Impact of a Femoral Fracture on Outcome after Traumatic Brain Injury—A Matched-Pair Analysis of the TraumaRegister DGU®
Abstract
1. Introduction
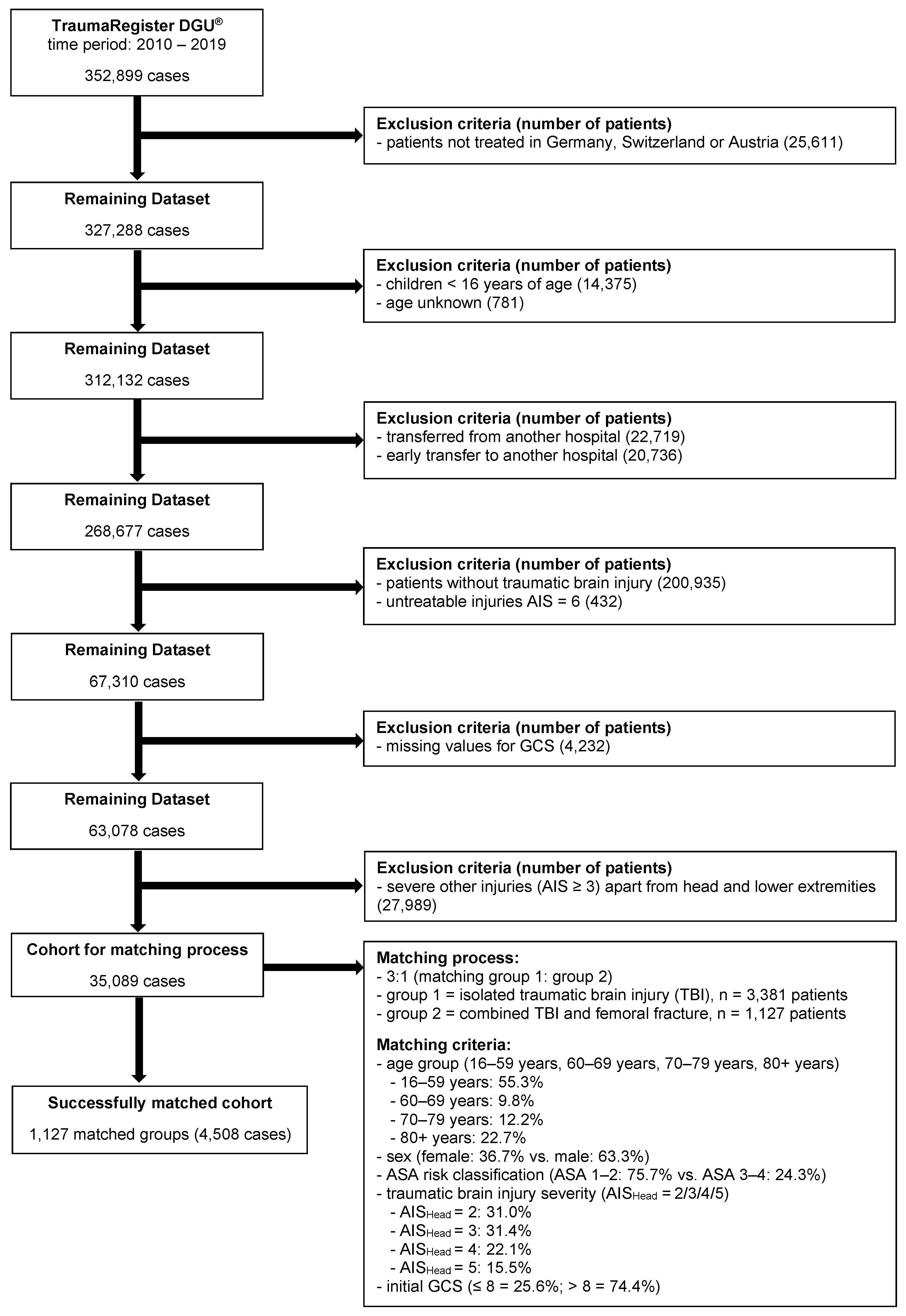
2. Materials and Methods
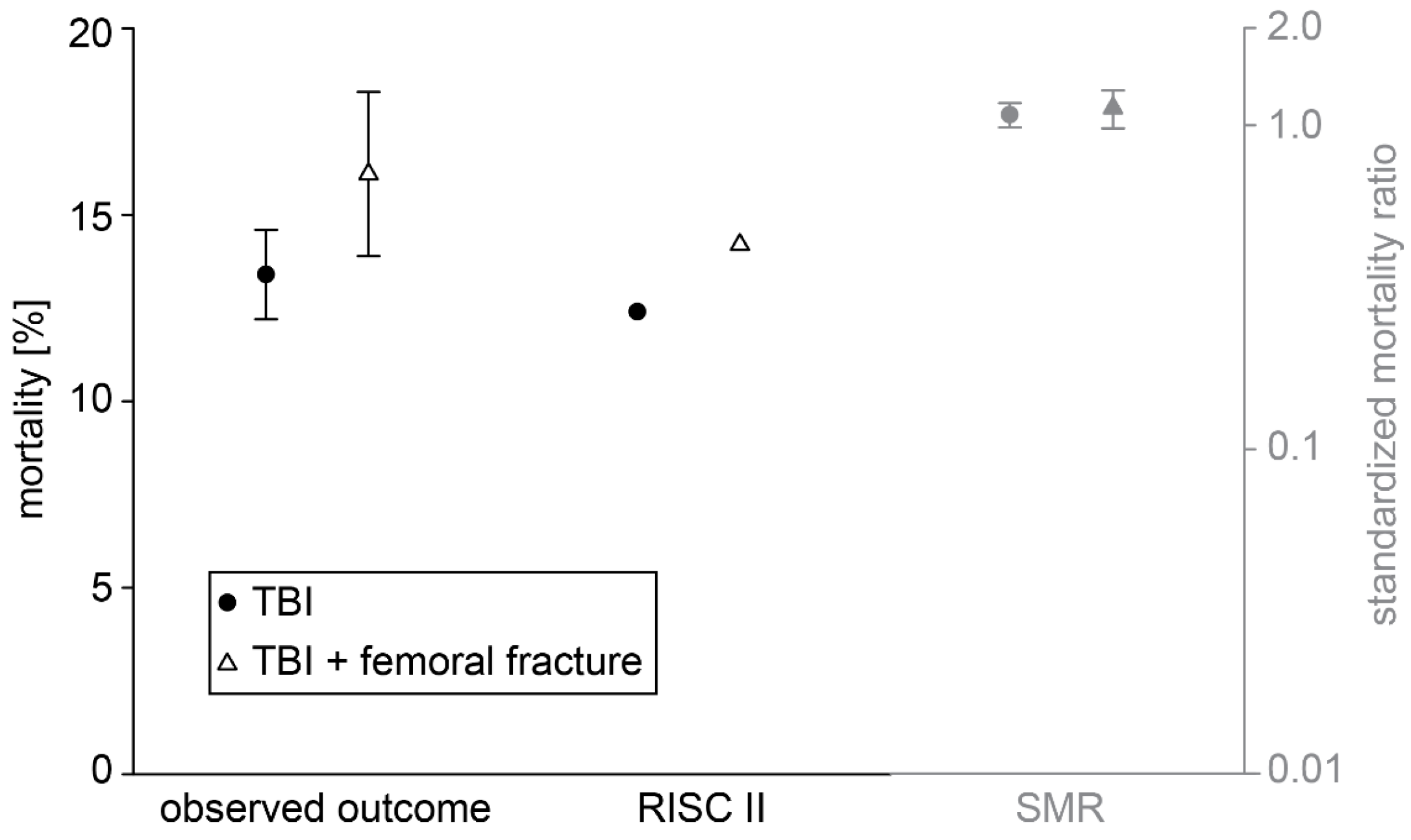
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Menon, D.K.; Schwab, K.; Wright, D.W.; Maas, A.I. Demographics and Clinical Assessment Working Group of the International and Interagency Initiative toward Common Data Elements for Research on Traumatic Brain Injury and Psychological Health. Position statement: Definition of traumatic brain injury. Arch. Phys. Med. Rehabil. 2010, 91, 1637–1640. [Google Scholar] [CrossRef] [PubMed]
- Maas, A.I.; Stocchetti, N.; Bullock, R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008, 7, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Peeters, W.; Brande, R.V.D.; Polinder, S.; Brazinova, A.; Steyerberg, E.W.; Lingsma, H.F.; Maas, A.I.R. Epidemiology of traumatic brain injury in Europe. Acta Neurochir. 2015, 157, 1683–1696. [Google Scholar] [CrossRef] [PubMed]
- Majdan, M.; Plancikova, D.; Brazinova, A.; Rusnak, M.; Nieboer, D.; Feigin, V.L.; Maas, A. Epidemiology of traumatic brain injuries in Europe: A cross-sectional analysis. Lancet Public. Health 2016, 1, e76–e83. [Google Scholar] [CrossRef]
- Bombardier, C.H.; Fann, J.R.; Temkin, N.R.; Esselman, P.C.; Barber, J.; Dikmen, S.S. Rates of major depressive disorder and clinical outcomes following traumatic brain injury. JAMA 2010, 303, 1938–1945. [Google Scholar] [CrossRef] [PubMed]
- Fann, J.R.; Burington, B.; Leonetti, A.; Jaffe, K.; Katon, W.J.; Thompson, R.S. Psychiatric illness following traumatic brain injury in an adult health maintenance organization population. Arch. Gen. Psychiatry 2004, 61, 53–61. [Google Scholar] [CrossRef]
- Gardner, R.C.; Burke, J.F.; Nettiksimmons, J.; Kaup, A.; Barnes, D.E.; Yaffe, K. Dementia risk after traumatic brain injury vs nonbrain trauma: The role of age and severity. JAMA Neurol. 2014, 71, 1490–1497. [Google Scholar] [CrossRef]
- Dikmen, S.S.; Machamer, J.E.; Powell, J.M.; Temkin, N.R. Outcome 3 to 5 years after moderate to severe traumatic brain injury. Arch. Phys. Med. Rehabil. 2003, 84, 1449–1457. [Google Scholar] [CrossRef]
- Gao, X.; Chen, J. Mild traumatic brain injury results in extensive neuronal degeneration in the cerebral cortex. J. Neuropathol. Exp. Neurol. 2011, 70, 183–191. [Google Scholar] [CrossRef]
- Dikranian, K.; Cohen, R.; Mac Donald, C.; Pan, Y.; Brakefield, D.; Bayly, P.; Parsadanian, A. Mild traumatic brain injury to the infant mouse causes robust white matter axonal degeneration which precedes apoptotic death of cortical and thalamic neurons. Exp. Neurol. 2008, 211, 551–560. [Google Scholar] [CrossRef]
- Manley, G.; Gardner, A.J.; Schneider, K.J.; Guskiewicz, K.M.; Bailes, J.; Cantu, R.C.; Castellani, R.J.; Turner, M.; Jordan, B.D.; Randolph, C.; et al. A systematic review of potential long-term effects of sport-related concussion. Br. J. Sports Med. 2017, 51, 969–977. [Google Scholar] [CrossRef]
- Leitgeb, J.; Mauritz, W.; Brazinova, A.; Majdan, M.; Wilbacher, I. Impact of concomitant injuries on outcomes after traumatic brain injury. Arch. Orthop. Trauma Surg. 2013, 133, 659–668. [Google Scholar] [CrossRef]
- Perel, P.; Arango, M.; Clayton, T.; Edwards, P.; Komolafe, E.; Poccock, S.; Roberts, I.; Shakur, H.; Steyerberg, E.; Yutthakasemsunt, S.; et al. Predicting outcome after traumatic brain injury: Practical prognostic models based on large cohort of international patients. BMJ 2008, 336, 425–429. [Google Scholar] [CrossRef]
- van Leeuwen, N.; Lingsma, H.F.; Perel, P.; Lecky, F.; Roozenbeek, B.; Lu, J.; Shakur, H.; Weir, J.; Steyerberg, E.W.; Maas, A.I.R.; et al. Prognostic value of major extracranial injury in traumatic brain injury: An individual patient data meta-analysis in 39,274 patients. Neurosurgery 2012, 70, 811–818, discussion 818. [Google Scholar] [CrossRef]
- Jahresbericht 2021—TraumaRegister DGU®. 2021. Deutsche Gesellschaft für Unfallchirurgie (DGU), Sektion Notfall- & Intensivmedizin und Schwerverletztenversorgung (NIS) der DGU Arbeitskreis TraumaRegister; AUC—Akademie der Unfallchirurgie GmbH. Available online: https://www.traumaregister-dgu.de/infos (accessed on 1 September 2020).
- Schieren, M.; Wappler, F.; Wafaisade, A.; Lefering, R.; Sakka, S.G.; Kaufmann, J.; Heiroth, H.-J.; Defosse, J.; Böhmer, A.B. Impact of blunt chest trauma on outcome after traumatic brain injury- a matched-pair analysis of the TraumaRegister DGU®. Scand. J. Trauma Resusc. Emerg. Med. 2020, 28, 21. [Google Scholar] [CrossRef]
- Mader, M.M.; Lefering, R.; Westphal, M.; Maegele, M.; Czorlich, P. Traumatic brain injury with concomitant injury to the spleen: Characteristics and mortality of a high-risk trauma cohort from the TraumaRegister DGU®. Eur. J. Trauma Emerg. Surg. 2022, 48, 4451–4459. [Google Scholar] [CrossRef]
- Boes, M.; Kain, M.; Kakar, S.; Nicholls, F.; Cullinane, D.; Gerstenfeld, L.; Einhorn, T.A.; Tornetta, P., 3rd. Osteogenic effects of traumatic brain injury on experimental fracture-healing. J. Bone Jt. Surg. Am. 2006, 88, 738–743. [Google Scholar] [CrossRef]
- Hofman, M.; Koopmans, G.; Kobbe, P.; Poeze, M.; Andruszkow, H.; Brink, P.R.G.; Pape, H.-C. Improved fracture healing in patients with concomitant traumatic brain injury: Proven or not? Mediat. Inflamm. 2015, 2015, 204842. [Google Scholar] [CrossRef]
- Beeton, C.A.; Chatfield, D.; Brooks, R.A.; Rushton, N. Circulating levels of interleukin-6 and its soluble receptor in patients with head injury and fracture. J. Bone Jt. Surg. Br. 2004, 86, 912–917. [Google Scholar] [CrossRef]
- Cadosch, D.; Gautschi, O.P.; Thyer, M.; Song, S.; Skirving, A.P.; Filgueira, L.; Zellweger, R. Humoral factors enhance fracture-healing and callus formation in patients with traumatic brain injury. J. Bone Jt. Surg. Am. 2009, 91, 282–288. [Google Scholar] [CrossRef]
- Pape, H.C.; Marcucio, R.; Humphrey, C.; Colnot, C.; Knobe, M.; Harvey, E. Trauma-induced inflammation and fracture healing. J. Orthop. Trauma 2010, 24, 522–525. [Google Scholar] [CrossRef]
- Terrando, N.; Monaco, C.; Ma, D.; Foxwell, B.M.J.; Feldmann, M.; Maze, M. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc. Natl. Acad. Sci. USA 2010, 107, 20518–20522. [Google Scholar] [CrossRef]
- Xia, W.; Xie, J.; Cai, Z.; Liu, X.; Wen, J.; Cui, Z.-K.; Zhao, R.; Zhou, X.; Chen, J.; Mao, X.; et al. Damaged brain accelerates bone healing by releasing small extracellular vesicles that target osteoprogenitors. Nat. Commun. 2021, 12, 604. [Google Scholar] [CrossRef]
- Suto, Y.; Nagata, K.; Ahmed, S.M.; Jacovides, C.; Browne, K.D.; Cognetti, J.; Weber, M.T.; Johnson, V.E.; Leone, R.; Kaplan, L.J.; et al. A concomitant bone fracture delays cognitive recovery from traumatic brain injury. J. Trauma Acute Care Surg. 2018, 85, 275–284. [Google Scholar] [CrossRef]
- Simon, D.W.; Vagni, V.M.; Kochanek, P.M.; Clark, R.S.B. Combined Neurotrauma Models: Experimental Models Combining Traumatic Brain Injury and Secondary Insults. Methods Mol. Biol. 2016, 1462, 393–411. [Google Scholar]
- McDonald, S.J.; Sun, M.; Agoston, D.V.; Shultz, S.R. The effect of concomitant peripheral injury on traumatic brain injury pathobiology and outcome. J. Neuroinflamm. 2016, 13, 90. [Google Scholar] [CrossRef]
- Shultz, S.R.; Sun, M.; Wright, D.K.; Brady, R.D.; Liu, S.; Beynon, S.; Schmidt, S.F.; Kaye, A.H.; Hamilton, J.A.; O’Brien, T.J.; et al. Tibial fracture exacerbates traumatic brain injury outcomes and neuroinflammation in a novel mouse model of multiTrauma. J. Cereb. Blood Flow. Metab. 2015, 35, 1339–1347. [Google Scholar] [CrossRef]
- Dasic, D.; Morgan, L.; Panezai, A.; Syrmos, N.; Ligarotti, G.K.; Zaed, I.; Chibbaro, S.; Khan, T.; Prisco, L.; Ganau, M. A scoping review on the challenges, improvement programs, and relevant output metrics for neurotrauma services in major trauma centers. Surg. Neurol. Int. 2022, 13, 171. [Google Scholar] [CrossRef]
- Pape, H.C.; Grimme, K.; Van Griensven, M.; Sott, A.H.; Giannoudis, P.; Morley, J.; Roise, O.; Ellingsen, E.; Hildebrand, F.; Wiese, B.; et al. Impact of intramedullary instrumentation versus damage control for femoral fractures on immunoinflammatory parameters: Prospective randomized analysis by the EPOFF Study Group. J. Trauma 2003, 55, 7–13. [Google Scholar] [CrossRef]
- Tschoeke, S.K.; Hellmuth, M.; Hostmann, A.; Ertel, W.; Oberholzer, A. The early second hit in trauma management augments the proinflammatory immune response to multiple injuries. J. Trauma 2007, 62, 1396—1403, discussion 1403—1404. [Google Scholar] [CrossRef]
- Bone, L.B.; Giannoudis, P. Femoral shaft fracture fixation and chest injury after polytrauma. J. Bone Jt. Surg. Am. 2011, 93, 311–317. [Google Scholar] [CrossRef]
- Stinner, D.J.; Edwards, D. Surgical Management of Musculoskeletal Trauma. Surg. Clin. N. Am. 2017, 97, 1119–1131. [Google Scholar] [CrossRef]
- Devendra, A.; P, G.N.; Raja, S.D.C.; Dheenadhayalan, J.; Rajasekaran, S. Current updates in management of extremity injuries in polytrauma. J. Clin. Orthop. Trauma 2021, 12, 113–122. [Google Scholar] [CrossRef]
- Blennow, K.; Hardy, J.; Zetterberg, H. The neuropathology and neurobiology of traumatic brain injury. Neuron 2012, 76, 886–899. [Google Scholar] [CrossRef]
- Xiong, Y.; Mahmood, A.; Chopp, M. Animal models of traumatic brain injury. Nat. Rev. Neurosci. 2013, 14, 128–142. [Google Scholar] [CrossRef]
- Marklund, N.; Bakshi, A.; Castelbuono, D.; Conte, V.; McIntosh, T. Evaluation of pharmacological treatment strategies in traumatic brain injury. Curr. Pharm. Des. 2006, 12, 1645–1680. [Google Scholar] [CrossRef]
- Sarrafzadeh, A.S.; Peltonen, E.E.; Kaisers, U.; Küchler, I.; Lanksch, W.R.; Unterberg, A.W. Secondary insults in severe head injury--do multiply injured patients do worse? Crit. Care Med. 2001, 29, 1116–1123. [Google Scholar] [CrossRef]
- Stulemeijer, M.; Van Der Werf, S.P.; Jacobs, B.; Biert, J.; Van Vugt, A.B.; Brauer, J.M.; Vos, P.E. Impact of additional extracranial injuries on outcome after mild traumatic brain injury. J. Neurotrauma 2006, 23, 1561–1569. [Google Scholar] [CrossRef]
- Lefering, R.; Paffrath, T.; Linker, R.; Bouillon, B.; Neugebauer, E.A.M. Deutsche Gesellschaft für Unfallchirurgie/German Society for Trauma Surgery. Head injury and outcome--what influence do concomitant injuries have? J. Trauma 2008, 65, 1036–1043, discussion 1043–1044. [Google Scholar] [CrossRef]
- Lingsma, H.; Andriessen, T.M.J.C.; Haitsema, I.; Horn, J.; van der Naalt, J.; Franschman, G.; Maas, A.I.R.; Vos, P.E.; Steyerberg, E.W. Prognosis in moderate and severe traumatic brain injury: External validation of the IMPACT models and the role of extracranial injuries. J. Trauma Acute Care Surg. 2013, 74, 639–646. [Google Scholar] [CrossRef]
- Hukkelhoven, C.W.; Steyerberg, E.W.; Rampen, A.J.J.; Farace, E.; Habbema, J.D.F.; Marshall, L.F.; Murray, G.D.; Maas, A.I.R.; Gardner, A.J.; Adamova, E.V.; et al. Patient age and outcome following severe traumatic brain injury: An analysis of 5600 patients. J. Neurosurg. 2003, 99, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.; Joannides, A.; Adeleye, A.O.; Bajamal, A.H.; Bashford, T.; Biluts, H.; Budohoski, K.; Ercole, A.; Fernández-Méndez, R.; Figaji, A.; et al. Casemix, management, and mortality of patients receiving emergency neurosurgery for traumatic brain injury in the Global Neurotrauma Outcomes Study: A prospective observational cohort study. Lancet Neurol. 2022, 21, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Leong, B.K.; Mazlan, M.; Rahim, R.B.A.; Ganesan, D. Concomitant injuries and its influence on functional outcome after traumatic brain injury. Disabil. Rehabil. 2013, 35, 1546–1551. [Google Scholar] [CrossRef]
- Davis, T.; Weintraub, A.; Makley, M.; Spier, E.; Forster, J. The intersection of cerebral fat embolism syndrome and traumatic brain injury: A literature review and case series. Brain Inj. 2020, 34, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Flierl, M.A.; Stoneback, J.W.; Beauchamp, K.M.; Hak, D.J.; Morgan, S.J.; Smith, W.R.; Stahel, P.F. Femur shaft fracture fixation in head-injured patients: When is the right time? J. Orthop. Trauma 2010, 24, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Mrozek, S.; Gaussiat, F.; Geeraerts, T. The management of femur shaft fracture associated with severe traumatic brain injury. Ann. Fr. Anesth. Reanim. 2013, 32, 510–515. [Google Scholar] [CrossRef]


| Outcome Variable | Group 1 TBI only | Group 2 TBI + femoral fracture | p—Value |
|---|---|---|---|
| Number of Patients | 3,381 | 1,127 | |
| Result presented as median and (IQR) | |||
| ICU length of stay in days | 3 (1–7) | 4 (2–11) | <0.001 |
| Length of hospital stay in days *1 | 10 (6–18) | 18 (10–27) | < 0.001 |
| Results presented as number of patients and in % per group | |||
| Neurosurgical intervention *2 | 268 (10.3) | 186 (22.4) | <0.001 |
| Multi-organ failure *3 | 214 (22.1) | 177 (30.6) | <0.001 |
| Sepsis *4 | 51 (5.4) | 34 (5.9) | 0.652 |
| Thromboembolic event *5 | 21 (2.2) | 15 (2.6) | 0.568 |
| Outcome | <0.001 | ||
| Death | 452 (13.4) | 181 (16.1) | 0.024 |
| —within 6 h | 52 (1.5) | 36 (3.2) | 0.001 |
| —within 24 h | 189 (5.6) | 89 (7.9) | 0.005 |
| Unresponsive *6 | 62 (1.9) | 25 (2.3) | - |
| Severe disability *6 | 281 (8.5) | 115 (10.4) | - |
| Moderate disability *6 | 716 (21.7) | 326 (29.4) | - |
| Good recovery *6 | 1792 (54.3) | 461 (41.6) | - |
| Discharge from hospital | <0.001 | ||
| —Discharge to home | 1,667 (49.3) | 419 (37.2) | - |
| —Discharge to rehabilitation facility | 864 (25.6) | 363 (32.2) | - |
| —Transfer to other hospital | 281 (8.3) | 99 (8.8) | - |
| —Other | 117 (3.5) | 65 (5.8) | - |
| Patient Characteristics | Group 1 TBI only | Group 2 TBI + femoral fracture | p—Value |
|---|---|---|---|
| Number of patients | 3,381 | 1,127 | |
| Results presented as means (SD) | |||
| Age (years) | 56.1 (22.7) | 53.9 (25.4) | 0.012 |
| ISS | 15.9 (7.5) | 23.4 (7.6) | <0.001 |
| Time from accident to hospital in minutes *1 | 61.0 (34.4) | 68.4 (34.0) | <0.001 |
| Results presented as number of patients in % per group | |||
| Mechanism of injury *2 | <0.001 | ||
| —Traffic, overall | 1,191 (36.4) | 667 (59.8) | <0.001 |
| —Traffic, car passenger | 400 (12.2) | 266 (23.9) | - |
| —Traffic, motorcyclists/socius | 182 (5.6) | 181 (16.2) | - |
| —Traffic, bicycle | 430 (13.1) | 103 (9.2) | - |
| —Traffic, pedestrian | 159 (4.9) | 101 (9.1) | - |
| —High fall ≥3 m | 448 (13.7) | 139 (12.5) | - |
| —Low fall <3 m | 1,301 (39.7) | 277 (24.8) | - |
| —Other | 353 (10.8) | 48 (4.3) | - |
| Level of care *3 | <0.001 | ||
| —Level 1 (supra-regional) | 1,890 (55.9) | 756 (67.1) | - |
| —Level 2 (regional) | 1,230 (36.4) | 286 (25.4) | - |
| —Level 3 (local) | 261 (7.7) | 85 (7.5) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul, M.M.; Mieden, H.J.; Lefering, R.; Kupczyk, E.K.; Jordan, M.C.; Gilbert, F.; Meffert, R.H.; Sirén, A.-L.; Hoelscher-Doht, S. Impact of a Femoral Fracture on Outcome after Traumatic Brain Injury—A Matched-Pair Analysis of the TraumaRegister DGU®. J. Clin. Med. 2023, 12, 3802. https://doi.org/10.3390/jcm12113802
Paul MM, Mieden HJ, Lefering R, Kupczyk EK, Jordan MC, Gilbert F, Meffert RH, Sirén A-L, Hoelscher-Doht S. Impact of a Femoral Fracture on Outcome after Traumatic Brain Injury—A Matched-Pair Analysis of the TraumaRegister DGU®. Journal of Clinical Medicine. 2023; 12(11):3802. https://doi.org/10.3390/jcm12113802
Chicago/Turabian StylePaul, Mila M., Hannah J. Mieden, Rolf Lefering, Eva K. Kupczyk, Martin C. Jordan, Fabian Gilbert, Rainer H. Meffert, Anna-Leena Sirén, and Stefanie Hoelscher-Doht. 2023. "Impact of a Femoral Fracture on Outcome after Traumatic Brain Injury—A Matched-Pair Analysis of the TraumaRegister DGU®" Journal of Clinical Medicine 12, no. 11: 3802. https://doi.org/10.3390/jcm12113802
APA StylePaul, M. M., Mieden, H. J., Lefering, R., Kupczyk, E. K., Jordan, M. C., Gilbert, F., Meffert, R. H., Sirén, A.-L., & Hoelscher-Doht, S. (2023). Impact of a Femoral Fracture on Outcome after Traumatic Brain Injury—A Matched-Pair Analysis of the TraumaRegister DGU®. Journal of Clinical Medicine, 12(11), 3802. https://doi.org/10.3390/jcm12113802








