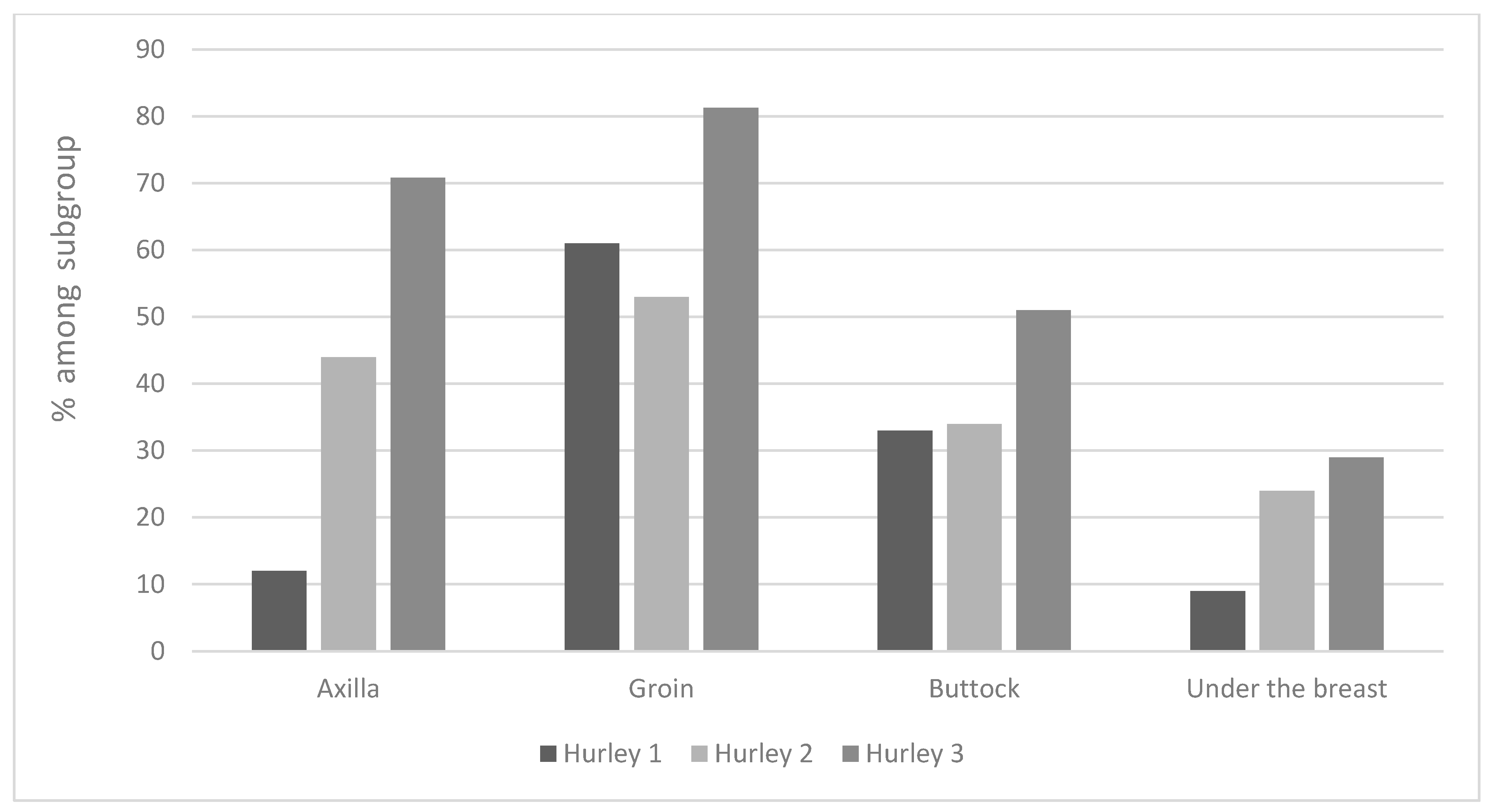
3.2. Diagnostic Factors for Predicting Disease Severity according to Hurley Stages
3.3. Treatment Response
4. Discussion
5. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jemec, G.B.E. Clinical practice. Hidradenitis suppurativa. N. Engl. J. Med. 2012, 366, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Revuz, J. Hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 985–998. [Google Scholar] [CrossRef] [PubMed]
- Gill, L.; Williams, M.; Hamzavi, I. Update on hidradenitis suppurativa: Connecting the tracts. F1000Prime Rep. 2014, 6, 112. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimmons, J.S.; Guilbert, P.R. A family study of hidradenitis suppurativa. J. Med. Genet. 1985, 22, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Lapins, J.; Jarstrand, C.; Emtestam, L. Coagulase-negative staphylococci are the most common bacteria found in cultures from the deep portions of hidradenitis suppurativa lesions, as obtained by carbon dioxide laser surgery. Br. J. Dermatol. 1999, 140, 90–95. [Google Scholar] [CrossRef] [Green Version]
- Roenigk, R.K.; Roenigk, H.H. Axillary Hyperhidrosis, Apocrine Bromhidrosis, Hidradenitis Suppurativa, and Familial Benign Pemphigus: Surgical Approach; Marcel Dekker: New York, NY, USA, 1989; pp. 729–739. [Google Scholar]
- Rabasseda, X. A report from the 22nd Congress of the European Academy of Dermatology and Venereology (October 2-6, 2013–Istanbul, Turkey). Drugs Today 2013, 49, 667–677. [Google Scholar] [CrossRef]
- Kraft, J.N.; Searles, G.E. Hidradenitis Suppurativa in 64 Female Patients: Retrospective Study Comparing Oral Antibiotics and Antiandrogen Therapy. J. Cutan. Med. Surg. 2007, 11, 125–131. [Google Scholar] [CrossRef]
- Lapins, J.; Ye, W.; Nyrén, O.; Emtestam, L. Incidence of Cancer Among Patients With Hidradenitis Suppurativa. Arch. Dermatol. 2001, 137, 730–734. [Google Scholar]
- Maclean, G.M.; Coleman, D.J. Three Fatal Cases of Squamous Cell Carcinoma Arising in Chronic Perineal Hidradenitis Suppurativa. Ann. R. Coll. Surg. Engl. 2007, 89, 709–712. [Google Scholar] [CrossRef] [Green Version]
- Shavit, E.; Dreiher, J.; Freud, T.; Halevy, S.; Vinker, S.; Cohen, A. Psychiatric comorbidities in 3207 patients with hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 2014, 29, 371–376. [Google Scholar] [CrossRef]
- Fimmel, S.; Zouboulis, C.C. Comorbidities of hidradenitis suppurativa (acne inversa). Dermato-Endocrinology 2010, 2, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jemec GB, E.; Wendelboe, P. Topical clindamycin versus systemic tetracycline in the treatment of hidradenitis suppurativa. J. Am. Acad. Dermatol. 1998, 39, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Gener, G.; Canoui-Poitrine, F.; Revuz, J.E.; Faye, O.; Poli, F.; Gabison, G.; Pouget, F.; Viallette, C.; Wolkenstein, P.; Bastuji-Garin, S. Combination Therapy with Clindamycin and Rifampicin for Hidradenitis Suppurativa: A Series of 116 Consecutive Patients. Dermatology 2009, 219, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Joseph, M.A.; Jayaseelan, E.; Ganapathi, B.; Stephen, J. Hidradenitis suppurativa treated with finasteride. J. Dermatol. Treat. 2005, 16, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Boer, J.; Nazary, M. Long-term results of acitretin therapy for hidradenitis suppurativa. Is acne inversa also a misnomer? Br. J. Dermatol. 2011, 164, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.; Gonzalez, T.; Montgomery, M.O.; Cardenas, V.; Kerdel, F.A. Infliximab therapy for patients with moderate to severe hidradenitis suppurativa: A randomized, double-blind, placebo-controlled crossover trial. J. Am. Acad. Dermatol. 2010, 62, 205–217. [Google Scholar] [CrossRef]
- Gupta, A.K.; Studholme, C. Adalimumab (Humira) for the Treatment of Hidradenitis Suppurativa. Skin Ther. Lett. 2016, 21, 1–4. [Google Scholar]
- Mahmoud, B.H.; Tierney, E.; Hexsel, C.L.; Pui j Ozog, D.M.; Hamzavi, I.H. Prospective controlled clinical and histopathologic study of hidradenitis suppurativa treated with the long-pulsed neodymium:yttrium-aluminium-garnet laser. J. Am. Acad. Dermatol. 2010, 62, 637–645. [Google Scholar] [CrossRef]
- Mikkelsen, P.R.; Dufour, D.N.; Zarchi, K.; Jemec, G.B. Recurrence rate and patient satisfaction of CO2 laser evaporation of lesions in patients with hidradenitis suppurativa: A retrospective study. Dermatol. Surg. 2015, 41, 255–260. [Google Scholar] [CrossRef]
- Lapins, J.; Marcusson, J.A.; Emtestam, L. Surgical treatment of chronic hidradenitis suppurativa: CO2 laser stripping-secondary intention technique. Br. J. Dermatol. 1994, 131, 551–556. [Google Scholar] [CrossRef]
- Shalom, G.; Babaev, M.; Freud, T.; Tiosano, S.; Pam, N.; Horev, A.; Diriher, J.; Vardy, D.A.; Comaneshter, D.; Cohen, A.D. Demographic and health care service utilization by 4417 patients with hidradenitis suppurativa. J. Am. Acad. Dermatol. 2017, 77, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, I.; Hayashi, N. Japan Acne Research Society. Questionnaire surveillance of hidradenitis suppurativa in Japan. J. Dermatol. 2015, 42, 747–749. [Google Scholar] [CrossRef] [PubMed]
- Canoui-Poitrine, F.; Revuz, J.E.; Wolkenstein, P.; Viallette, C.; Gabison, G.; Pouget, F.; Poli, F.; Faye, O.; Bastuji-Garin, S. Clinical characteristics of a series of 302 French patients with hidradenitis suppurativa, with an analysis of factors associated with disease severity. J. Am. Acad. Dermatol. 2009, 61, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Bettoli, V.; Pasquinucci, S.; Caracciolo, S.; Piccolo, D.; Cazzaniga, S.; Fantini, F.; Binello, L.; Pintori, G.; Naldi, L. The Hidradenitis supurativa patient journey in Italy: Current status, unmet needs and opportunities. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1965–1970. [Google Scholar] [CrossRef]
- Kim, W.B.; Sibbald, R.G.; Hu, H.; Bashash, M.; Anooshirvani, N.; Coutts, P.; Alavi, A. Clinical features and patient out-comes of hidradenitis suppurativa: A cross-sectional retrospective study. J. Cutan. Med. Surg. 2016, 20, 52–57. [Google Scholar] [CrossRef] [Green Version]
- Von Der Werth, J.M.; Williams, H.C.; Raeburn, J.A. The clinical genetics of hidradenitis suppurativa revisited. Br. J. Dermatol. 2000, 142, 947–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrader, A.M.; Deckers, I.E.; Van Der Zee, H.H.; Boer, J.; Prens, E.P. Hidradenitis suppurativa: A retrospective study of 846 Dutch patients to identify factors associated with disease severity. J. Am. Acad. Dermatol. 2014, 71, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Gulliver, W.; Zouboulis, C.C.; Prens, E.; Jemec, G.B.; Tzellos, T. Evidence-based approach to the treatment of hidradenitis suppurativa/acne inversa, based on the European guidelines for hidradenitis suppurativa. Rev. Endocr. Metab. Disord. 2016, 17, 343–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sbidian, E.; Hotz, C.; Seneschal, J.; Maruani, A.; Amelot, F.; Aubin, F.; Paul, C.; Barry, M.B.; Humbert, P.; Dupuy, A.; et al. Antitumour necrosis factor-alpha therapy for hidradenitis suppurativa: Results from a national cohort study between 2000 and 2013. Br. J. Dermatol. 2016, 174, 667–700. [Google Scholar] [CrossRef] [Green Version]
- Martin-Ezquerra, G.; Masferrer, E.; Masferrer-Niubo, M.; Ferran, M.; Sánchez-Regaña, M.; Collgros, H.; Bordas, X.; Notario, J.; Alsina, M.; Gil, I.; et al. Use of biological treatments in patients with hidradenitis suppurativa. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 56–60. [Google Scholar] [CrossRef]
- Kimball, A.B.; Okun, M.M.; Williams, D.A.; Gottlieb, A.B.; Papp, K.A.; Zouboulis, C.C.; Armstrong, A.W.; Kerdel, F.; Gold, M.H.; Forman, S.B.; et al. Two Phase 3 Trials of Adalimumab for Hidradenitis Suppurativa. N. Engl. J. Med. 2016, 375, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Garnock-Jones, K.P.; Keam, S.J. Adalimumab: A Review in Hidradenitis Suppurativa. Am. J. Clin. Dermatol. 2016, 17, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Burzi, L.; Repetto, F.; Ribero, S.; Mastorino, L.; Quaglino, P.; Dapavo, P. Paradoxical psoriasiform reactions during treatment with adalimumab for hidradenitis suppurativa: Real-life experience and therapeutic response to other biological drugs. Dermatol. Ther. 2022, 35, e15866. [Google Scholar] [CrossRef] [PubMed]
- Pink, A.E.; Simpson, M.A.; Brice, G.W.; Smith, C.H.; Desai, N.; Mortimer, P.S.; Barker, J.N.; Trembath, R.C. PSENEN and NCSTN mutations in familial hidradenitis suppurativa (Acne Inversa). J. Investig. Dermatol. 2011, 131, 70–1568. [Google Scholar] [CrossRef] [Green Version]
- Nazzaro, G.; Passoni, E.; Guanziroli, E.; Casazza, G.; Muratori, S.; Barbareschi, M.; Veraldi, S.; Marzano, A.V. Comparison of clinical and sonographic scores in a cohort of 140 patients with hidradenitis suppurativa from an Italian referral centre: A retrospective observational study. Eur. J. Dermatol. 2018, 28, 845–847. [Google Scholar] [CrossRef]



| N = 164 (%) | |
|---|---|
| Gender | |
| Male | 96 (58.5%) |
| Female | 68 (41.5%) |
| Age at diagosis; range (years) | 33.9 ± 13.8; 11–75 |
| Ethnicity | |
| Jew | 93 (57%, 44% adjusted) |
| Arab | 71 (43%, 56% adjusted) |
| Hurley | |
| 1 | 33 (20%) |
| 2 | 83 (51%) |
| 3 | 48 (29%) |
| Hypertension | 5 (3%) |
| Overweight | 46 (28%) |
| Hyperlipidaemia | 34 (21%) |
| Diabetes mellitus | 8 (5%) |
| Smoker | 90 (55%) |
| Time until diagnosis (years) | 4 [1–10] |
| Family history of HS | 29 (18%) |
| Past medical history | 57 (35%) |
| Affected body area | |
| Groin | 122 (74%) |
| Axilla | 103 (63%) |
| Buttocks | 71 (43%) |
| Sub-mammary | 44 (27%) |
| Associated condition | |
| Acne | 18 (11%) |
| Eczema | 20 (12%) |
| Psoriasis | 11 (7%) |
| Others | 10 (6%) |
| N | Male | Female | |
|---|---|---|---|
| No. of HS Patients | 164 | 96 (58.5%) | 68 (41.4%) |
| 9–0 (year) | 0 | 0 | 0 |
| 19–10 (year) | 25 (15.2%) | 10 (10.4%) | 15 (22%) |
| 29–20 (year) | 50 (30.4%) | 30 (31.25%) | 20 (29.4%) |
| 39–30 (year) | 37 (22.5%) | 16 (16.6%) | 21 (30.8%) |
| 49–40 (year) | 26 (15.8%) | 17 (17.7%) | 9 (13.2%) |
| 50+ (year) | 26 (15.8%) | 23 (23.9%) | 3 (4.4%) |
| Gender | p < 0.0001 | ||
| Male | 96 (58.5%) | 8270 (37.4%) | |
| Female | 68 (41.5%) | 13,815 (62.6%) | |
| Age; range | 33.9 ± 13.8; 11–75 | 38.5 ±15.5 | p < 0.0001 |
| Ethnicity | p < 0.0001 | ||
| Jew | 93 (57%) | 19,580 (88.7%) | |
| Other | 71 (43%) | 2505 (11.3%) | |
| Overweight | 46 (28%) | 1554 (7%) | p < 0.0001 |
| Smoker | 90 (55%) | 2838 (12.9%) | p < 0.0001 |
| Treatment/Response | N = 164 | Complete Remission | Significant Response | Mild Response | No Effect |
|---|---|---|---|---|---|
| Topical treatment | n = 156 | - | - | 15/156 (10%) | 141/156 (90%) |
| surgical treatment | |||||
| Laser deroofing | n = 7 | 7/7 (100%) | - | - | - |
| Wide excision | n = 24 | - | 2 (8%) | 5 (21%) | 17 (71%) |
| Surgical deroofing | n = 10 | - | 1 (10%) | 4 (40%) | 5 (50%) |
| Incision and drainage | n = 61 | 1 (2%) | 7 (11%) | 11 (18%) | 42 (69%) |
| systemic pharmaceutical treatment | |||||
| Dapsone | n = 5 | - | 2/5 (40%) | - | 4/5 (60%) |
| Rifampin | n = 62 | 2 (3%) | 8 (13%) | 15 (24%) | 37 (60%) |
| Clindamycin | n = 128 | 8 (6%) | 27 (21%) | 40 (31%) | 53 (41%) |
| Augmentin/cephalosporin | n = 26 | 1 (4%) | 1 (4%) | 7 (27%) | 17 (65%) |
| Minocycline | n = 29 | 1 (3%) | 3 (10%) | 5 (17%) | 20 (69%) |
| Doxycycline | n = 29 | - | 2 (7%) | 11 (38%) | 16 (55%) |
| Systemic steroids | n = 24 | - | 1 (4%) | 6 (25%) | 17 (71%) |
| Acitretin | n = 27 | 1 (4%) | 6 (22%) | 2 (7%) | 18 (67%) |
| Isotretinoin | n = 40 | - | 4 (10%) | 10 (25%) | 26 (65%) |
| Adalimumab | n = 36 | 3 (8%) | 12 (33%) | 15 (42%) | 6 (17%) |
| Infliximab | n = 5 | 1 (20%) | 1 (20%) | 2 (40%) | 1 (20%) |
| N = 36 | Complete Remission + Significant; n = 15 (1) | Mild; n = 15 (3) | No Effect; n = 6 (4) | p-Value |
|---|---|---|---|---|
| Ethnicity (Jews) | 7 (47%) | 10 (67%) | 2 (33%) | p = 0.32 |
| Arabs | 8 (53%) | 5 (33%) | 4 (67%) | |
| Gender (male) | 9 (60%) | 12 (80%) | 5 (83%) | p = 0.38 |
| Female | 6 (40%) | 3 (20%) | 1 (17%) | |
| Hurley | p = 0.78 | |||
| 1 | 0 | 1 (7%) | 0 | |
| 2 | 4 (27%) | 5 (33%) | 2 (33%) | |
| 3 | 11 (73%) | 9 (60%) | 4 (67%) | |
| Age (years) | 38.7 ± 12.8 | 39.5 ± 14.6 | 39.8 ± 16.0 | p = 0.98 |
| Time until diagnosis (years) | 2 [0.33–6.0] | 6 [3–15] | 5 [0.48–24.5] | 1 vs. 3 p = 0.049 |
| Hypertension | 2 (13%) | 1 (7%) | 0 | NA |
| Obesity | 7 (47%) | 5 (33%) | 2 (33%) | p = 0.72 |
| Hyperlipidaemia | 3 (20%) | 2 (13%) | 1 (17%) | p = 0.88 |
| Diabetes mellitus | 2 (13%) | 2 (13%) | 0 | p = 0.64 |
| Smoker | 7 (47%) | 10 (67%) | 3 (50%) | p = 0.52 |
| Location (groin) | 13 (87%) | 11 (73%) | 6 (100%) | p = 0.30 |
| Axilla | 12 (80%) | 8 (53%0 | 5 (83%) | p = 0.21 |
| Buttocks | 7 (47%) | 6 (40%) | 6 (100%) | p = 0.037 1 vs. 4—0.045 3 vs. 4—0.02 |
| Sub-mammary | 9 (60%) | 2 (13%) | 2 (33%) | Small numbers |
| White blood cells | 5 (33%) | 3 (20%) | 0 | p = 0.24 |
| Liver enzymes | 2 (13%) | 5 (33%0 | 0 | p = 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hammud, A.; Avitan-Hersh, E.; Khamaysi, Z. Clinical-Epidemiological Characteristics of Hidradenitis Suppurativa: A Retrospective Cohort Study from a Tertiary Care Centre in Northern Israel. J. Clin. Med. 2023, 12, 3921. https://doi.org/10.3390/jcm12123921
Hammud A, Avitan-Hersh E, Khamaysi Z. Clinical-Epidemiological Characteristics of Hidradenitis Suppurativa: A Retrospective Cohort Study from a Tertiary Care Centre in Northern Israel. Journal of Clinical Medicine. 2023; 12(12):3921. https://doi.org/10.3390/jcm12123921
Chicago/Turabian StyleHammud, Anan, Emily Avitan-Hersh, and Ziad Khamaysi. 2023. "Clinical-Epidemiological Characteristics of Hidradenitis Suppurativa: A Retrospective Cohort Study from a Tertiary Care Centre in Northern Israel" Journal of Clinical Medicine 12, no. 12: 3921. https://doi.org/10.3390/jcm12123921
APA StyleHammud, A., Avitan-Hersh, E., & Khamaysi, Z. (2023). Clinical-Epidemiological Characteristics of Hidradenitis Suppurativa: A Retrospective Cohort Study from a Tertiary Care Centre in Northern Israel. Journal of Clinical Medicine, 12(12), 3921. https://doi.org/10.3390/jcm12123921








