Pearls and Pitfalls of Weaning an Infant with Severe Atopic Dermatitis and Sensitization/Allergy to Food
Abstract
1. Introduction
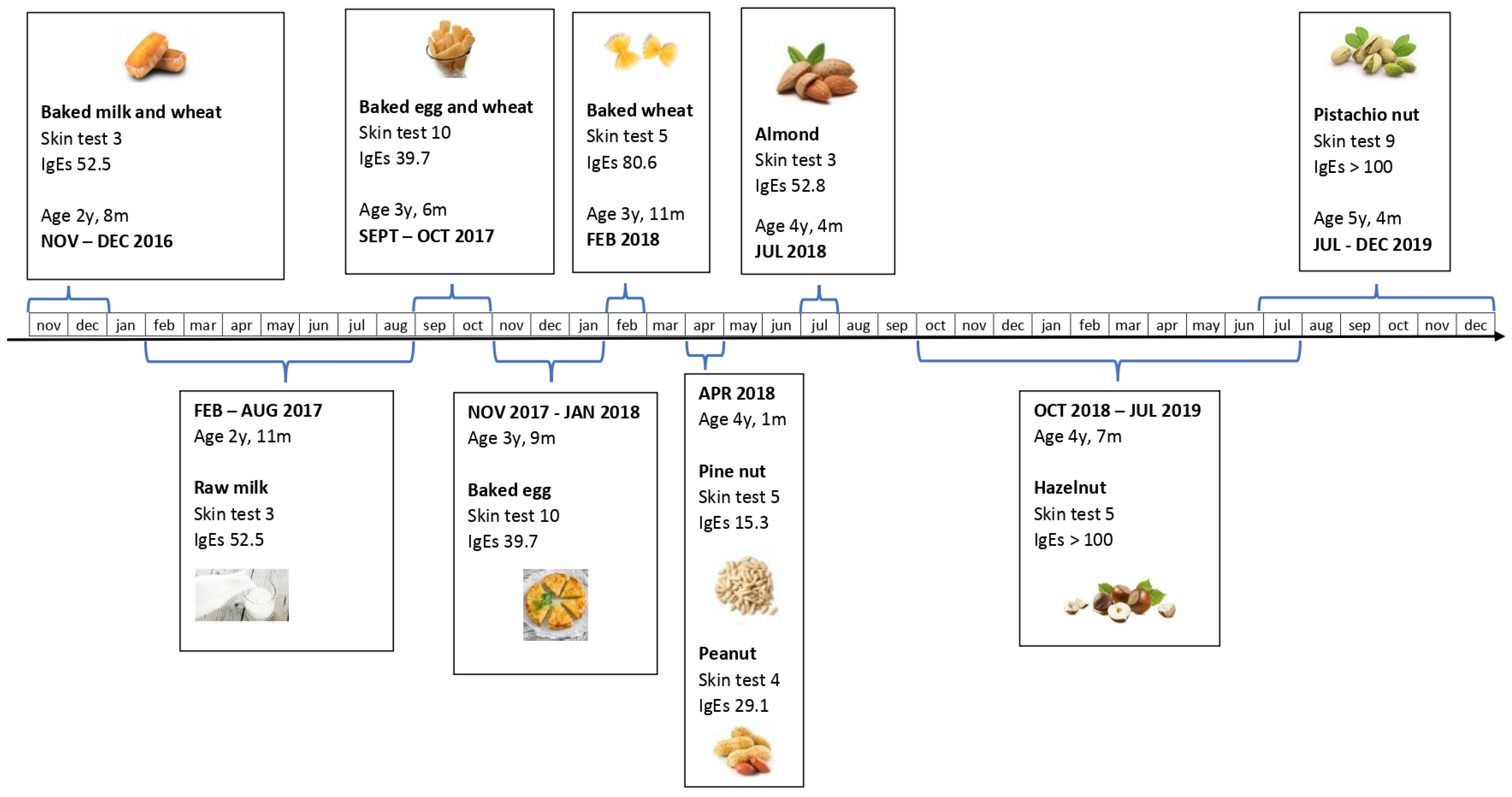
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Çetinkaya, P.G.; Şahiner, Ü.M. Childhood atopic dermatitis: Current developments, treatment approaches, and future expectations. Turk. J. Med. Sci. 2019, 49, 963. [Google Scholar] [CrossRef] [PubMed]
- Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Beyer, K.; Bindslev-Jensen, C.; Cardona, V.; Dubois, A.; Du Toit, G.; Eigenmann, P.; et al. EAACI Food Allergy and Anaphylaxis Guidelines: Diagnosis and management of food allergy. Allergy 2014, 17, 1008–1025. [Google Scholar] [CrossRef] [PubMed]
- Geat, D.; Giovannini, M.; Barlocco, G.; Pertile, R.; Pace, M.; Mori, F.; Novembre, E.; Girolomoni, G.; Cristofolini, M.; Baldo, E. Assessing patients’ characteristics and treatment patterns among children with atopic dermatitis. Ital. J. Pediatr. 2021, 47, 92. [Google Scholar] [CrossRef]
- Galli, E.; Fortina, A.B.; Ricci, G.; Maiello, N.; Neri, I.; Baldo, E.; Berti, I.; Bonamonte, D.; Capra, L.; Carboni, E.; et al. Narrative review on the management of moderate-severe atopic dermatitis in pediatric age of the Italian Society of Pediatric Allergology and Immunology (SIAIP), of the Italian Society of Pediatric Dermatology (SIDerP) and of the Italian Society of Pediatr. Ital. J. Pediatr. 2022, 48, 95. [Google Scholar] [CrossRef]
- Mortz, C.G.; du Toit, G.; Beyer, K.; Bindslev-Jensen, C.; Brockow, K.; Brough, H.A.; Comberiati, P.; Eiwegger, T.; Santos, A.; Worm, M.; et al. When and how to evaluate for immediate type food allergy in children with atopic dermatitis. Allergy 2021, 76, 3845–3848. [Google Scholar] [CrossRef]
- Miyaji, Y.; Yang, L.; Yamamoto-Hanada, K.; Narita, M.; Saito, H.; Ohya, Y. Earlier aggressive treatment to shorten the duration of eczema in infants resulted in fewer food allergies at 2 years of age. J. Allergy Clin. Immunol. Pract. 2020, 8, 1721–1724.e6. [Google Scholar] [CrossRef]
- Schäfer, T. The impact of allergy on atopic eczema from data from epidemiological studies. Curr. Opin. Allergy Clin. Immunol. 2008, 8, 418–422. [Google Scholar] [CrossRef]
- Eller, E.; Kjaer, H.F.; Høst, A.; Andersen, K.E.; Bindslev-Jensen, C. Food allergy and food sensitization in early childhood: Results from the DARC cohort. Allergy 2009, 64, 1023–1029. [Google Scholar] [CrossRef]
- Hon, K.-L.E.; Leung, T.-F.; Ching, G.; Chow, C.-M.; Luk, V.; Ko, W.-S.F.; Ng, P.-C. Patterns of food and aeroallergen sensitization in childhood eczema. Acta Paediatr. 2008, 97, 1734–1737. [Google Scholar] [CrossRef]
- Pónyai, G.; Hidvégi, B.; Németh, I.; Sas, A.; Temesvári, E.; Kárpáti, S. Contact and aeroallergens in adulthood atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 1346–1355. [Google Scholar] [CrossRef]
- Atherton, D.J.; Soothill, J.F.; Sewell, M.; Wells, R.S.; Chilvers, C.D. A double-blind controlled crossover trial of an antigen-avoidance diet in atopic eczema. Lancet 1978, 311, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Juto, P.; Engberg, S.; Winberg, J. Treatment of infantile atopic dermatitis with a strict elimination diet. Clin. Exp. Allergy 1978, 8, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Businco, L.; Businco, E.; Cantani, A.; Galli, E.; Infussi, R.; Benincori, N. Results of a milk and/or egg free diet in children with atopic dermatitis. Allergol. Immunopathol. 1982, 10, 283–288. [Google Scholar]
- Lee, K.S.; Rha, Y.H.; Oh, I.H.; Choi, Y.S.; Kim, Y.E.; Choi, S.H. Does Breast-feeding Relate to Development of Atopic Dermatitis in Young Korean Children?: Based on the Fourth and Fifth Korea National Health and Nutrition Examination Survey 2007–2012. Allergy. Asthma Immunol. Res. 2017, 9, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Mostrom, M.; Ludvigsson, J.; Duchen, K. Exclusive breastfeeding and risk of atopic dermatitis in some 8300 infants. Pediatr. Allergy Immunol. 2005, 16, 201–208. [Google Scholar] [CrossRef]
- Yang, Y.W.; Tsai, C.L.; Lu, C.Y. Exclusive breastfeeding and incident atopic dermatitis in childhood: A systematic review and meta-analysis of prospective cohort studies. Br. J. Dermatol. 2009, 161, 373–383. [Google Scholar] [CrossRef]
- Kull, I.; Böhme, M.; Wahlgren, C.F.; Nordvall, L.; Pershagen, G.; Wickman, M. Breast-feeding reduces the risk for childhood eczema. J. Allergy Clin. Immunol. 2005, 116, 657–661. [Google Scholar] [CrossRef]
- Chiu, C.Y.; Liao, S.L.; Su, K.W.; Tsai, M.H.; Hua, M.C.; Lai, S.H.; Chen, L.C.; Yao, T.C.; Yeh, K.W.; Huang, J.L. Exclusive or Partial Breastfeeding for 6 Months Is Associated with Reduced Milk Sensitization and Risk of Eczema in Early Childhood: The PATCH Birth Cohort Study. Medicine 2016, 95, 15. [Google Scholar] [CrossRef]
- Gdalevich, M.; Mimouni, D.; David, M.; Mimouni, M. Breast-feeding and the onset of atopic dermatitis in childhood: A systematic review and meta-analysis of prospective studies. J. Am. Acad. Dermatol. 2001, 45, 520–527. [Google Scholar] [CrossRef]
- Schoetzau, A.; Filipiak-Pittroff, B.; Franke, K.; Koletzko, S.; Von Berg, A.; Gruebl, A.; Bauer, C.P.; Berdel, D.; Reinhardt, D.; Wichmann, H.-E.; et al. Effect of exclusive breast-feeding and early solid food avoidance on the incidence of atopic dermatitis in high-risk infants at 1 year of age. Pediatr. Allergy Immunol. 2002, 13, 234–242. [Google Scholar] [CrossRef]
- Purvis, D.J.; Thompson, J.M.D.; Clark, P.M.; Robinson, E.; Black, P.N.; Wild, C.J.; Mitchell, E.A. Risk factors for atopic dermatitis in New Zealand children at 3.5 years of age. Br. J. Dermatol. 2005, 152, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.; Fujiwara, T. Breastfeeding and risk of atopic dermatitis up to the age 42 months: A birth cohort study in Japan. Ann. Epidemiol. 2014, 24, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Pesonen, M.; Kallio, M.J.T.; Ranki, A.; Siimes, M.A. Prolonged exclusive breastfeeding is associated with increased atopic dermatitis: A prospective follow-up study of unselected healthy newborns from birth to age 20 years. Clin. Exp. Allergy 2006, 36, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, D.; Soto-Ramírez, N.; Kurukulaaratchy, R.J.; Holloway, J.W.; Karmaus, W.; Ewart, S.L.; Arshad, S.H.; Erlewyn-Lajeunesse, M. Filaggrin loss-of-function mutations are associated with food allergy in childhood and adolescence. J. Allergy Clin. Immunol. 2014, 134, 876–882.e4. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.J.; Asai, Y.; Cordell, H.J.; Campbell, L.E.; Zhao, Y.; Liao, H.; Northstone, K.; Henderson, J.; Alizadehfar, R.; Ben-Shoshan, M.; et al. Loss-of-function variants in the filaggrin gene are a significant risk factor for peanut allergy. J. Allergy Clin. Immunol. 2011, 127, 661–667. [Google Scholar] [CrossRef] [PubMed]
- Brough, H.A.; Liu, A.H.; Sicherer, S.; Makinson, K.; Douiri, A.; Brown, S.J.; Stephens, A.C.; McLean, W.I.; Turcanu, V.; Wood, R.A.; et al. Atopic dermatitis increases the effect of exposure to peanut antigen in dust on peanut sensitization and likely peanut allergy. J. Allergy Clin. Immunol. 2015, 135, 164–170.e4. [Google Scholar] [CrossRef]
- Flohr, C.; Perkin, M.; Logan, K.; Marrs, T.; Radulovic, S.; Campbell, L.E.; MacCallum, S.F.; McLean, W.I.; Lack, G. Atopic dermatitis and disease severity are the main risk factors for food sensitization in exclusively breastfed infants. J. Investig. Dermatol. 2014, 134, 345–350. [Google Scholar] [CrossRef]
- Perkin, M.R.; Logan, K.; Marrs, T.; Radulovic, S.; Craven, J.; Boyle, R.J.; Chalmers, J.R.; Williams, H.C.; Versteeg, S.A.; van Ree, R.; et al. Association of frequent moisturizer use in early infancy with the development of food allergy. J. Allergy Clin. Immunol. 2021, 147, 967–976.e1. [Google Scholar] [CrossRef]
- Eigenmann, P.A.; Beyer, K.; Lack, G.; Muraro, A.; Ong, P.Y.; Sicherer, S.H.; Sampson, H.A. Are avoidance diets still warranted in children with atopic dermatitis? Pediatr. Allergy Immunol. 2020, 31, 19–26. [Google Scholar] [CrossRef]
- Halken, S.; Muraro, A.; de Silva, D.; Khaleva, E.; Angier, E.; Arasi, S.; Arshad, H.; Bahnson, H.T.; Beyer, K.; Boyle, R.; et al. EAACI guideline: Preventing the development of food allergy in infants and young children (2020 update). Pediatr. Allergy Immunol. 2021, 32, 843–858. [Google Scholar] [CrossRef]
- Du Toit, G.; Roberts, G.; Sayre, P.H.; Bahnson, H.T.; Radulovic, S.; Santos, A.F.; Brough, H.A.; Phippard, D.; Basting, M.; Feeney, M.; et al. Randomized Trial of Peanut Consumption in Infants at Risk for Peanut Allergy. N. Engl. J. Med. 2015, 372, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Perkin, M.R.; Logan, K.; Tseng, A.; Raji, B.; Ayis, S.; Peacock, J.; Brough, H.; Marrs, T.; Radulovic, S.; Craven, J.; et al. Randomized Trial of Introduction of Allergenic Foods in Breast-Fed Infants. N. Engl. J. Med. 2016, 374, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Natsume, O.; Kabashima, S.; Nakazato, J.; Yamamoto-Hanada, K.; Narita, M.; Kondo, M.; Saito, M.; Kishino, A.; Takimoto, T.; Inoue, E.; et al. Two-step egg introduction for prevention of egg allergy in high-risk infants with eczema (PETIT): A randomised, double-blind, placebo-controlled trial. Lancet 2017, 389, 276–286. [Google Scholar] [CrossRef]
- Foong, R.-X.; Giovannini, M.; Toit, G.D. Food-dependent exercise-induced anaphylaxis. Curr. Opin. Allergy Clin. Immunol. 2019, 19, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Tagliati, S.; Barni, S.; Giovannini, M.; Liccioli, G.; Sarti, L.; Alicandro, T.; Paladini, E.; Perferi, G.; Azzari, C.; Novembre, E.; et al. Nut Allergy: Clinical and Allergological Features in Italian Children. Nutrients 2021, 13, 4076. [Google Scholar] [CrossRef] [PubMed]
- de las Vecillas, L.; Eguiluz-Gracia, I.; Giovannini, M. You might owe your mother more than you think. Allergy 2021, 76, 3236–3237. [Google Scholar] [CrossRef]
- Barbati, F.; Giovannini, M.; Oranges, T.; Lodi, L.; Barni, S.; Novembre, E.; Baldo, E.; Cristofolini, M.; Stagi, S.; Ricci, S.; et al. Netherton Syndrome in Children: Management and Future Perspectives. Front. Pediatr. 2021, 9, 645259. [Google Scholar] [CrossRef]
- Werfel, T.; Ballmer-Weber, B.; Eigenmann, P.; Niggemann, B.; Rancé, F.; Turjanmaa, K.; Worm, M. Eczematous reactions to food in atopic eczema: Position paper of the EAACI and GA2LEN. Allergy 2007, 62, 723–728. [Google Scholar] [CrossRef]
- Kvenshagen, B.; Jacobsen, M.; Halvorsen, R. Atopic dermatitis in premature and term children. Arch. Dis. Child. 2009, 94, 202–205. [Google Scholar] [CrossRef]
- Hill, D.J.; Heine, R.G.; Hosking, C.S. The diagnostic value of skin prick testing in children with food allergy. Pediatr. Allergy Immunol. 2004, 15, 435–441. [Google Scholar] [CrossRef]
- Sampson, H.A. Food allergy. Part 2: Diagnosis and management. J. Allergy Clin. Immunol. 1999, 103, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.J.; Heine, R.G.; Hosking, C.S.; Brown, J.; Thiele, L.; Allen, K.J.; Su, J.; Varigos, G.; Carlin, J.B. IgE food sensitization in infants with eczema attending a dermatology department. J. Pediatr. 2007, 151, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Eigenmann, P.A.; Sicherer, S.H.; Borkowski, T.A.; Cohen, B.A.; Sampson, H.A. Prevalence of IgE-mediated food allergy among children with atopic dermatitis. Pediatrics 1998, 101, 3. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.E.; Eckert, J.K.; Koplin, J.J.; Lowe, A.J.; Gurrin, L.C.; Dharmage, S.C.; Vuillermin, P.; Tang, M.L.K.; Ponsonby, A.-L.; Matheson, M.; et al. Which infants with eczema are at risk of food allergy? Results from a population-based cohort. Clin. Exp. Allergy 2015, 45, 255–264. [Google Scholar] [CrossRef]
- Roduit, C.; Frei, R.; Depner, M.; Karvonen, A.M.; Renz, H.; Braun-Fahrländer, C.; Schmausser-Hechfellner, E.; Pekkanen, J.; Riedler, J.; Dalphin, J.-C.; et al. Phenotypes of Atopic Dermatitis Depending on the Timing of Onset and Progression in Childhood. JAMA Pediatr. 2017, 171, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Paller, A.S.; Spergel, J.M.; Mina-Osorio, P.; Irvine, A.D. The atopic march and atopic multimorbidity: Many trajectories, many pathways. J. Allergy Clin. Immunol. 2019, 143, 46–55. [Google Scholar] [CrossRef]
- Kramer, M.S.; Kakuma, R. Maternal dietary antigen avoidance during pregnancy or lactation, or both, for preventing or treating atopic disease in the child. Evid. Based. Child Health 2014, 9, 447–483. [Google Scholar] [CrossRef] [PubMed]
- Kajosaari, M.; Saarinen, U.M. Prophylaxis of atopic disease by six months’ total solid food elimination. Evaluation of 135 exclusively breast-fed infants of atopic families. Acta Paediatr. Scand. 1983, 72, 411–414. [Google Scholar] [CrossRef]
- Fergusson, D.M.; Horwood, L.J.; Shannon, F.T. Early solid feeding and recurrent childhood eczema: A 10-year longitudinal study. Pediatrics 1990, 86, 541–546. [Google Scholar] [CrossRef]
- Fergusson, D.M.; Horwood, L.J.; Beautrais, A.L.; Shannon, F.T.; Taylor, B. Eczema and infant diet. Clin. Exp. Allergy 1981, 11, 325–331. [Google Scholar] [CrossRef]
- Du Toit, G.; Katz, Y.; Sasieni, P.; Mesher, D.; Maleki, S.J.; Fisher, H.R.; Fox, A.T.; Turcanu, V.; Amir, T.; Zadik-Mnuhin, G.; et al. Early consumption of peanuts in infancy is associated with a low prevalence of peanut allergy. J. Allergy Clin. Immunol. 2008, 122, 984–991. [Google Scholar] [CrossRef] [PubMed]
- Koplin, J.J.; Osborne, N.J.; Wake, M.; Martin, P.E.; Gurrin, L.C.; Robinson, M.N.; Tey, D.; Slaa, M.; Thiele, L.; Miles, L.; et al. Can early introduction of egg prevent egg allergy in infants? A population-based study. J. Allergy Clin. Immunol. 2010, 126, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Greer, F.R.; Sicherer, S.H.; Burks, A.W.; Committee on Nutrition and Section on Allergy and Immunology. Effects of early nutritional interventions on the development of atopic disease in infants and children: The role of maternal dietary restriction, breastfeeding, timing of introduction of complementary foods, and hydrolyzed formulas. Pediatrics 2008, 121, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Thygarajan, A.; Burks, A.W. American Academy of Pediatrics recommendations on the effects of early nutritional interventions on the development of atopic disease. Curr. Opin. Pediatr. 2008, 20, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Perkin, M.R.; Logan, K.; Marrs, T.; Radulovic, S.; Craven, J.; Flohr, C.; Lack, G.; Young, L.; Offord, V.; DeSousa, M.; et al. Enquiring About Tolerance (EAT) study: Feasibility of an early allergenic food introduction regimen. J. Allergy Clin. Immunol. 2016, 137, 1477–1486.e8. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, D.M.; Spergel, J.M.; Assa’ad, A.H.; Pongracic, J.A. Primary Prevention of Allergic Disease Through Nutritional Interventions. J. Allergy Clin. Immunol. Pract. 2013, 1, 29–36. [Google Scholar] [CrossRef]
- Australasian Society of Clinical Immunology and Allergy. Infant Feeding and Allergy Prevention. Available online: http://www.allergy.org.au/images/pcc/ASCIA_guidelines_infant_feeding_and_allergy_prevention.pdf (accessed on 4 April 2023).
- Tham, E.H.; Shek LP, C.; Van Bever, H.P.; Vichyanond, P.; Ebisawa, M.; Wong, G.W.; Lee, B.W. Early introduction of allergenic foods for the prevention of food allergy from an Asian perspective—An Asia Pacific Association of Pediatric Allergy, Respirology & Immunology (APAPARI) consensus statement. Pediatr. Allergy Immunol. 2018, 29, 18–27. [Google Scholar]
- Comberiati, P.; Costagliola, G.; D’Elios, S.; Peroni, D. Prevention of Food Allergy: The Significance of Early Introduction. Medicina 2019, 55, 323. [Google Scholar] [CrossRef]
- Togias, A.; Cooper, S.F.; Acebal, M.L.; Assa’Ad, A.; Baker, J.R.; Beck, L.A.; Block, J.; Byrd-Bredbenner, C.; Chan, E.S.; Eichenfield, L.F.; et al. Addendum guidelines for the prevention of peanut allergy in the United States: Report of the National Institute of Allergy and Infectious Diseases–sponsored expert panel. J. Allergy Clin. Immunol. 2017, 139, 29–44. [Google Scholar] [CrossRef]
- Barni, S.; Liccioli, G.; Sarti, L.; Giovannini, M.; Novembre, E.; Mori, F. Immunoglobulin E (IgE)-Mediated Food Allergy in Children: Epidemiology, Pathogenesis, Diagnosis, Prevention, and Management. Medicina 2020, 56, 111. [Google Scholar] [CrossRef]
- Cafarotti, A.; Giovannini, M.; Begìn, P.; Brough, H.A.; Arasi, S. Management of IgE-mediated food allergy in the 21st century. Clin. Exp. Allergy 2023, 53, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Abrams, E.M.; Becker, A.B. Oral food challenge outcomes in a pediatric tertiary care center. Allergy Asthma Clin. Immunol. 2017, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Perry, T.T.; Matsui, E.C.; Conover-Walker, M.K.; Wood, R.A. Risk of oral food challenges. J. Allergy Clin. Immunol. 2004, 114, 1164–1168. [Google Scholar] [CrossRef]
- DunnGalvin, A.; Daly, D.; Cullinane, C.; Stenke, E.; Keeton, D.; Erlewyn-Lajeunesse, M.; Roberts, G.C.; Lucas, J.; Hourihane, J.O. Highly accurate prediction of food challenge outcome using routinely available clinical data. J. Allergy Clin. Immunol. 2011, 127, 633–639.e3. [Google Scholar] [CrossRef]
- Ballini, G.; Gavagni, C.; Guidotti, C.; Ciolini, G.; Liccioli, G.; Giovannini, M.; Sarti, L.; Ciofi, D.; Novembre, E.; Mori, F.; et al. Frequency of positive oral food challenges and their outcomes in the allergy unit of a tertiary-care pediatric hospital. Allergol. Immunopathol. 2021, 49, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Barni, S.; Mori, F.; Piccorossi, A.; Sarti, L.; Pucci, N.; Maresca, M.; Giovannini, M.; Liccioli, G.; Novembre, E. Low-Dose Oral Food Challenge with Hazelnut: Efficacy and Tolerability in Children. Int. Arch. Allergy Immunol. 2019, 178, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Yanagida, N.; Sato, S.; Nagakura, K.; Asaumi, T.; Ebisawa, M. Oral food challenge using different target doses and time intervals between doses. Curr. Opin. Allergy Clin. Immunol. 2018, 18, 222–227. [Google Scholar] [CrossRef]
- Switkowski, K.M.; Oken, E.; Rifas-Shiman, S.L.; Camargo, C.A., Jr.; Gold, D.R.; Sordillo, J.E.; Lightdale, J.R. Timing of Cow’s Milk Protein Introduction and Childhood Adverse Reactions to Cow’s Milk. J. Allergy Clin. Immunol. Pract. 2022, 10, 2713–2721.e2. [Google Scholar] [CrossRef]
- Urisu, A.; Ebisawa, M.; Ito, K.; Aihara, Y.; Ito, S.; Mayumi, M.; Kohno, Y.; Kondo, N. Japanese guideline for food allergy 2014. Allergol. Int. 2014, 63, 399–419. [Google Scholar] [CrossRef]
- Ebisawa, M.; Ito, K.; Fujisawa, T. Japanese guidelines for food allergy 2017. Allergol. Int. 2017, 66, 248–264. [Google Scholar] [CrossRef]
| AGE | 6 Months | 2 Years | 4 Years | |||
|---|---|---|---|---|---|---|
| tIgE (kU/L) | 1777 | 2863 | 4155 | |||
| Eosinophils (n °/mmc) | 6087 | 708 | 378 | |||
| Foods | SPT/PbP (mm) | sIgE (kU/L) | SPT/PbP (mm) | sIgE (kU/L) | SPT/PbP (mm) | SIgE (kU/L) |
| MILK | 6 | 47.60 | 3 | 52.50 | - | 17.00 |
| EGG WHITE | 4 | >100.00 | 10 | 39.70 | - | 19.10 |
| WHEAT | 4 | >100.00 | 5 | 80.60 | - | 41.10 |
| PEANUT | np | np | 2 | 19.30 | 4 | 29.10 |
| PISTACHIO | np | np | 7 | >100.00 | 9 | >100.00 |
| CASHEW | np | np | 7 | >100.00 | 10 | >100.00 |
| HAZELNUT | np | np | 3 | >100.00 | 5 | >100.00 |
| ALMOND | np | np | 2 | >100.00 | 3 | 52.80 |
| WALNUT | np | np | 0 | 14.40 | 4 | 26.00 |
| PINE NUT | np | np | 0 | 3.81 | 5 | 15.30 |
| FISH | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giovannini, M.; Bolis, M.; Barni, S.; Liccioli, G.; Sarti, L.; Morelli, S.; Pontone, M.; Pessina, B.; Tomei, L.; Valleriani, C.; et al. Pearls and Pitfalls of Weaning an Infant with Severe Atopic Dermatitis and Sensitization/Allergy to Food. J. Clin. Med. 2023, 12, 3889. https://doi.org/10.3390/jcm12123889
Giovannini M, Bolis M, Barni S, Liccioli G, Sarti L, Morelli S, Pontone M, Pessina B, Tomei L, Valleriani C, et al. Pearls and Pitfalls of Weaning an Infant with Severe Atopic Dermatitis and Sensitization/Allergy to Food. Journal of Clinical Medicine. 2023; 12(12):3889. https://doi.org/10.3390/jcm12123889
Chicago/Turabian StyleGiovannini, Mattia, Marta Bolis, Simona Barni, Giulia Liccioli, Lucrezia Sarti, Susanna Morelli, Matteo Pontone, Benedetta Pessina, Leonardo Tomei, Claudia Valleriani, and et al. 2023. "Pearls and Pitfalls of Weaning an Infant with Severe Atopic Dermatitis and Sensitization/Allergy to Food" Journal of Clinical Medicine 12, no. 12: 3889. https://doi.org/10.3390/jcm12123889
APA StyleGiovannini, M., Bolis, M., Barni, S., Liccioli, G., Sarti, L., Morelli, S., Pontone, M., Pessina, B., Tomei, L., Valleriani, C., Novembre, E., & Mori, F. (2023). Pearls and Pitfalls of Weaning an Infant with Severe Atopic Dermatitis and Sensitization/Allergy to Food. Journal of Clinical Medicine, 12(12), 3889. https://doi.org/10.3390/jcm12123889








