Minimally Invasive Staging of Early-Stage Epithelial Ovarian Cancer versus Open Surgery in Terms of Feasibility and Safety: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Subsection
2.2. Search Method
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
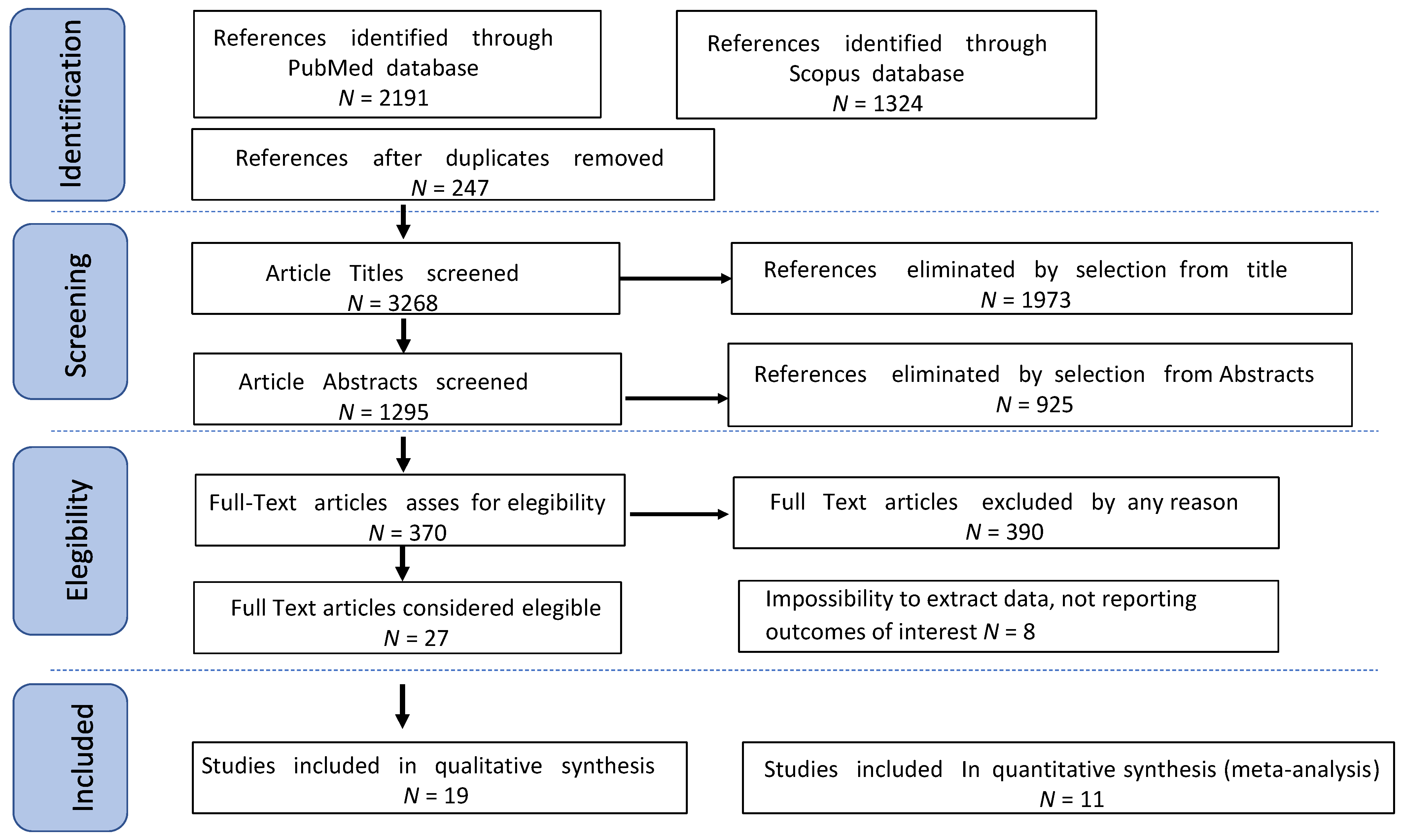
3. Results
3.1. Studies’ Characteristics
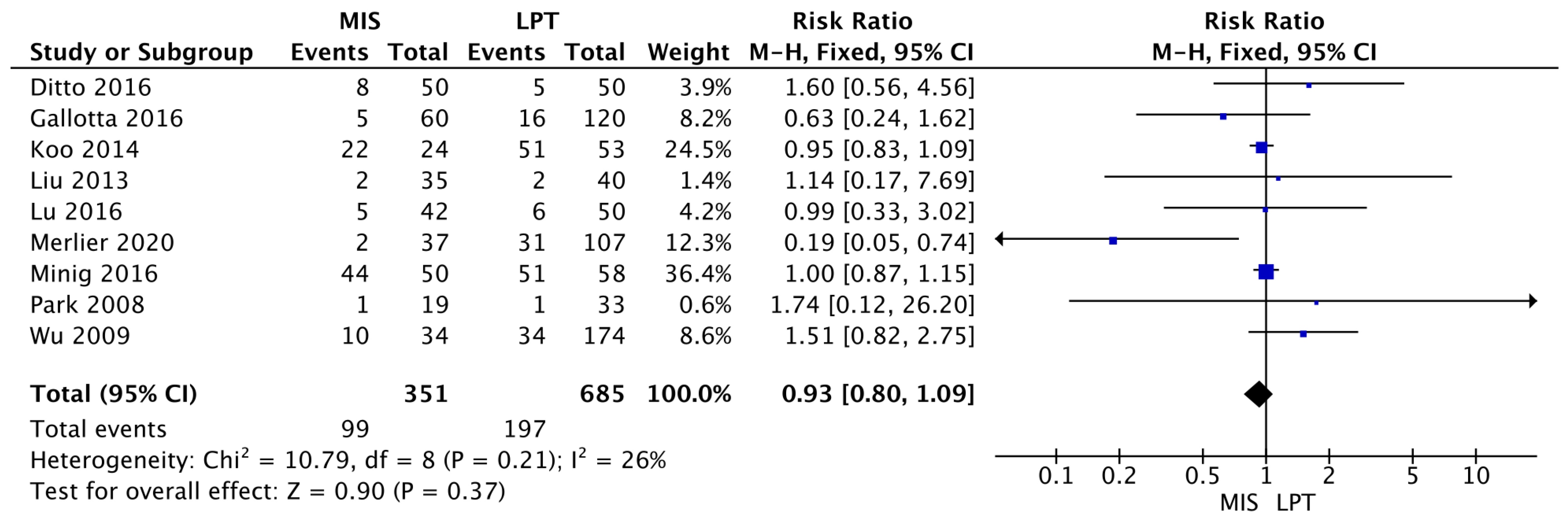
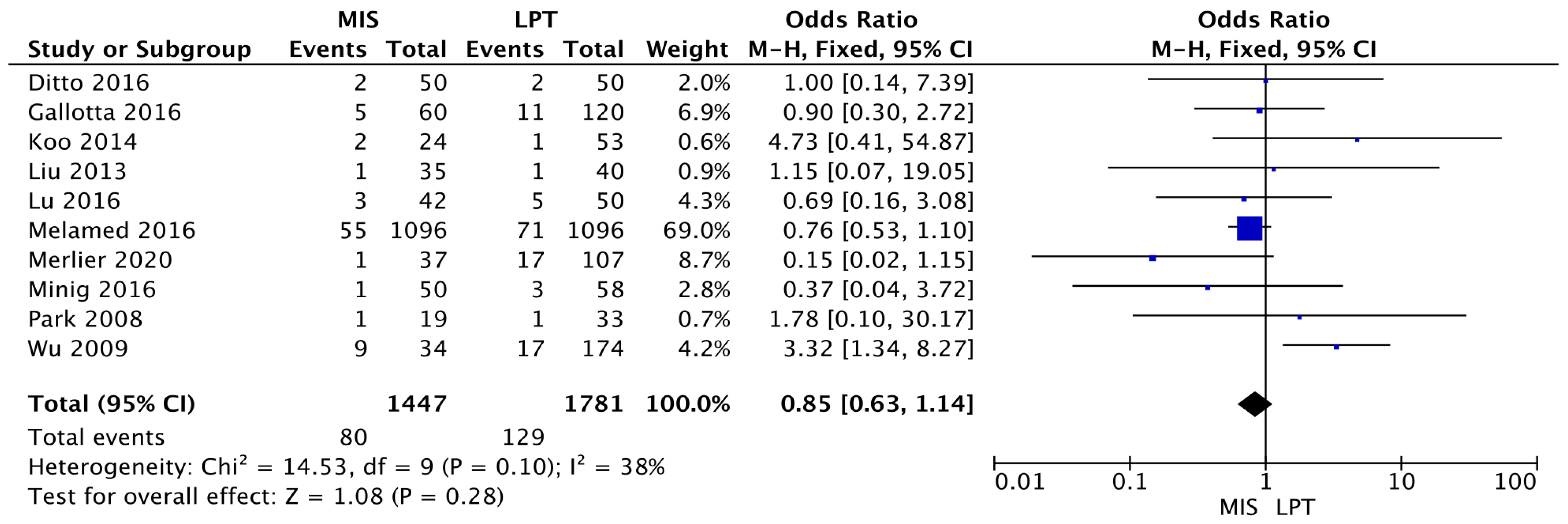
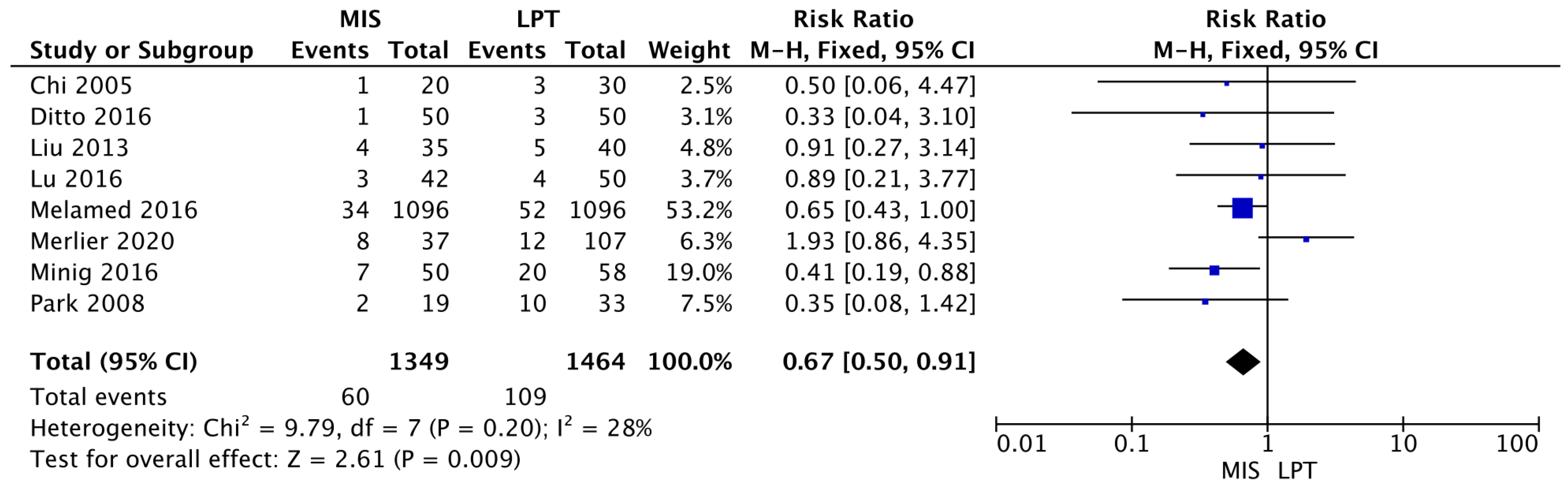
3.2. Outcomes
| Name | 3Y DFS * (%) | 3Y OS ° (%) | 5Y DFS * (%) | 5Y OS ° (%) |
|---|---|---|---|---|
| Colomer 2008 [8] | 95 | 100 | / | / |
| Ditto 2016 [28] | 90 | 95 | 83 | 95 |
| Facer 2019 [21] | / | / | / | 86.4 |
| Gallotta 2016 [29] | 89 | 92 | / | / |
| Gallotta 2021 [22] | / | / | 83 | 93.8 |
| Ghezzi 2011 [23] | 91.2 | 97 | / | / |
| Koo 2014 [30] | 86.1 | / | 86.1 | / |
| Lee 2017 [24] | / | 95 | / | 95 |
| Liu 2013 [31] | / | 97.1 | / | / |
| Lu 2016 [32] | / | 100 | 91.3 | 92.9 |
| Melamed 2016 [33] | / | 94.1 | / | / |
| Merlier 2020 [34] | 93 | 97.3 | 88 | 97.3 |
| Minig 2016 [35] | 87 | 98 | 84 | 98 |
| Muzii 2008 [25] | 95.6 | 100 | / | / |
| Nezhat 2008 [10] | 91.6 | 100 | / | / |
| Park 2018 [11] | 100 | 100 | / | / |
| Tozzi 2003 [26] | 91.6 | 100 | / | / |
| Wu 2009 [36] | / | / | 69.5 | 67.4 |
| Name | Group MIS Recurrence Rate (%) | Group OSS Recurrence Rate (%) | p |
|---|---|---|---|
| Colomer 2018 [8] | 5 | NA | |
| Gallotta 2016 [29] | 8.3 | 16.3 | 0.651 |
| Gallotta 2021 [22] | 15.3 | NA | |
| Ghezzi 2011 [23] | 7.3 | NA | |
| Koo 2014 [30] | 8.3 | 3.8 | 0.585 |
| Lee 2017 [24] | 8.3 | NA | |
| Liu 2013 [31] | 5.7 | 5.0 | >0.05 |
| Lu 2016 [32] | 13 | 13 | |
| Merlier 2020 [34] | 5.4 | 29 | 0.08 |
| Minig 2016 [35] | 12 | 12 | 0.785 |
| Muzii 2008 [25] | 4.3 | NA | |
| Nezhat 2008 [10] | 8.3 | NA | |
| Park 2018 [11] | 0 | 0 | |
| Tozzi 2003 [26] | 8.3 | NA |
| Name | Group MIS% | Group OSS% | p |
|---|---|---|---|
| Colomer 2018 [8] | 20 | / | / |
| Ditto 2016 [28] | 20 | 26 | 0.63 |
| Facer 2019 [21] | 11.3 | / | / |
| Gallotta 2021 [22] | 18.1 | / | / |
| Ghezzi 2011 [23] | 25.6 | / | / |
| Liu 2013 [31] | 17.1 | 22.5 | NA |
| Lu 2016 [32] | 21.4 | 20.0 | 0.86 |
| Melamed 2016 [33] | 12.2 | 19.2 | <0.001 |
| Minig 2016 [35] | 24 | 14 | 0.173 |
| Muzii 2008 [25] | 26 | / | / |
| Nezhat 2008 [10] | 19.4 | / | / |
| Park 2018 [11] | 21.1 | 21.2 | 0.989 |
3.3. Meta-Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Single Arm Studies | ||||||
|---|---|---|---|---|---|---|
| Name | Country | Study Design | Selection (Max 5) | Comparability (Max 1) | Exposure (Max 2) | Tot |
| Colomer 2008 [8] | Spain | Prospective Monocentric Study | 5 | 0 | 1 | 6 |
| Facer 2019 [21] | USA | Retrospective Cohort Multicenter Study | 3 | 1 | 1 | 5 |
| Gallotta 2021 [22] | Italy | Retrospective Observational Multicenter Study | 4 | 1 | 2 | 7 |
| Ghezzi 2011 [23] | Italy | Prospective monocentrc Study | 4 | 1 | 2 | 7 |
| Lee 2017 [24] | Taiwan | Retrospective Observational Monocentric study | 4 | 1 | 1 | 6 |
| Muzii 2008 [25] | Italy | Prospective Observational Study | 3 | 1 | 2 | 6 |
| Nezhat 2008 [10] | USA | Retrospective Observational Monocentric study | 3 | 1 | 1 | 5 |
| Tozzi 2003 [26] | Germany | Prospective Observational Study | 4 | 1 | 1 | 6 |
| Comparative Studies, Included for meta-analysis | ||||||
| Chi 2005 [27] | USA | Retrospective Case-Control Monocentric Study | 3 | 0 | 1 | 4 |
| Ditto 2016 [28] | Italy | Retrospective Case-Control Multicentric study | 4 | 1 | 1 | 6 |
| Gallotta 2016 [29] | Italy | Retrospective Case-Control Multicentric study | 5 | 1 | 1 | 7 |
| Koo 2014 [30] | Korea | Retrospective Case-Control Monocentric study | 3 | 1 | 1 | 5 |
| Liu 2013 [31] | China | Retrospective Case-Control Monocentric study | 4 | 1 | 2 | 7 |
| Lu 2016 [32] | Chin | Retrospective Case-Control Monocentric study | 4 | 1 | 1 | 6 |
| Melamed 2016 [33] | USA | Retrospective Observational Multicentric study | 4 | 1 | 1 | 6 |
| Merlier 2020 [34] | France | Retrospective Case-Control Multicentric study | 5 | 1 | 2 | 8 |
| Minig 2016 [35] | Spain | retrospective comparative observational study | 5 | 1 | 2 | 7 |
| Park 2018 [11] | USA | Retrospective Case-Control Monocentric study | 4 | 1 | 1 | 6 |
| Wu 2009 [36] | Taiwan | Retrospective Case-Control Monocentric study | 4 | 1 | 2 | 7 |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 2015, 65, 5–29. [Google Scholar] [CrossRef]
- Petersson, F. Annual report on the results of treatment in gynaecological cancer. Int. J. Gynaecol. Obs. 1991, 21, 238–277. [Google Scholar]
- Oncology Committee of the International Federation of Gynecology and Obstetrics. Changes in definitions of clinical staging for carcinoma of the cervix and ovary: International Federation of Gynaecology and Obstetrics. Am. J. Obs. Gynecol. 1987, 156, 263–264. [Google Scholar]
- Schorge, J.O.; Eisenhauer, E.E.; Chi, D.S. Current surgical management of ovarian cancer. Hematol./Oncol. Clin. N. Am. 2012, 26, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Zanetta, G.; Rota, S.; Chiari, S.; Bonazzi, C.; Bratina, G.; Torri, V.; Mangioni, C. The accuracy of staging: An important prognostic determinator in stage I ovarian carcinoma. A multivariate analysis. Ann. Oncol. 1998, 9, 1097–1101. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Chang, W.H.; Chiu, L.H.; Chiu, Y.H.; Wang, I.D.; Yen, Y.K.; Liu, W.M. Surgical advantages of laparoscopic pelvic and para-aortic lymph node dissection using the thermal welding instrument compared with conventional laparotomy for lymph node dissection. Gynecol. Minim. Invasive Ther. 2013, 2, 132–134. [Google Scholar] [CrossRef]
- Kotani, Y.; Umemoto, M.; Tobiume, T.; Shiota, M. Ovarian tumor cases that were preoperatively diagnosed as benign but postoperatively confirmed as borderline or malignant after laparoscopic surgery. Gynecol. Minim. Invasive Ther. 2013, 2, 122–125. [Google Scholar] [CrossRef]
- Colomer, A.T.; Jiménez, A.M.; Barceló, M.I. Laparoscopic treatment and staging of early ovarian cancer. J. Minim. Invasive Gynecol. 2008, 15, 414–419. [Google Scholar] [CrossRef]
- Pomel, C.; Provencher, D.; Dauplat, J.; Gauthier, P.; Le Bouedec, G.; Drouin, P.; Audet-Lapointe, P.; Dubuc-Lissoir, J. Laparoscopy staging of early ovarian cancer. Int. J. Gynecol. Cancer 2009, 19, S7–S13. [Google Scholar]
- Nezhat, F.R.; Ezzati, M.; Chuang, L.; Shamshirsaz, A.A.; Rahaman, J.; Gretz, H. Laparoscopic management of early ovarian and fallopian tube cancers: Surgical and survival outcome. Am. J. Obstet. Gynecol. 2009, 200, e1–e6. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, D.Y.; Suh, D.S.; Kim, J.H.; Kim, Y.M.; Kim, Y.T.; Nam, J.H. Comparison of laparoscopy and laparotomy in surgical staging of early-stage ovarian and fallopian tubal cancer. Ann. Surg. Oncol. 2008, 15, 2012–2019. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, J.J.; Kim, D.Y.; Kim, J.H.; Kim, Y.M.; Kim, Y.T.; Nam, J.H. Laparoscopic management of early stage epithelial ovarian cancer. J. Minim. Invasive Gynecol. 2010, 17, S90. [Google Scholar] [CrossRef]
- Gad, M.S.; El Khouly, N.I.; Soto, E.; Brodman, M.; Chuang, L.; Nezhat, F.R.; Gretz, H.F. Differences in perioperative outcomes of laparoscopic management of benign and malignant adnexal masses. Gynecol. Oncol. 2011, 22, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, F.; Cromi, A.; Uccella, S.; Bergamini, V.; Tomera, S.; Franchi, M.; Bolis, P. Laparoscopy versus laparotomy for the surgical management of apparent early stage ovarian cancer. Gynecol. Oncol. 2007, 105, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kim, S.W.; Paek, J.; Lee, S.H.; Yim, G.W.; Kim, J.H.; Kim, J.W.; Kim, Y.T.; Nam, E.J. Comparisons of surgical outcomes, complications, and costs between laparotomy and laparoscopy in early-stage ovarian cancer. Int. J. Gynecol. Cancer 2011, 21, 251–256. [Google Scholar] [CrossRef]
- Muzii, L.; Angioli, R.; Zullo, M.; Panici, P.B. The unexpected ovarian malignancy found during operative laparoscopy: Incidence, management, and implications for prognosis. J. Minim. Invasive Gynecol. 2005, 12, 81–89. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal 8 with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Kansagara, D.; O’Neil, M.; Nugent, S.; Freeman, M.; Low, A.; Kondo, K.; Elven, C.; Zakher, B.; Motu’apuaka, M.; Paynter, R. Quality Assessment Criteria for Observational Studies, Based on the Newcastle-Ottawa Scale. 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK476448/table/appc.t4 (accessed on 27 March 2022).
- Chaimani, A.; Higgins, J.P.T.; Mavridis, D.; Spyridonos, P.; Salanti, G. Graphical Tools for Network Meta-Analysis in STATA. PLoS ONE 2013, 8, e76654. [Google Scholar] [CrossRef]
- Facer, B.; Wang, F.; Grijalva, C.G.; Alvarez, R.D.; Shu, X.-O. Survival outcomes for robotic-assisted laparoscopy versus traditional laparoscopy in clinical stage I epithelial ovarian cancer. Am. J. Obstet. Gynecol. 2020, 222, 474.e1–474.e12. [Google Scholar] [CrossRef]
- Gallotta, V.; Jeong, S.Y.; Conte, C.; Trozzi, R.; Cappuccio, S.; Moroni, R.; Ferrandina, G.; Scambia, G.; Kim, T.-J.; Fagotti, A. Minimally invasive surgical staging for early stage ovarian cancer: A long-term follow up. Eur. J. Surg. Oncol. 2021, 47, 1698–1704. [Google Scholar] [CrossRef] [PubMed]
- Ghezzi, F.; Malzoni, M.; Vizza, E.; Cromi, A.; Perone, C.; Corrado, G.; Uccella, S.; Cosentino, F.; Mancini, E.; Franchi, M. Laparoscopic Staging of Early Ovarian Cancer: Results of a Multi-Institutional Cohort Study. Ann. Surg. Oncol. 2011, 19, 1589–1594. [Google Scholar] [CrossRef]
- Lee, C.-L.; Kusunoki, S.; Huang, C.-Y.; Wu, K.-Y.; Lee, P.-S.; Huang, K.-G. Surgical and survival outcomes of laparoscopic staging surgery for patients with stage I ovarian cancer. Taiwan. J. Obstet. Gynecol. 2018, 57, 7–12. [Google Scholar] [CrossRef]
- Muzii, L.; Palaia, I.; Sansone, M.; Calcagno, M.; Plotti, F.; Angioli, R.; Panici, P.B. Laparoscopic fertility-sparing staging in unexpected early stage ovarian malignancies. Fertil. Steril. 2009, 91, 2632–2637. [Google Scholar] [CrossRef]
- Tozzi, R.; Köhler, C.; Ferrara, A.; Schneider, A. Laparoscopic treatment of early ovarian cancer: Surgical and survival outcomes. Gynecol. Oncol. 2004, 93, 199–203. [Google Scholar] [CrossRef]
- Chi, D.S.; Abu-Rustum, N.R.; Sonoda, Y.; Ivy, J.; Rhee, E.; Moore, K.; Levine, D.A.; Barakat, R.R. The safety and efficacy of laparoscopic surgical staging of apparent stage I ovarian and fallopian tube cancers. Am. J. Obstet. Gynecol. 2005, 192, 1614–1619. [Google Scholar] [CrossRef]
- Ditto, A.; Bogani, G.; Martinelli, F.; Signorelli, M.; Chiappa, V.; Scaffa, C.; Indini, A.; Maggiore, U.L.R.; Lorusso, D.; Raspagliesi, F. Minimally Invasive Surgical Staging for Ovarian Carcinoma: A Propensity-Matched Comparison with Traditional Open Surgery. J. Minim. Invasive Gynecol. 2017, 24, 98–102. [Google Scholar] [CrossRef]
- Gallotta, V.; Petrillo, M.; Conte, C.; Vizzielli, G.; Fagotti, A.; Ferrandina, G.; Fanfani, F.; Costantini, B.; Carbone, V.; Scambia, G. Laparoscopic Versus Laparotomic Surgical Staging for Early-Stage Ovarian Cancer: A Case-Control Study. J. Minim. Invasive Gynecol. 2016, 23, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.-J.; Kim, J.-E.; Kim, Y.-H.; Hahn, H.-S.; Lee, I.-H.; Kim, T.-J.; Lee, K.-H.; Shim, J.-U.; Lim, K.-T. Comparison of laparoscopy and laparotomy for the management of early-stage ovarian cancer: Surgical and oncological outcomes. J. Gynecol. Oncol. 2014, 25, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, L.; He, Y.; Peng, D.; Wang, X.; Chen, W.; Fu, X.; Ma, Y. Comparison of Laparoscopy and Laparotomy in the Surgical Management of Early-Stage Ovarian Cancer. Int. J. Gynecol. Cancer 2014, 24, 352–357. [Google Scholar] [CrossRef]
- Lu, Q.; Qu, H.; Liu, C.; Wang, S.; Zhang, Z.; Zhang, Z. Comparison of Laparoscopy and Laparotomy in Surgical Staging of Apparent Early Ovarian Cancer. Medicine 2016, 95, e3655. [Google Scholar] [CrossRef] [PubMed]
- Melamed, A.; Keating, N.L.; Clemmer, J.T.; Bregar, A.J.; Wright, J.D.; Boruta, D.M.; Schorge, J.O.; del Carmen, M.G.; Rauh-Hain, J.A. Laparoscopic staging for apparent stage I epithelial ovarian cancer. Am. J. Obstet. Gynecol. 2017, 216, 50.e1–50.e12. [Google Scholar] [CrossRef] [PubMed]
- Merlier, M.; Kerbage, Y.; Pierache, A.; Ramdane, N.; Canlorbe, G.; Bolze, P.-A.; Ballester, M.; Bendifallah, S.; Ouldamer, L.; Touboul, C.; et al. Impact on Prognosis of the Surgical Route, Laparoscopy or Laparotomy, for the Surgical Staging of Early Stage Ovarian Cancer—A Study from the FRANCOGYN Group. J. Clin. Med. 2020, 9, 3528. [Google Scholar] [CrossRef]
- Minig, L.; Saadi, J.; Patrono, M.G.; Giavedoni, M.E.; Cárdenas-Rebollo, J.M.; Perrotta, M. Laparoscopic surgical staging in women with early stage epithelial ovarian cancer performed by recently certified gynecologic oncologists. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 201, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.I.; Lee, C.L.; Liao, P.J.; Huang, K.G.; Chang, T.C.; Chou, H.H.; Wang, C.J.; Soong, Y.K.; Hsueh, S.; Lai, C.H. Survival impact of initial surgical approach in stage I ovarian cancer. Chang Gung Med. J. 2010, 33, 558–567. [Google Scholar]
- Hiett, A.K.; Sonek, J.D.; Guy, M.; Reid, T.J. Performance of IOTA Simple Rules, Simple Rules risk assessment, ADNEX model and O-RADS in differentiating between benign and malignant adnexal lesions in North American women. Ultrasound Obstet. Gynecol. 2022, 59, 668–676. [Google Scholar] [CrossRef]
- Lucidi, A.; Buca, D.; Ronsini, C.; Tinari, S.; Bologna, G.; Buca, D.; Leombroni, M.; Liberati, M.; D’Antonio, F.; Scambia, G.; et al. Role of Extracellular Vesicles in Epithelial Ovarian Cancer: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 8762. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, M.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef]
- Ronsini, C.; Köhler, C.; De Franciscis, P.; La Verde, M.; Mosca, L.; Solazzo, M.C.; Colacurci, N. Laparo-assisted vaginal radical hysterectomy as a safe option for Minimal Invasive Surgery in early stage cervical cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2022, 166, 188–195. [Google Scholar] [CrossRef]
- Volz, J.; Köster, S.; Spacek, Z.; Paweletz, N. The influence of pneumoperitoneum used in laparoscopic surgery on an intraabdominal tumor growth. Cancer 1999, 86, 770–774. [Google Scholar] [CrossRef]
- Meinhold-Heerlein, I.; Fotopoulou, C.; Harter, P.; Kurzeder, C.; Mustea, A.; Wimberger, P.; Hauptmann, S.; Sehouli, J. The new WHO classification of ovarian, fallopian tube, and primary peritoneal cancer and its clinical implications. Arch. Gynecol. Obstet. 2016, 293, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Trimbos, J.B. Staging of early ovarian cancer and the impact of lymph node sampling. Int. J. Gynecol. Cancer 2000, 10, 8–11. [Google Scholar] [CrossRef]
- Harter, P.; Mouret-Reynier, M.A.; Pignata, S.; Cropet, C.; González-Martín, A.; Bogner, G.; Fujiwara, K.; Vergote, I.; Colombo, N.; Nøttrup, T.J.; et al. Efficacy of maintenance olaparib plus bevacizumab according to clinical risk in patients with newly diagnosed, advanced ovarian cancer in the phase III PAOLA-1/ENGOT-ov25 trial. Gynecol. Oncol. 2022, 164, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.R.; Coleman, R.L.; González-Martín, A.; Moore, K.N.; Colombo, N.; Ray-Coquard, I.; Pignata, S. The forefront of ovarian cancer therapy: Update on PARP inhibitors. Ann. Oncol. 2020, 31, 1148–1159, Correction in Ann. Oncol. 2021, 32, 1066–1067. [Google Scholar] [CrossRef] [PubMed]
- Trimbos, J.B.; Vergote, I.; Bolis, G.; Vermorken, J.B.; Mangioni, C.; Madronal, C.; Franchi, M.; Tateo, S.; Zanetta, G.; Scarfone, G.; et al. Impact of adjuvant chemotherapy and surgical staging in early-stage ovarian carcinoma: European Organisation for Research and Treatment of Cancer-Adjuvant ChemoTherapy in Ovarian Neoplasm trial. J. Natl. Cancer Inst. 2003, 95, 113–125. [Google Scholar] [CrossRef]
- Matsuo, K.; Huang, Y.; Matsuzaki, S.; Klar, M.; Roman, L.D.; Sood, A.K.; Wright, J.D. Minimally invasive surgery and risk of capsule rupture for women with early-stage ovarian cancer. JAMA Oncol. 2020, 6, 1110–1113. [Google Scholar] [CrossRef]
- Ghirardi, V.; De Felice, F.; Rosati, A.; Ergasti, R.; Alletti, S.G.; Mascilini, F.; Scambia, G.; Fagotti, A. A Laparoscopic Adjusted Model Able to Predict the Risk of Intraoperative Capsule Rupture in Early-stage Ovarian Cancer: Laparoscopic Ovarian Cancer Spillage Score (LOChneSS Study). J. Minim. Invasive Gynecol. 2022, 29, 961–967. [Google Scholar] [CrossRef]
- Morice, P.; Camatte, S.; Larregain-Fournier, D.; Thoury, A.; Duvillard, P.; Castaigne, D. Port-site implantation after laparoscopic treatment of borderline ovarian tumors. Obstet. Gynecol. 2004, 104, 1167–1170. [Google Scholar] [CrossRef]
- Liu, C.S.; Nagarsheth, N.P.; Nezhat, F.R. Laparoscopy and Ovarian Cancer: A Paradigm Change in the Management of Ovarian Cancer? J. Minim. Invasive Gynecol. 2009, 16, 250–262. [Google Scholar] [CrossRef]
- Zivanovic, O.; Sonoda, Y.; Diaz, J.P.; Levine, D.A.; Brown, C.L.; Chi, D.S.; Barakat, R.R.; Abu-Rustum, N.R. The rate of port-site metastases after 2251 laparoscopic procedures in women with underlying malignant disease. Gynecol. Oncol. 2008, 111, 431–437. [Google Scholar] [CrossRef]
- Schuurman, T.; Zilver, S.; Samuels, S.; Schats, W.; Amant, F.; van Trommel, N.; Lok, C. Fertility- sparing surgery in epithelial ovarian cancer: A systematic review of onco- logical issues. Ann. Oncol. 2016, 27, 1994–2004. [Google Scholar] [CrossRef]
- Bogani, G.; Borghi, C.; Leone Roberti Maggiore, U.; Ditto, A.; Signorelli, M.; Martinelli, F.; Chiappa, V.; Lopez, C.; Sabatucci, I.; Scaffa, C.; et al. Minimally Invasive Surgical Staging in Early-stage Ovarian. Carcinoma: A Systematic Review and Meta-analysis. J. Minim. Invasive Gynecol. 2017, 24, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Alletti, S.G.; Restaino, S.; Finelli, A.; Ronsini, C.; Lucidi, A.; Scambia, G.; Fanfani, F. Step by Step Total Laparoscopic Hysterectomy with Uterine Arteries Ligation at the Origin. J. Minim. Invasive Gynecol. 2020, 27, 22–23. [Google Scholar] [CrossRef] [PubMed]
- Scambia, G.; Nero, C.; Uccella, S.; Vizza, E.; Ghezzi, F.; Cosentino, F.; Chiantera, V.; Fagotti, A. Sentinel-node biopsy in early stage ovarian cancer: A prospective multicentre study (SELLY). Int. J. Gynecol. Cancer 2019, 29, 1437–1439. [Google Scholar] [CrossRef] [PubMed]
- Restaino, S.; Ronsini, C.; Finelli, A.; Perrone, E.; Scambia, G.; Fanfani, F. Role of blue dye for sentinel lymph node detection in early endometrial cancer. Gynecol. Surg. 2017, 14, 23. [Google Scholar] [CrossRef]
- Kong, Q.; Wei, H.; Zhang, J.; Li, Y.; Wang, Y. Comparison of the survival outcomes of laparoscopy versus laparotomy in treatment of early-stage ovarian cancer: A systematic review and meta-analysis. J. Ovarian Res. 2021, 14, 45. [Google Scholar] [CrossRef]
| Single Arm Studies | ||||||
|---|---|---|---|---|---|---|
| Name | Country | Study Design | Study Year | Population | N of Participant, Total (MIS/OSS) ^ | Mean FUP Months |
| Colomer 2008 [8] | Spain | Prospective Monocentric study | 2003–2007 | Apparent early-stage epithelial ovarian cancer undergoing MIS staging | 19 | 24.7 |
| Facer 2019 [21] | USA | Retrospective Cohort Multicentric Study | 2010–2014 | Apparent early-stage epithelial ovarian cancer undergoing MIS staging | 1901 | 37.6 |
| Gallotta 2021 [22] | Italy | Retrospective Observational Multicentric Study | 2008–2016 | Apparent early-stage epithelial ovarian cancer undergoing MIS staging | 254 | 61 |
| Ghezzi 2011 [23] | Italy | Retrospective Observational Multicentric study | Apparent early-stage epithelial ovarian cancer undergoing MIS staging | 82 | 28.5 | |
| Lee 2017 [24] | Taiwan | Retrospective Observational Monocentric study | 2002–2014 | Apparent early-stage epithelial ovarian cancer undergoing MIS staging | 24 | 31.5 |
| Muzii 2008 [25] | Italy | Prospective Observational Monocentric study | 2003–2013 | Apparent early-stage epithelial ovarian cancer undergoing MIS staging | 27 | 20 |
| Nezhat 2008 [10] | USA | Retrospective Observational Monocentric study | 1995–2007 | Apparent early-stage epithelial ovarian cancer undergoing MIS staging | 36 | 55.9 |
| Tozzi 2003 [26] | Germany | Prospective Observational Monocentric study | 1996–2003 | Apparent early-stage epithelial ovarian cancer undergoing MIS staging | 24 | 46.4 |
| Comparative Studies | ||||||
| Chi 2005 [27] | USA | Retrospective Case-Control Monocentric Study | 2000–2003 | Apparent early-stage epithelial ovarian cancer undergoing MIS vs. OSS staging | 50 (20/30) | 46 |
| Ditto 2016 [28] | Italy | Retrospective Case-Control Multicentric study | 2005–2015 | Apparent early-stage epithelial ovarian cancer undergoing MIS vs. OSS staging | 100 (50/50) | 51.1 |
| Gallotta 2016 [29] | Italy | Retrospective Case-Control Multicentric study | 2000–2013 | Apparent early-stage epithelial ovarian cancer undergoing MIS vs. OSS staging | 180 (60/120) | 38 |
| Koo 2014 [30] | Korea | Retrospective Case-Control Monocentric study | 2006–2012 | Apparent early stage epithelial ovarian cancer undergoing MIS vs. OSS staging | 77 (24/53) | 31 |
| Liu 2013 [31] | China | Retrospective Case-Control Monocentric study | 2002–2010 | Apparent early-stage epithelial ovarian cancer undergoing MIS vs. OSS staging | 75 (35/40) | 84 |
| Lu 2016 [32] | China | Retrospective Case-Control Monocentric study | 2002–2014 | Apparent early-stage epithelial ovarian cancer undergoing MIS vs. OSS staging | 92 (42/50) | 82 |
| Melamed 2016 [33] | USA | Retrospective Observational Multicentric study | 2010–2012 | Apparent early-stage epithelial ovarian cancer undergoing MIS vs. OSS staging | 4798 (1112/3686) | 29.9 |
| Merlier 2020 [34] | France | Retrospective Case-Control Multicentric study | 2000–2018 | Apparent early-stage epithelial ovarian cancer undergoing MIS vs. OSS staging | 144 (37/107) | 36 |
| Minig 2016 [35] | Spain | retrospective comparative observational study | 2006–2014 | Apparent early-stage epithelial ovarian cancer undergoing MIS vs. OSS staging | 108 (50/58) | 30.4 |
| Park 2008 [11] | USA | Retrospective Case-Control Monocentric study | 2004–2007 | Apparent early-stage epithelial ovarian cancer undergoing MIS vs. OSS staging | 52 (19/33) | 17 |
| Wu 2009 [36] | Taiwan | Retrospective Case-Control Monocentric study | 1984–2006 | Apparent early-stage epithelial ovarian cancer undergoing MIS vs. OSS staging | 208 (34/174) | 65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ronsini, C.; Pasanisi, F.; Molitierno, R.; Iavarone, I.; Vastarella, M.G.; De Franciscis, P.; Conte, C. Minimally Invasive Staging of Early-Stage Epithelial Ovarian Cancer versus Open Surgery in Terms of Feasibility and Safety: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 3831. https://doi.org/10.3390/jcm12113831
Ronsini C, Pasanisi F, Molitierno R, Iavarone I, Vastarella MG, De Franciscis P, Conte C. Minimally Invasive Staging of Early-Stage Epithelial Ovarian Cancer versus Open Surgery in Terms of Feasibility and Safety: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(11):3831. https://doi.org/10.3390/jcm12113831
Chicago/Turabian StyleRonsini, Carlo, Francesca Pasanisi, Rossella Molitierno, Irene Iavarone, Maria Giovanna Vastarella, Pasquale De Franciscis, and Carmine Conte. 2023. "Minimally Invasive Staging of Early-Stage Epithelial Ovarian Cancer versus Open Surgery in Terms of Feasibility and Safety: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 11: 3831. https://doi.org/10.3390/jcm12113831
APA StyleRonsini, C., Pasanisi, F., Molitierno, R., Iavarone, I., Vastarella, M. G., De Franciscis, P., & Conte, C. (2023). Minimally Invasive Staging of Early-Stage Epithelial Ovarian Cancer versus Open Surgery in Terms of Feasibility and Safety: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 12(11), 3831. https://doi.org/10.3390/jcm12113831