Impact of Clinical Pharmacist Running Anticoagulation Clinic in Saudi Arabia
Abstract
:1. Introduction
2. Method
2.1. Study Design and Setting
2.2. Models of Care
2.3. Eligibility Criteria
2.4. Data Extraction
2.5. Study Outcome
2.6. Sample Size Calculation
2.7. Statistical Analysis
2.8. Ethics Approval
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention Heart Disease Facts. Available online: https://www.cdc.gov/heartdisease/facts.htm (accessed on 13 April 2023).
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef]
- Ansell, J.; Hirsh, J.; Poller, L.; Bussey, H.; Jacobson, A.; Hylek, E. The Pharmacology and Management of the Vitamin K Antagonists: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004, 126, 204S–233S. [Google Scholar] [CrossRef] [Green Version]
- Koda-Kimble, M.A.; Young, L.Y.; Kradjan, W.A.; Guglielmo, B.J.; Alldredge, B.K.; Corelli, R.L.; Williams, B.R. (Eds.) Applied Therapeutics: The Clinical Use of Drugs, 9th ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2008; ISBN 9780781765558. [Google Scholar]
- Chisholm-Burns, M.A.; Schwinghammer, T.L.; Wells, B.G.; Malone, P.M.; DiPiro, J.T. Pharmacotherapy Principles and Practice, 3rd ed.; McGraw-Hill Medical: New York, NY, USA, 2013; ISBN 9780071780469. [Google Scholar]
- DiPiro, J.; Yee, G.; Haines, S.T.; Nolin, T.D.; Ellingrod, V.; Posey, L.M. DiPiro’s Pharmacotherapy: A Pathophysiologic Approach, 12th ed.; McGraw-Hill Education: Columbus, OH, USA, 2023; ISBN 9781264264544. [Google Scholar]
- Shields, L.B.E.; Fowler, P.; Siemens, D.M.; Lorenz, D.J.; Wilson, K.C.; Hester, S.T.; Honaker, J.T. Standardized Warfarin Monitoring Decreases Adverse Drug Reactions. BMC Fam. Pract. 2019, 20, 151. [Google Scholar] [CrossRef] [Green Version]
- Crader, M.F.; Johns, T.; Arnold, J.K. Warfarin Drug Interactions; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Testa, S.; Alatri, A.; Paoletti, O.; Morstabilini, G.; Medagliani, M.A.; Denti, N.; Martellenghi, E. Reorganisation of an Anticoagulation Clinic Using a Telemedicine System: Description of the Model and Preliminary Results. Intern. Emerg. Med. 2006, 1, 24–29. [Google Scholar] [CrossRef]
- Chiquette, E.; Amato, M.G.; Bussey, H.I. Comparison of an Anticoagulation Clinic with Usual Medical Care: Anticoagulation Control, Patient Outcomes, and Health Care Costs. Arch. Intern. Med. 1998, 158, 1641. [Google Scholar] [CrossRef] [Green Version]
- Ryan, F.; Byrne, S.; O’Shea, S. Managing Oral Anticoagulation Therapy: Improving Clinical Outcomes. A Review. J. Clin. Pharm. Ther. 2008, 33, 581–590. [Google Scholar] [CrossRef]
- Damaske, D.L.; Baird, R.W. Development and Implementation of a Pharmacist-Managed Inpatient Warfarin Protocol. Bayl. Univ. Med. Cent. Proc. 2005, 18, 397–400. [Google Scholar] [CrossRef] [Green Version]
- Witt, D.M.; Sadler, M.A.; Shanahan, R.L.; Mazzoli, G.; Tillman, D.J. Effect of a Centralized Clinical Pharmacy Anticoagulation Service on the Outcomes of Anticoagulation Therapy. Chest 2005, 127, 1515–1522. [Google Scholar] [CrossRef] [Green Version]
- Young, S.; Bishop, L.; Twells, L.; Dillon, C.; Hawboldt, J.; O’Shea, P. Comparison of Pharmacist Managed Anticoagulation with Usual Medical Care in a Family Medicine Clinic. BMC Fam. Pract. 2011, 12, 88. [Google Scholar] [CrossRef] [Green Version]
- Rudd, K.M.; Dier, J.G. Comparison of Two Different Models of Anticoagulation Management Services with Usual Medical Care. Pharmacotherapy 2010, 30, 330–338. [Google Scholar] [CrossRef]
- Lewis, S.M.; Kroner, B.A. Patient Survey of a Pharmacist-Managed Anticoagulation Clinic. Manag. Care Interface 1997, 10, 66–70. [Google Scholar]
- Bond, C.A.; Raehl, C.L. Pharmacist-Provided Anticoagulation Management in United States Hospitals: Death Rates, Length of Stay, Medicare Charges, Bleeding Complications, and Transfusions. Pharmacotherapy 2004, 24, 953–963. [Google Scholar] [CrossRef]
- Beyth, R.J.; Quinn, L.M.; Landefeld, C.S. Prospective Evaluation of an Index for Predicting the Risk of Major Bleeding in Outpatients Treated with Warfarin. Am. J. Med. 1998, 105, 91–99. [Google Scholar] [CrossRef]
- Dib, J.G.; Mohammed, K.; Momattin, H.I.; Alshehri, A.M. Implementation of Pharmacist-Managed Anticoagulation Clinic in a Saudi Arabian Health Center. Hosp. Pharm. 2014, 49, 260–268. [Google Scholar] [CrossRef] [Green Version]
- Garwood, C.L.; Dumo, P.; Baringhaus, S.N.; Laban, K.M. Quality of Anticoagulation Care in Patients Discharged from a Pharmacist-Managed Anticoagulation Clinic after Stabilization of Warfarin Therapy. Pharmacotherapy 2008, 28, 20–26. [Google Scholar] [CrossRef]
- Clark, N.P. Role of the Anticoagulant Monitoring Service in 2018: Beyond Warfarin. Hematol. Am. Soc. Hematol. Educ. Program 2018, 2018, 348–352. [Google Scholar] [CrossRef] [Green Version]
- Rodgers, J.L.; Jones, J.; Bolleddu, S.I.; Vanthenapalli, S.; Rodgers, L.E.; Shah, K.; Karia, K.; Panguluri, S.K. Cardiovascular Risks Associated with Gender and Aging. J. Cardiovasc. Dev. Dis. 2019, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Länsitie, M.; Kangas, M.; Jokelainen, J.; Venojärvi, M.; Timonen, M.; Keinänen-Kiukaanniemi, S.; Korpelainen, R. Cardiovascular Disease Risk and All-Cause Mortality Associated with Accelerometer-Measured Physical Activity and Sedentary Time—A Prospective Population-Based Study in Older Adults. BMC Geriatr. 2022, 22, 729. [Google Scholar] [CrossRef]
- Akinosun, A.S.; Polson, R.; Diaz-Skeete, Y.; De Kock, J.H.; Carragher, L.; Leslie, S.; Grindle, M.; Gorely, T. Digital Technology Interventions for Risk Factor Modification in Patients with Cardiovascular Disease: Systematic Review and Meta-Analysis. JMIR MHealth UHealth 2021, 9, e21061. [Google Scholar] [CrossRef]
- Shakoor, H.; Platat, C.; Ali, H.I.; Ismail, L.C.; Al Dhaheri, A.S.; Bosevski, M.; Apostolopoulos, V.; Stojanovska, L. The Benefits of Physical Activity in Middle-Aged Individuals for Cardiovascular Disease Outcomes. Maturitas 2023, 168, 49–52. [Google Scholar] [CrossRef]
- Bartus, K.; Sadowski, J.; Litwinowicz, R.; Filip, G.; Jasinski, M.; Deja, M.; Kusmierczyk, M.; Pawlak, S.; Jemielity, M.; Jagielak, D.; et al. Changing Trends in Aortic Valve Procedures over the Past Ten Years-from Mechanical Prosthesis via Stented Bioprosthesis to TAVI Procedures-Analysis of 50,846 Aortic Valve Cases Based on a Polish National Cardiac Surgery Database. J. Thorac. Dis. 2019, 11, 2340–2349. [Google Scholar] [CrossRef]
- Boudoulas, K.D.; Ravi, Y.; Garcia, D.; Saini, U.; Sofowora, G.G.; Gumina, R.J.; Sai-Sudhakar, C.B. Type of Valvular Heart Disease Requiring Surgery in the 21st Century: Mortality and Length-of-Stay Related to Surgery. Open Cardiovasc. Med. J. 2013, 7, 104–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rose, A.J.; Ozonoff, A.; Henault, L.E.; Hylek, E.M. Anticoagulation for Valvular Heart Disease in Community-Based Practice. Thromb. Haemost. 2010, 103, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, E.; Udeani, G.; Horseman, M.; Hintze, T.D.; Surani, S. Role of Clinical Pharmacists in Intensive Care Units. Cureus 2021, 13, e17929. [Google Scholar] [CrossRef] [PubMed]
- Althomali, A.; Altowairqi, A.; Alghamdi, A.; Alotaibi, M.; Althubaiti, A.; Alqurashi, A.; Harbi, A.A.; Algarni, M.A.; Haseeb, A.; Elnaem, M.H.; et al. Impact of Clinical Pharmacist Intervention on Clinical Outcomes in the Critical Care Unit, Taif City, Saudi Arabia: A Retrospective Study. Pharmacy 2022, 10, 108. [Google Scholar] [CrossRef]
- Manzoor, B.S.; Cheng, W.-H.; Lee, J.C.; Uppuluri, E.M.; Nutescu, E.A. Quality of Pharmacist-Managed Anticoagulation Therapy in Long-Term Ambulatory Settings: A Systematic Review. Ann. Pharmacother. 2017, 51, 1122–1137. [Google Scholar] [CrossRef]
- Wang, N.; Qiu, S.; Yang, Y.; Zhang, C.; Gu, Z.-C.; Qian, Y. Physician-Pharmacist Collaborative Clinic Model to Improve Anticoagulation Quality in Atrial Fibrillation Patients Receiving Warfarin: An Analysis of Time in Therapeutic Range and a Nomogram Development. Front. Pharmacol. 2021, 12, 673302. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.M.; Quek, Y.-N.; Tay, J.C.; Chadachan, V.; Lee, H.K. Efficacy and Safety of a Pharmacist-Managed Inpatient Anticoagulation Service for Warfarin Initiation and Titration: Pharmacist-Managed Inpatient Anticoagulation Service. J. Clin. Pharm. Ther. 2011, 36, 585–591. [Google Scholar] [CrossRef]
- Mifsud, E.M.; Wirth, F.; Camilleri, L.; Azzopardi, L.M.; Serracino-Inglott, A. Pharmacist-Led Medicine Use Review in Community Pharmacy for Patients on Warfarin. Int. J. Clin. Pharm. 2019, 41, 741–750. [Google Scholar] [CrossRef]
- Falamić, S.; Lucijanić, M.; Ortner-Hadžiabdić, M.; Marušić, S.; Bačić-Vrca, V. Pharmacists’ Interventions Improve Health-Related Quality of Life of Rural Older Person on Warfarin: A Randomized Controlled Trial. Sci. Rep. 2021, 11, 21897. [Google Scholar] [CrossRef]
- Daniels, P.R.; Manning, D.M.; Moriarty, J.P.; Bingener-Casey, J.; Ou, N.N.; O’Meara, J.G.; Roellinger, D.L.; Naessens, J.M. Improving Inpatient Warfarin Therapy Safety Using a Pharmacist-Managed Protocol. BMJ Open Qual. 2018, 7, e000290. [Google Scholar] [CrossRef] [PubMed]
- Aidit, S.; Soh, Y.C.; Yap, C.S.; Khan, T.M.; Neoh, C.F.; Shaharuddin, S.; Kassab, Y.W.; Patel, R.P.; Ming, L.C. Effect of Standardized Warfarin Treatment Protocol on Anticoagulant Effect: Comparison of a Warfarin Medication Therapy Adherence Clinic with Usual Medical Care. Front. Pharmacol. 2017, 8, 637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, N.O.; Osman, B.; Abdelhai, Y.M.; El-Hadiyah, T.M.H. Impact of Clinical Pharmacist Intervention in Anticoagulation Clinic in Sudan. Int. J. Clin. Pharm. 2017, 39, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Sheng, X.Y.; Xiang, Q.; Wang, Z.N.; Zhou, Y.; Cui, Y.M. Comparing the Effectiveness of Pharmacist-Managed Warfarin Anticoagulation with Other Models: A Systematic Review and Meta-Analysis. J. Clin. Pharm. Ther. 2016, 41, 602–611. [Google Scholar] [CrossRef]
- Al-Jazairi, A.; Hijazi, H.; Samarkandi, H.; Akhras, N.; Devol, E.; Hamasni, I. What is the ideal clinical pharmacy practice model? A satisfaction comparative study. J. Am. Coll. Clin. Pharm. 2021, 4, 441–449. [Google Scholar] [CrossRef]
- Mills, T.; Madden, M.; Stewart, D.; Gough, B.; McCambridge, J. Integration of a clinical pharmacist workforce into newly forming primary care networks: A qualitatively driven, complex systems analysis. BMJ Open 2022, 12, e066025. [Google Scholar] [CrossRef]
- Weir, K.R.; Bonner, C.; McCaffery, K.; Naganathan, V.; Carter, S.M.; Rigby, D.; Trevena, L.; McLachlan, A.; Jansen, J. Pharmacists and patients sharing decisions about medicines: Development and feasibility of a conversation guide. Res. Soc. Adm. Pharm. 2019, 15, 682–690. [Google Scholar] [CrossRef] [Green Version]


| Gender | Male N (%) | Female N (%) | p-Value |
| 50 (52.08) | 46 (47.92) | p = 0.683 | |
| Age Group (Years) | Male N (%) | Female N (%) | p-Value |
| 15–24 | 4 (8) | 0 (0) | --------- |
| 25–34 | 6 (12) | 4 (8.7) | p = 0.527 |
| 35–44 | 4 (8) | 4 (8.7) | p = 1.000 |
| 45–54 | 16 (32) | 6 (13.04) | p = 0.033 |
| 55–64 | 10 (20) | 22 (47.83) | p = 0.034 |
| 65–74 | 2 (4) | 8 (17.39) | p = 0.058 |
| 75–84 | 2 (4) | 2 (4.35) | p = 1.000 |
| 85–94 | 6 (12) | 0 (0) | --------- |
| Total | 50 (52.08) | 46 (47.92) | |
| Smoking Habits | Male N (%) | Female N (%) | p-Value |
| 88 (91.6) | 8 (8.4) | p = 0.000 | |
| Type of Diseases | Male N (%) | Female N (%) | p-Value |
| AF | 4 (8) | 0 (0) | --------- |
| HTN | 4 (8) | 4 (8.7) | p = 1.000 |
| VD | 28 (56) | 28 (60.87) | p = 1.000 |
| ACS | 4 (8) | 2 (4.35) | p = 0.414 |
| HTN + Diabetes | 10 (20) | 12 (26.09) | p = 0.670 |
| Total | 50 (100) | 46 (100) |
| Indications | N (%) | p-Value |
|---|---|---|
| AVR–Mechanical | 4 (4.17) | p = 0.000 |
| Atrial fibrillation | 8 (8.33) | |
| Dual AVR and MVR | 16 (16.67) | |
| Intracardiac thrombus | 12 (12.5) | |
| MVR–Bioprosthetic | 18 (18.75) | |
| MVR–Mechanical | 32 (33.33) | |
| PE and Stroke | 2 (2.02) | |
| Stroke | 2 (2.02) | |
| Valvular Disease | 2 (2.02) | |
| Total | 96 (100) |
| Type of Clinical Pharmacist’s Intervention | 1st Visit | 2nd Visit | 3rd Visit | 4th Visit | 5th Visit | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | ||
| Medication reconciliation (Yes) | 96 | 100 | 96 | 100 | 96 | 100 | 96 | 100 | 96 | 100 | |
| Medication discontinuation (Yes) | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Hold the medication (Yes) | 4 | 4.2 | 2 | 2.1 | 0 | 0.0 | 0 | 0.0 | 2 | 2.1 | |
| Drug addition (Yes) | 0 | 0.0 | 0 | 0.0 | 2 | 2.1 | 0 | 0.0 | 0 | 0.0 | |
| Drug change (Yes) | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
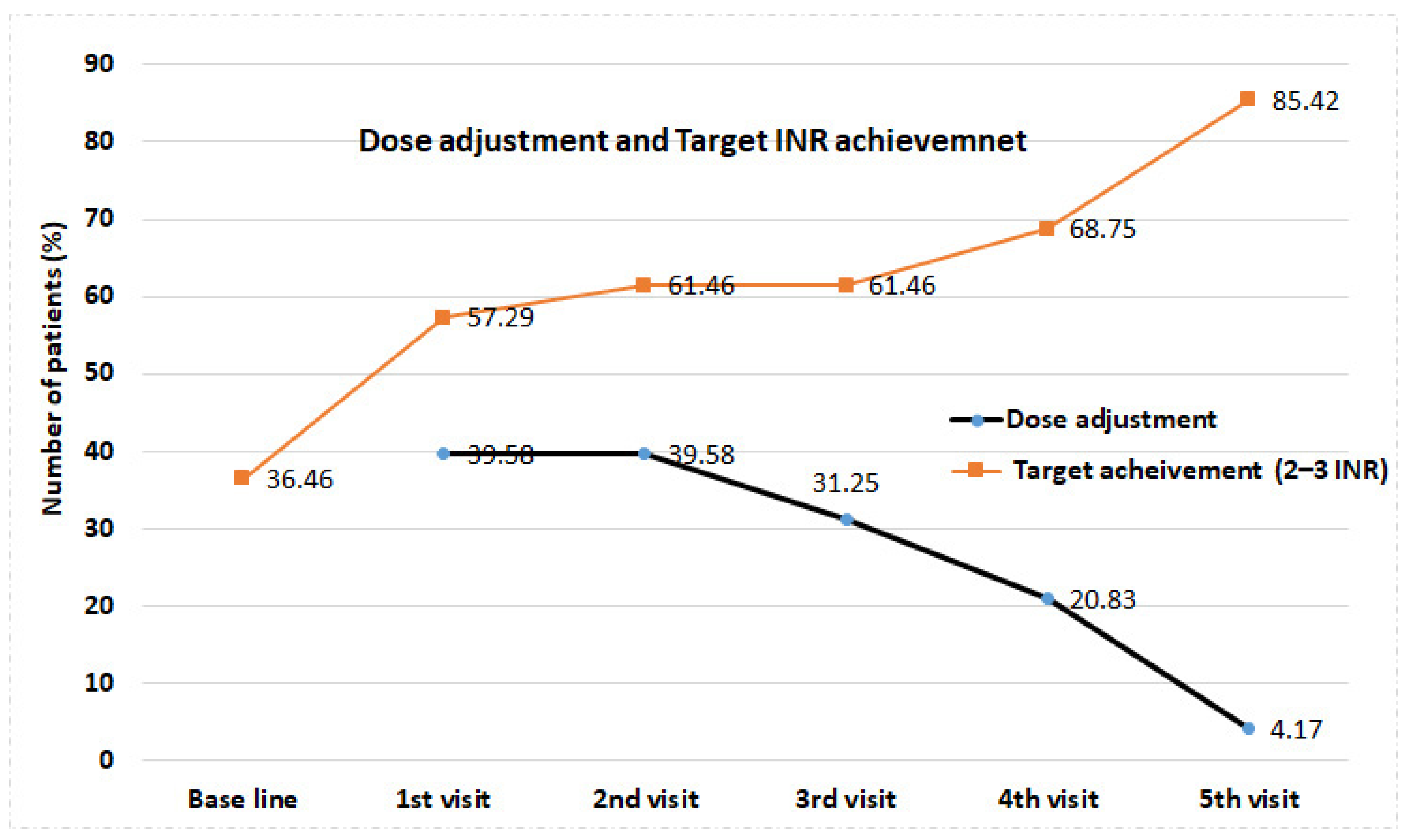
| Dose adjustment (Yes) | 38 | 39.6 | 38 | 39.6 | 30 | 31.2 | 20 | 20.8 | 4 | 4.2 | p = 0.000 |
| ADR reporting (Yes) | 4 | 4.2 | 2 | 2.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Patient counseling (Yes) | 96 | 100 | 96 | 100 | 96 | 100 | 96 | 100 | 96 | 100 | |
| Physicians’ recommendation (Yes) | 50 | 52.1 | 50 | 52.1 | 50 | 52.1 | 20 | 20.8 | 14 | 14.6 | |
| Change in Weekly Dose | |||||||
|---|---|---|---|---|---|---|---|
| Drug | Baseline | 1st Visit | 2nd Visit | 3rd Visit | 4th Visit | 5th Visit | p-Value |
| Weekly Dose (mg) (Mean ± SD) | 28.99 ± 11.81 | 27.74 ± 12.28 | 28.33 ± 11.45 | 29.48 ± 12.05 | 30.70 ± 12.58 | 31.60 ± 13.35 * | 0.001 |
| Dose Range (Min–Max) | 52.50 (3.50–56) | 47 (8–55) | 47 (9–56) | 43 (10–56) | 43 (12–55) | 42.5 (12–54.5) | 0.001 |
| Change in Weekly INR | |||||||
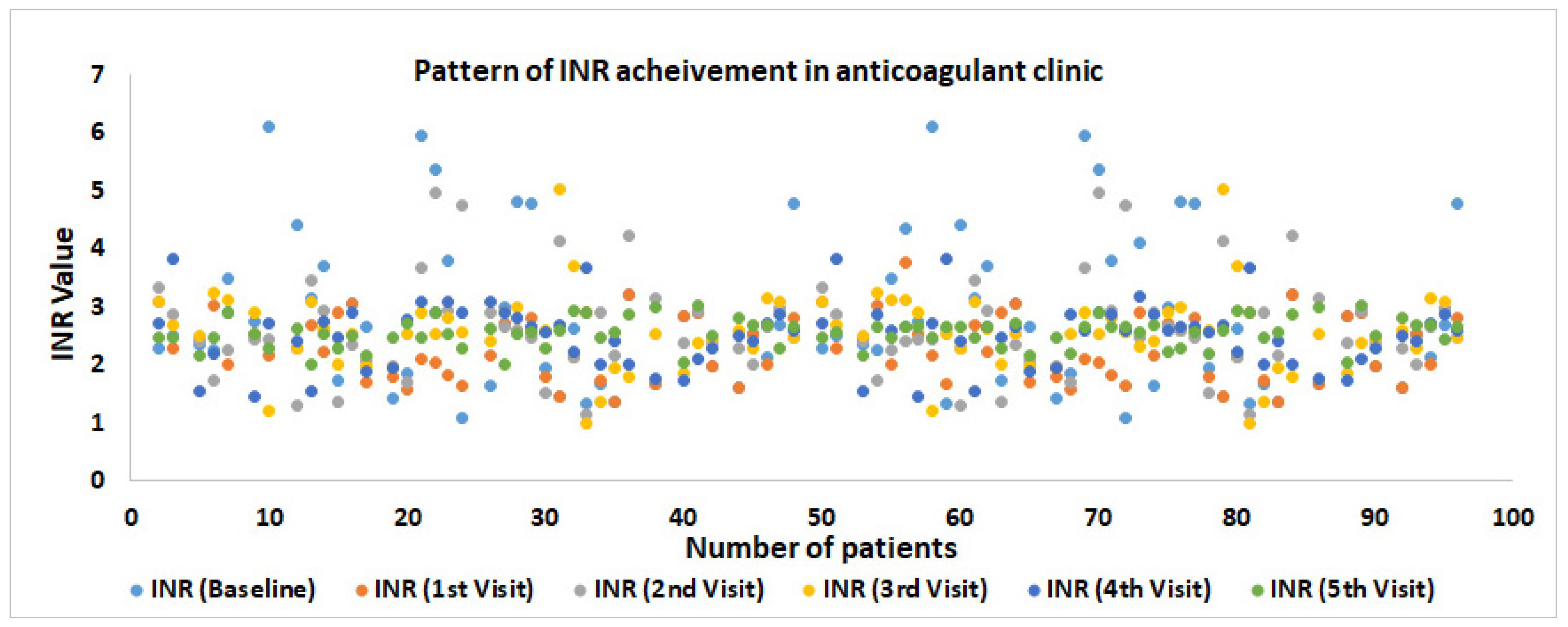
| INR Value | Baseline | 1st Visit | 2nd Visit | 3rd Visit | 4th Visit | 5th Visit | p-Value |
| INR (Mean ± SD) | 2.87 ± 1.31 | 2.5 ± 0.95 * | 2.63 ± 0.82 * | 2.62 ± 0.79 * | 2.58 ± 0.58 * | 2.63 ± 0.47 * | 0.001 |
| INR Range (Min–Max) | 5.02 (1.10–6.12) | 5.32 (1.38–6.70) | 3.8 (1.1604.96) | 4.55 (1.01–5.56) | 2.64 (1.47–3.91) | 0.4 (1.64–4.26) | 0.001 |
| Number of Patients | ||||||
|---|---|---|---|---|---|---|
| Adverse Drug Reactions | Baseline N (%) | 1st Visit N (%) | 2nd Visit N (%) | 3rd Visit N (%) | 4th Visit N (%) | 5th Visit N (%) |
| Nose bleeding | 6 (6.3) | 4 (4.2) | 2 (2.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Reasons of Not Achieving Target INR | Baseline N (%) | 1st Visit N (%) | 2nd Visit N (%) | 3rd Visit N (%) | 4th Visit N (%) | 5th Visit N (%) |
| Non-compliance | 18 (18.8) | 24 (25) | 16 (16.7) | 8 (8.3) | 4 (4.2) | 2 (2.1) |
| Dose hypersensitivity | 2 (2.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (2.1) | 0 (0.0) |
| Sub-therapeutic dose | 2 (2.1) | 0 (0.0) | 0 (0.0) | 2 (2.1) | 0 (0.0) | 0 (0.0) |
| No follow-up | 16 (16.7) | 6 (6.3) | 6 (6.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Drug–Drug interaction | 0 (0.0) | 0 (0.0) | 4 (4.2) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Unknown reason | 40 (41.7) | 12 (12.5) | 12 (12.5) | 18 (18.8) | 2 (2.1) | 0 (0.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshaiban, A.; Alavudeen, S.S.; Alshahrani, I.; Kardam, A.M.; Alhasan, I.M.; Alasiri, S.A.; Imam, M.T.; Almalki, Z.S.; Akhtar, M.S. Impact of Clinical Pharmacist Running Anticoagulation Clinic in Saudi Arabia. J. Clin. Med. 2023, 12, 3887. https://doi.org/10.3390/jcm12123887
Alshaiban A, Alavudeen SS, Alshahrani I, Kardam AM, Alhasan IM, Alasiri SA, Imam MT, Almalki ZS, Akhtar MS. Impact of Clinical Pharmacist Running Anticoagulation Clinic in Saudi Arabia. Journal of Clinical Medicine. 2023; 12(12):3887. https://doi.org/10.3390/jcm12123887
Chicago/Turabian StyleAlshaiban, Abdulrahman, Sirajudeen S. Alavudeen, Ibrahim Alshahrani, Abdulaziz M. Kardam, Ibrahim Mohammed Alhasan, Saleh Abdulrahman Alasiri, Mohammad Tarique Imam, Ziyad Saeed Almalki, and Md Sayeed Akhtar. 2023. "Impact of Clinical Pharmacist Running Anticoagulation Clinic in Saudi Arabia" Journal of Clinical Medicine 12, no. 12: 3887. https://doi.org/10.3390/jcm12123887
APA StyleAlshaiban, A., Alavudeen, S. S., Alshahrani, I., Kardam, A. M., Alhasan, I. M., Alasiri, S. A., Imam, M. T., Almalki, Z. S., & Akhtar, M. S. (2023). Impact of Clinical Pharmacist Running Anticoagulation Clinic in Saudi Arabia. Journal of Clinical Medicine, 12(12), 3887. https://doi.org/10.3390/jcm12123887






