Plasma-Free Strategy for Cardiac Surgery with Cardiopulmonary Bypass in Infants < 10 kg: A Retrospective, Propensity-Matched Study
Abstract
:1. Introduction
2. Materials and Methods
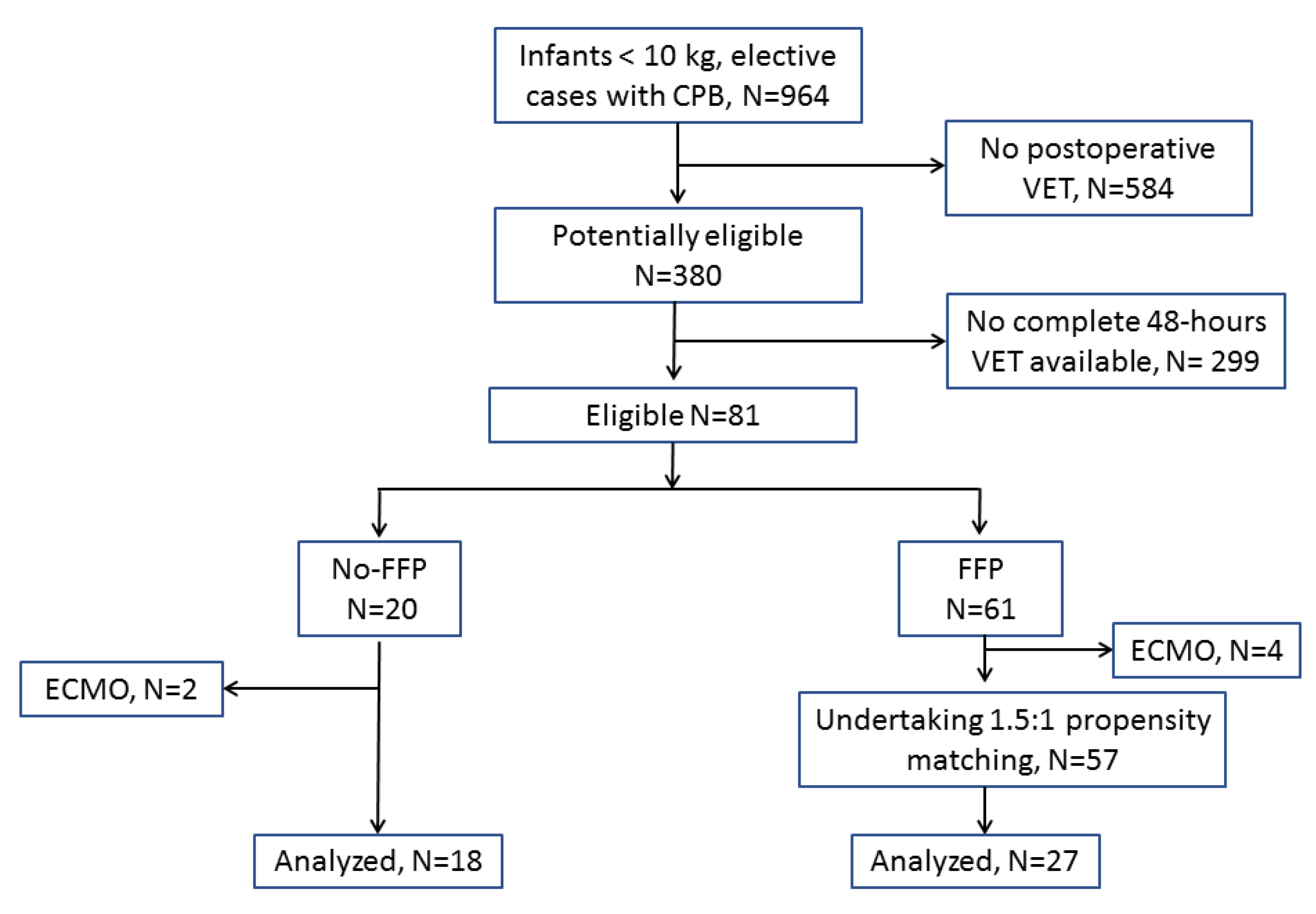
2.1. Patient Population and Propensity Matching
2.2. Anesthesia, CPB, Surgery, and ICU Management
2.3. Interventions
2.4. Data Collection and Measurements
2.5. Bleeding Treatment Protocol
- Correction of residual heparin, if the CT at INTEM exceeded by 20%, the CT at HEPTEM
- Fibrinogen concentrate (Haemocomplettan or Riastap, CSL Behring, Marburg, Germany) 30 mg kg−1 if FIBTEM MCF < 8 mm or Clauss fibrinogen < 150 mg dL−1 in the FFP-free group, and/or FFP in the FFP-based group.
- Platelet concentrates if EXTEM MCF < 35 mm and FIBTEM MCF ≥ 8 mm or platelet count < 100,000 cells/µL. In case of persisting bleeding with an EXTEM MCF < 35 mm, platelet concentrates could be repeated regardless of FIBTEM MCF values.
- 4-factors PCC (Confidex, CSL Behring, Marburg, Germany or Pronativ, Octapharma, Lachen, Switzerland) 20 IU kg−1 if EXTEM CT > 100 (after correction of fibrinogen and platelet values) or prothrombin activity < 50% in the FFP-free group, and/or FFP in the FFP-based group.
- Additional tranexamic acid (30 mg kg−1) in the presence of VET signs of hyperfibrinolysis.
- Surgical re-exploration if bleeding persisted after correction of the above factors.
2.6. Sample Size and Statistics
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McCall, M.M.; Blackwell, M.M.; Smyre, J.T.; Sistino, J.J.; Acsell, J.R.; Dorman, B.; Bradley, S.M. Fresh frozen plasma in the pediatric pump prime: A prospective, randomized trial. Ann. Thorac. Surg. 2004, 77, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Oliver, W.C., Jr.; Beynen, F.M.; Nuttall, G.A.; Schroeder, D.R.; Ereth, M.H.; A Dearani, J.; Puga, F.J. Blood loss in infants and children for open heart operations: Albumin 5% versus Fresh-Frozen plasma in the prime. Ann. Thorac. Surg. 2003, 75, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Yoo, Y.C.; Park, H.K.; Bang, S.O.; Lee, K.Y.; Bai, S.J. Fresh frozen plasma in pump priming for congenital heart surgery: Evaluation of effects on postoperative coagulation profiles using a fibrinogen assay and rotational thromboelastometry. Yonsei Med. J. 2013, 54, 752–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchi, P.; Cotza, M.; Beccaris, C.; Silvetti, S.; Isgrò, G.; Pomè, G.; Giamberti, A.; Ranucci, M. Early or late fresh frozen plasma administration in newborns and small infants undergoing cardiac surgery: The APPEAR randomized trial. Br. J. Anaesth. 2017, 118, 788–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, X.; Liu, J.; Zhao, M.; Cui, Y.; Feng, Z.; Zhao, J.; Long, C.; Li, S.; Yan, F.; Wang, X.; et al. The influence of cardiopulmonary bypass priming without FFP on postoperative coagulation and recovery in pediatric patients with cyanotic congenital heart disease. Eur. J. Pediatr. 2014, 173, 1437–1443. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.; Liu, J.; Zhao, M.; Cui, Y.; Feng, Z.; Zhao, J.; Long, C.; Li, S.; Yan, F.; Wang, X.; et al. Evidence-based use of FFP: The influence of a priming strategy without FFP during CPB on postoperative coagulation and recovery in pediatric patients. Perfusion 2015, 30, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Dieu, A.; Martins, M.R.; Eeckhoudt, S.; Matta, A.; Kahn, D.; Khalifa, C.; Rubay, J.; Poncelet, A.; Haenecour, A.; Derycke, E.; et al. Fresh Frozen Plasma versus Crystalloid Priming of Cardiopulmonary Bypass Circuit in Pediatric Surgery: A Randomized Clinical Trial. Anesthesiology 2020, 132, 95–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranucci, M.; Bianchi, P.; Cotza, M.; Beccaris, C.; Silvetti, S.; Isgrò, G.; Giamberti, A.; Baryshnikova, E. Fibrinogen levels and postoperative chest drain blood loss in low-weight (<10 kg) children undergoing cardiac surgery. Perfusion 2019, 34, 629–636. [Google Scholar] [PubMed]
- Ranucci, M.; Baryshnikova, E.; Crapelli, G.B.; Rahe-Meyer, N.; Menicanti, L.; Frigiola, A. Surgical Clinical Outcome REsearch (SCORE) Group. Randomized, double-blinded, placebo-controlled trial of fibrinogen concentrate supplementation after complex cardiac surgery. J. Am. Heart Assoc. 2015, 4, e002066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faraoni, D.; Meier, J.; New, H.V.; Van der Linden, P.J.; Hunt, B.J. Patient Blood Management for Neonates and Children Undergoing Cardiac Surgery: 2019 NATA Guidelines. J. Cardiothorac. Vasc. Anesth. 2019, 33, 3249–3263. [Google Scholar] [CrossRef] [PubMed]
- Staffa, S.J.; Zurakowski, D. Five steps to successfully implement and evaluate propensity score matching in clinical research studies. Anesth. Analg. 2018, 127, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Schulte, P.J.; Mascha, E.J. Propensity score methods: Theory and practice for anesthesia research. Anesth. Analg. 2018, 127, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Matthay, E.C.; Hagan, E.; Gottlieb, L.M.; Tan, M.L.; Vlahov, D.; Adler, N.; Glymour, M.M. Powering population health research: Considerations for plausible and actionable effect sizes. SSM-Popul. Health 2021, 14, 100789. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.T.; Barnes, S.L.; Lo, S.T.; Leung, D.Y. Non-inferiority trials in cardiology: What clinicians need to know. Heart 2020, 106, 99–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siemens, K.; Hunt, B.J.; Harris, J.; Nyman, A.G.; Parmar, K.; Tibby, S.M. Individualized, intraoperative dosing of fibrinogen concentrate for the prevention of bleeding in neonatal and infant cardiac surgery using cardiopulmonary bypass (FIBCON). A phase 1b/2 randomized controlled trial. Circ. Cardiovasc. Int. 2020, 13, e009465. [Google Scholar] [CrossRef] [PubMed]
- Davenport, P.; Sola-Visner, M. Hemostatic Challenges in Neonates. Front. Pediatr. 2021, 9, 627715. [Google Scholar] [CrossRef] [PubMed]

| Variable | FFP-Free (n = 18) | FFP-Based before Matching (n = 53) | ASMD Pre-Match | FFP-Based after Matching (n = 27) | ASMD Post-Match |
|---|---|---|---|---|---|
| Age (months) | 7.00 (7.0) | 7.02 (7.3) | 0.00 | 6.3 (4.6) | 0.12 |
| Weight (kg) | 5.46 (2.01) | 5.62 (2.14) | −0.08 | 5.6 (2.0) | −0.07 |
| Preoperative hematocrit (%) | 39.4 (6.3) | 36.6 (7.5) | 0.40 | 38.5 (8.6) | 0.12 |
| aPTT (sec) | 33.2 (12.5) | 32.7 (4.9) | 0.05 | 32.4 (4.9) | 0.08 |
| PT (%) | 88.3 (20.5) | 90.3 (13.1) | −0.12 | 90.8 (13.7) | −0.14 |
| Platelet count (×1000 cells/µL) | 363 (113) | 334 (122) | 0.25 | 357 (136) | 0.05 |
| Cardiopulmonary bypass time (min) | 91 (38) | 108 (48) | −0.39 | 96 (45) | −0.12 |
| Nadir hematocrit on CPB (%) | 30.2 (3.2) | 30 (2.8) | 0.07 | 30.7 (3.0) | −0.16 |
| Nadir temperature on CPB (°C) | 31.1 (3.6) | 30.9 (2.9) | 0.06 | 31.6 (3.1) | −0.15 |
| RACHS II | 2.5 (1.5) | 2.1 (0.91) | 0.32 | 2.36 (1.05) | 0.15 |
| Redo surgery | 4 (22.2) | 4 (7.5) | 0.42 | 5 (18.5) | 0.09 |
| Urgent surgery | 5 (27.8) | 8 (15.1) | 0.31 | 7 (25.9) | 0.04 |
| Item | FFP-Free (n = 18) | FFP-Based (n = 27) | Mean Difference (95% CI) | p | |
|---|---|---|---|---|---|
| aPTT (sec) | |||||
| Baseline | 33.2 (12.5) | 32.7 (4.9) | −0.87 (−6.24 to 4.48) | 0.743 | |
| Arrival ICU | 39.0 (7.5) | 33.6 (4.1) | −5.5 (−9.0 to −2.2) | 0.003 | |
| 24 h | 37.8 (8.3) | 36.5 (7.5) | −1.32 (−6.14 to 3.48) | 0.581 | |
| 48 h | 40.4 (7.5) | 40.7 (11.3) | 0.22 (−6.66 to 7.11) | 0.948 | |
| Prothrombin activity (%) | |||||
| Baseline | 88.3 (20.5) | 90.3 (13.1) | 2.53 (−9.83 to 14.9) | 0.679 | |
| Arrival ICU | 48.8 (11) | 69.1 (10.5) | 20.3 (12.8 to 27.8) | 0.001 | |
| 24 h | 55.3 (12.6) | 62.3 (8) | 7.54 (0.06 to 15) | 0.048 | |
| 48 h | 57.8 (9.4) | 65.3 (12.9) | 7.52 (−5.64 to 20.7) | 0.250 | |
| Platelet count (×1000 cells/µL) | |||||
| Baseline | 363 (113) | 357 (136) | −6.5 (−85 to 72) | 0.868 | |
| Arrival ICU | 147 (48) | 146 (74) | −1.7 (−41 to 38) | 0.931 | |
| 24 h | 153 (51) | 146 (54) | −7.8 (−41 to 25) | 0.635 | |
| 48 h | 141 (50) | 159 (71) | 18.4 (−20 to 57) | 0.345 | |
| Fibrinogen (mg dL−1) | |||||
| Arrival ICU | 156 (50) | 202 (45) | 46 (17 to 75) | 0.002 | |
| 24 h | 214 (71) | 258 (55) | 44 (6 to 82) | 0.023 | |
| 48 h | 271 (110) | 393 (91) | 123 (50 to 195) | 0.002 | |
| EXTEM CT (sec) | |||||
| Post-protamine | 108 (27) | 93 (39) | −14.8 (−36.5 to 6.98) | 0.178 | |
| 24 h | 88 (19) | 78 (16) | −10.2 (−21 to 0.6) | 0.064 | |
| 48 h | 82 (20) | 87 (22) | 5.1 (−8.8 to 19) | 0.462 | |
| HEPTEM CT (sec) | |||||
| Post-protamine | 312 (76) | 272 (92) | −40.3 (−101 to 20.5) | 0.186 | |
| 24 h | 294 (107) | 290 (138) | −4.1 (−91.5 to 83) | 0.924 | |
| 48 h | 270 (81) | 247 (70) | −23.2 (−86 to 39.6) | 0.454 | |
| EXTEM MCF (mm) | |||||
| Post-protamine | 44 (8) | 52 (8) | 8.7 (3.6 to 13.8) | 0.001 | |
| 24 h | 54 (8) | 58 (6) | 4.4 (0.20 to 8.7) | 0.041 | |
| 48 h | 55 (10) | 60 (6) | 5.5 (0.22 to 10.7) | 0.041 | |
| FIBTEM MCF (mm) | |||||
| Post-protamine | 4.7 (2.1) | 11 (6.3) | 6.3 (3.2 to 9.4) | 0.001 | |
| 24 h | 10 (3.8) | 15.7 (6.2) | 5.7 (2.3 to 9) | 0.001 | |
| 48 h | 12.5 (6.5) | 20.5 (5.9) | 8.0 (3.9 to 12) | 0.001 | |
| Item | FFP-Free (n = 18) | FFP-Based (n = 27) | Mean Difference (95% CI) | p |
|---|---|---|---|---|
| Bleeding (cumulative, mL kg−1) | ||||
| 12 h | 16.7 (12) | 14.5 (9.4) | −2.2 (−8.7 to 4.3) | 0.501 |
| 24 h | 31.9 (27.1) | 24.2 (16.3) | −7.7 (−20.8 to 5.3) | 0.239 |
| 48 h | 58.5 (66) | 37 (25.6) | −21.5 (−49.8 to 7.8) | 0.133 |
| Packed red cells transfusions (mL kg−1) | ||||
| Priming volume | 22.9 (7.2) | 23.1 (8.2) | 2.4 (−4.7 to 4.9) | 0.863 |
| After protamine in the OR | 11.5 (7.1) | 11 (8.4) | 2.4 (−5.4 to 4.3) | 0.821 |
| Postoperative 24 h | 11.2 (9.3) | 11.6 (9.5) | 2.9 (−5.3 to 6.24) | 0.875 |
| Postoperative 48 h | 4.3 (7.3) | 2.3 (6.0) | −2 (−6 to 2) | 0.312 |
| Total | 50 (19.2) | 48 (23) | −2 (−15 to 11) | 0.761 |
| FFP transfusions (mL kg−1) | ||||
| Priming volume | 0 | 29.4 (13.8) | 29.4 (22.8 to 36) | 0.001 |
| After protamine in the OR | 0 | 4.4 (5.1) | 4.4 (2 to 6.9) | 0.001 |
| Postoperative 24 h | 0 | 1.8 (5.9) | 1.9 (−0.01 to 3.74) | 0.051 |
| Postoperative 48 h | 0 | 0 | 0 | N/A |
| Total | 0 | 8.1 (18.3) | 33.9 (26.3 to 41.4) | 0.001 |
| Platelet transfusions (mL kg−1) | ||||
| After protamine in the OR | 1.14 (3.3) | 1.28 (3.5) | 0.15 (−1.95 to 2.24) | 0.890 |
| Postoperative 24 h | 0 | 0.18 (1.0) | 0.19 (−0.27 to 0.64) | 0.420 |
| Postoperative 48 h | 0 | 0 | 0 | N/A |
| Total | 1.13 (3.3) | 1.46 (3.8) | 0.33 (−1.9 to 2.6) | 0.766 |
| Fibrinogen concentrate (mg kg−1) | ||||
| OR and ICU | 23.4 (20) | 4.3 (10.5) | −19.1 (−28.3 to −9.84) | 0.001 |
| PCC (IU kg−1) | ||||
| OR and ICU | 5.6 (9.2) | 0.74 (3.8) | −4.8 (−8.8 to −0.81) | 0.020 |
| Item | FFP-Free (n = 18) | FFP-Based (n = 27) | p |
|---|---|---|---|
| Surgical revision due to bleeding | 0 (0) | 0 (0) | N/A |
| Inotropic drugs > 48 h | 9 (50) | 13 (52) | 0.897 |
| Bloodstream infection | 3 (17) | 1 (4) | 0.190 |
| Hospital mortality | 1 (5.6) | 0 (0) | 0.419 |
| Peak serum creatinine (mg dL−1) | 0.59 (0.20) | 0.46 (0.16) | 0.020 |
| Blood lactates arrival ICU (mmol L−1) | 1.85 (1.30) | 1.74 (0.67) | 0.722 |
| Mechanical ventilation (hours) | 77 (84) | 53 (55) | 0.257 |
| ICU stay (days) | 4 (2–9) | 4 (2–7) | 0.674 |
| Postoperative hospital stay (days) | 15 (12–23) | 14 (8–17) | 0.236 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranucci, M.; Di Dedda, U.; Isgrò, G.; Giamberti, A.; Cotza, M.; Cornara, N.; Baryshnikova, E. Plasma-Free Strategy for Cardiac Surgery with Cardiopulmonary Bypass in Infants < 10 kg: A Retrospective, Propensity-Matched Study. J. Clin. Med. 2023, 12, 3907. https://doi.org/10.3390/jcm12123907
Ranucci M, Di Dedda U, Isgrò G, Giamberti A, Cotza M, Cornara N, Baryshnikova E. Plasma-Free Strategy for Cardiac Surgery with Cardiopulmonary Bypass in Infants < 10 kg: A Retrospective, Propensity-Matched Study. Journal of Clinical Medicine. 2023; 12(12):3907. https://doi.org/10.3390/jcm12123907
Chicago/Turabian StyleRanucci, Marco, Umberto Di Dedda, Giuseppe Isgrò, Alessandro Giamberti, Mauro Cotza, Noemi Cornara, and Ekaterina Baryshnikova. 2023. "Plasma-Free Strategy for Cardiac Surgery with Cardiopulmonary Bypass in Infants < 10 kg: A Retrospective, Propensity-Matched Study" Journal of Clinical Medicine 12, no. 12: 3907. https://doi.org/10.3390/jcm12123907





