Development of a Statistical Shape Model and Assessment of Anatomical Shape Variations in the Hemipelvis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Dataset
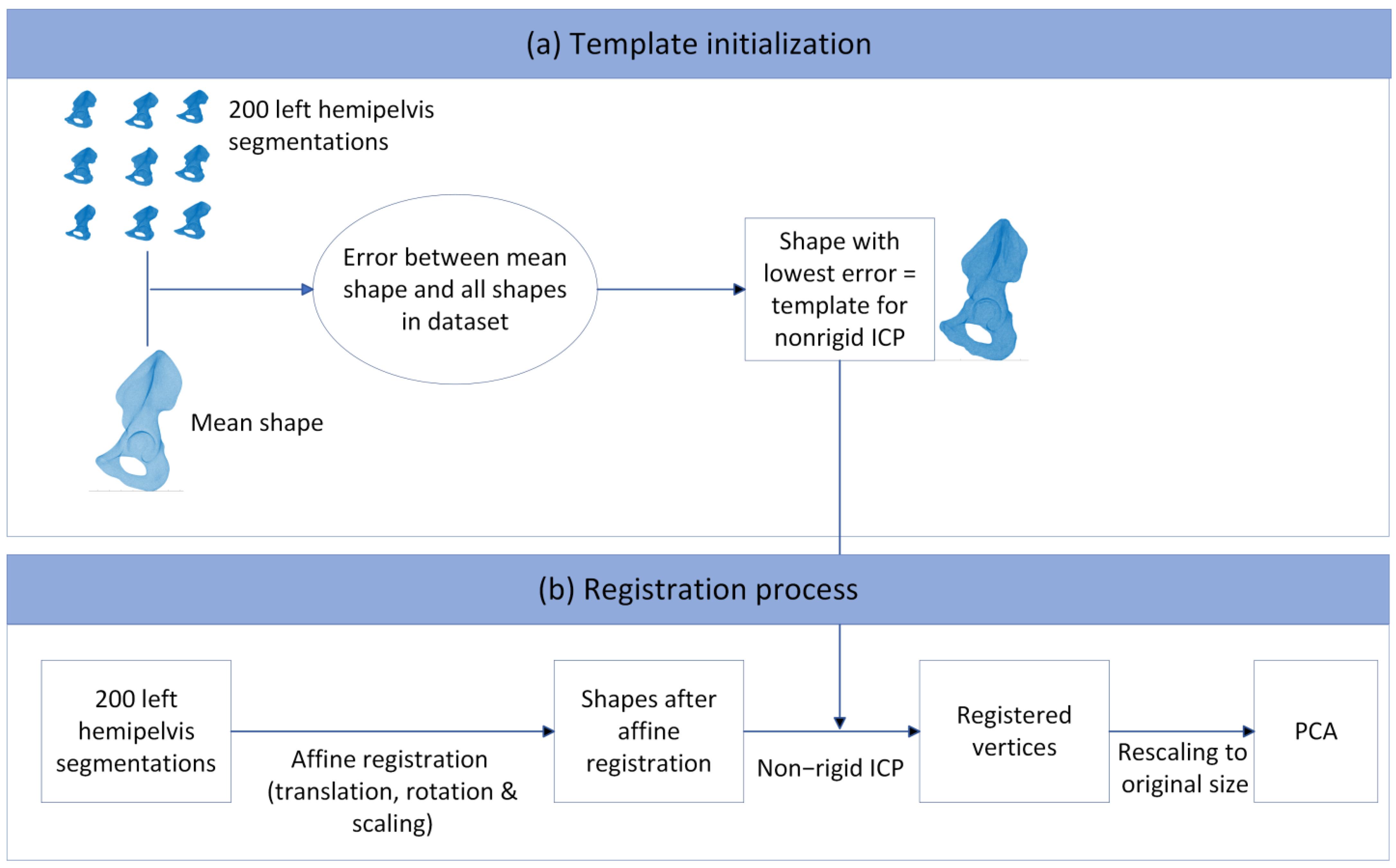
2.3. Three-Dimensional Model Preparation
2.4. Principal Component Analysis and Statistical Shape Modeling
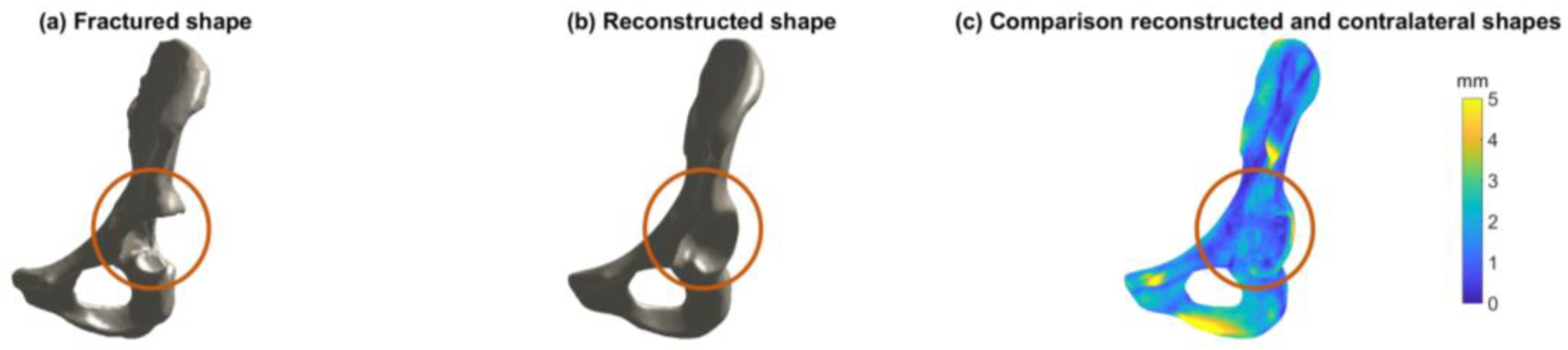
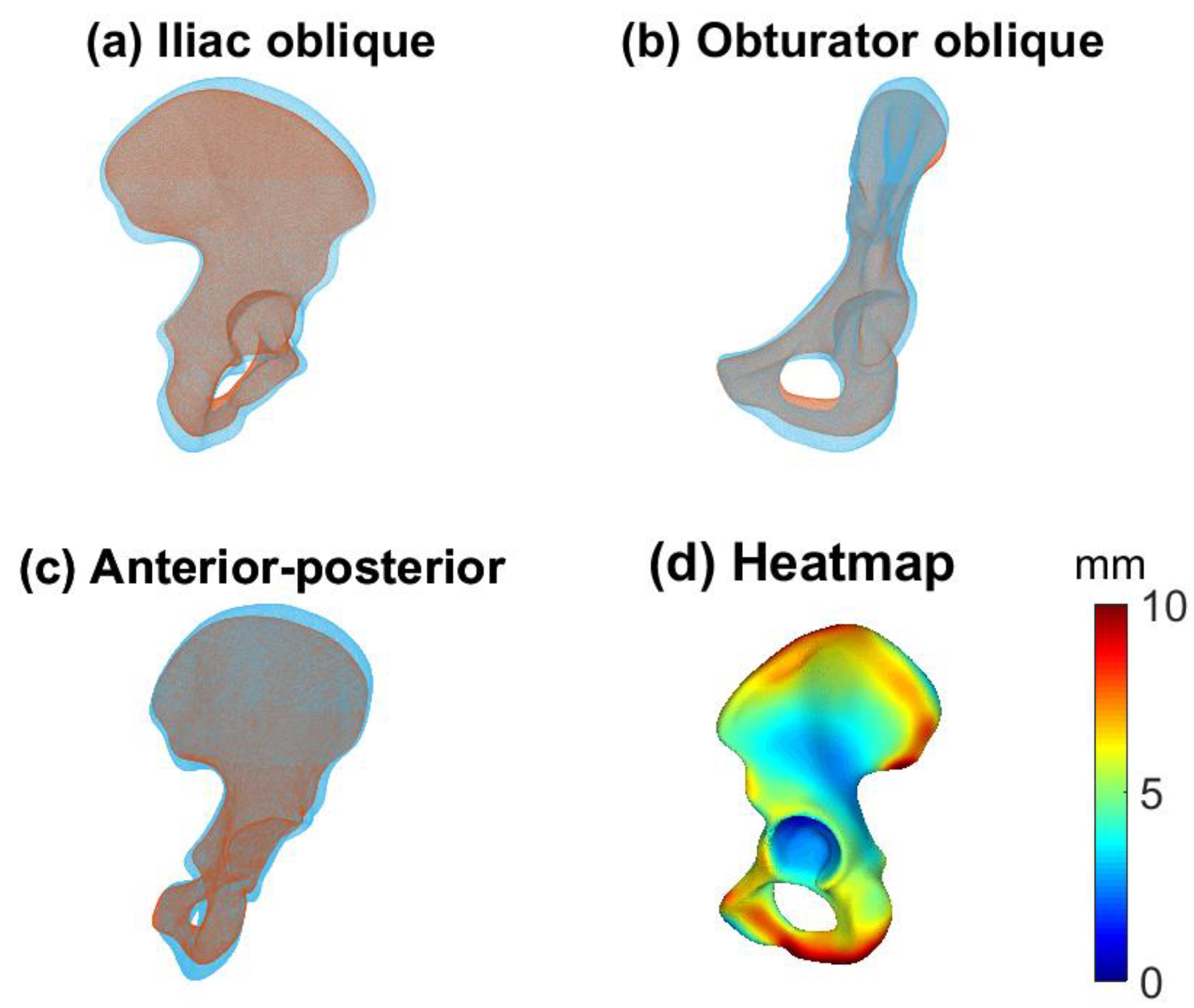
2.5. Evaluation
2.6. Statistical Analyses
3. Results
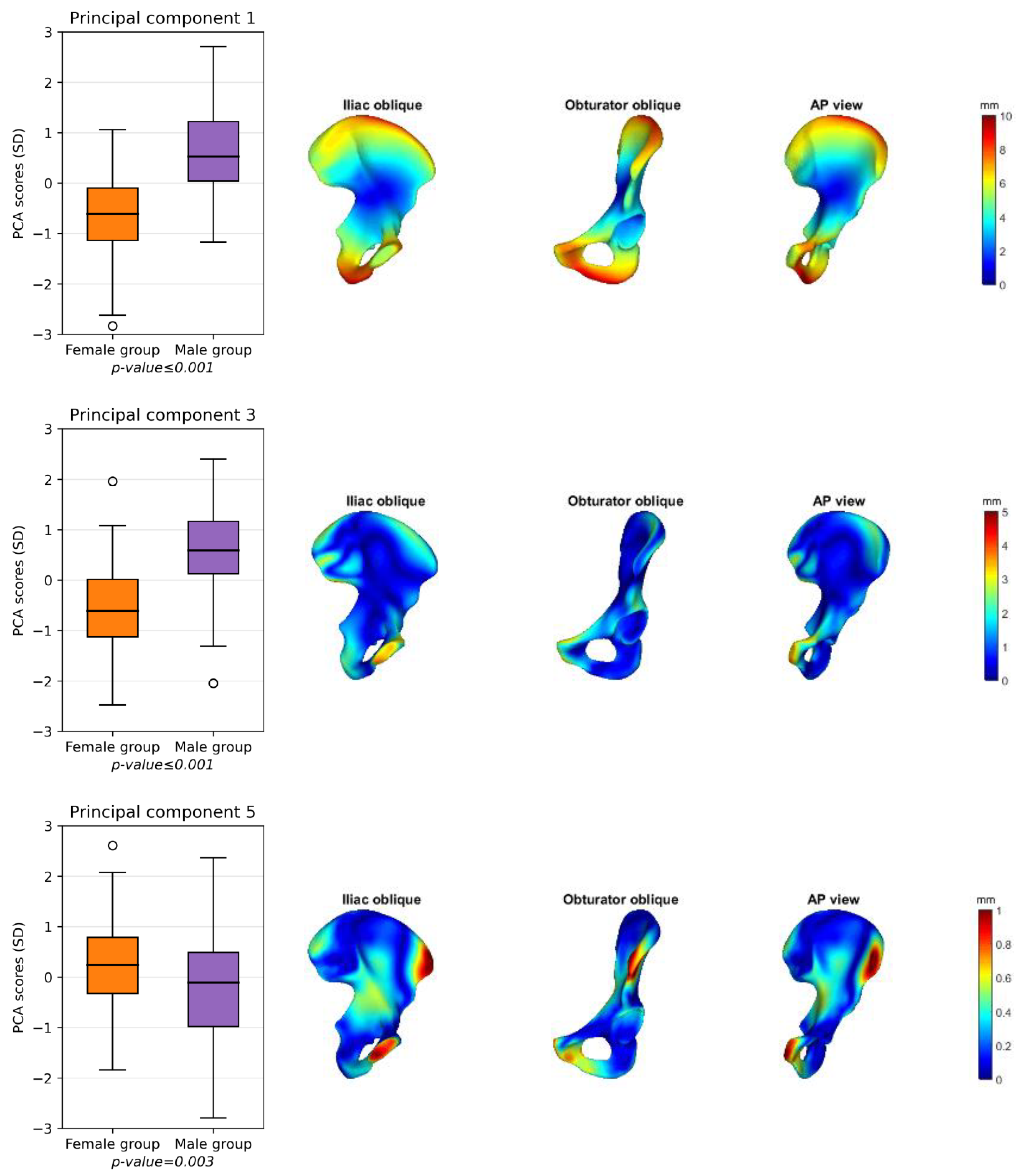
3.1. Shape Variation in the Pelvis
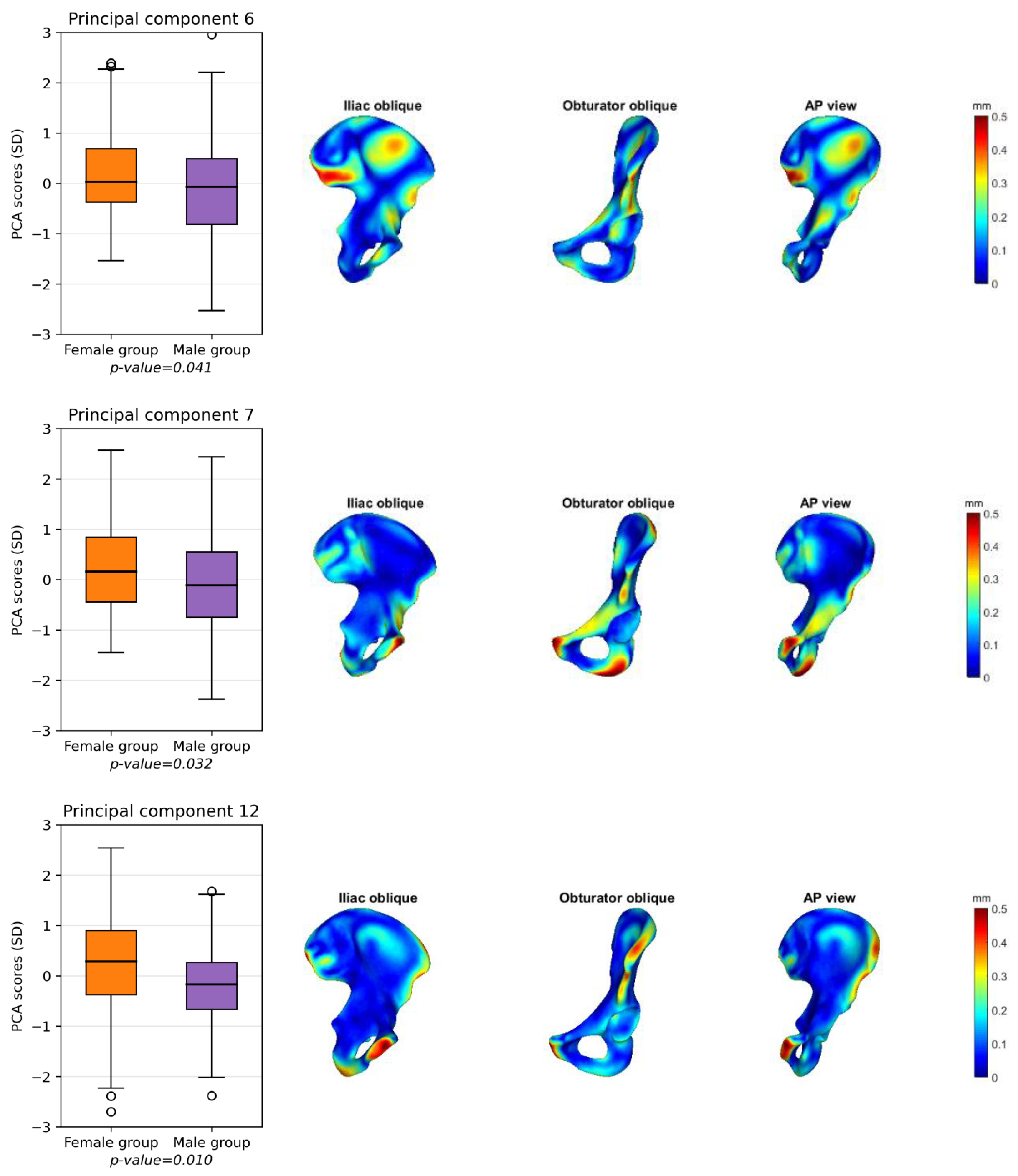
3.2. Male and Female Hemipelvis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Letournel, E. Acetabulum Fractures: Classification and Management. J. Orthop. Trauma 2019, 33, S1–S2. [Google Scholar] [CrossRef]
- Maini, L.; Verma, T.; Sharma, A.; Sharma, A.; Mishra, A.; Jha, S. Evaluation of Accuracy of Virtual Surgical Planning for Patient-Specific Pre-Contoured Plate in Acetabular Fracture Fixation. Arch. Orthop. Trauma Surg. 2018, 138, 495–504. [Google Scholar] [CrossRef]
- Zeng, C.; Xing, W.; Wu, Z.; Huang, H.; Huang, W. A Combination of Three-Dimensional Printing and Computer-Assisted Virtual Surgical Procedure for Preoperative Planning of Acetabular Fracture Reduction. Injury 2016, 47, 2223–2227. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Chou, Y.-C.; Li, Y.-T.; Chen, J.-E.; Hung, C.-C.; Wu, C.-C.; Shen, H.-C.; Yeh, T.-T. Pre-Operative Virtual Simulation and Three-Dimensional Printing Techniques for the Surgical Management of Acetabular Fractures. Int. Orthop. 2019, 43, 1969–1976. [Google Scholar] [CrossRef]
- Fang, C.; Cai, H.; Kuong, E.; Chui, E.; Siu, Y.C.; Ji, T.; Drstvenšek, I. Surgical Applications of Three-Dimensional Printing in the Pelvis and Acetabulum: From Models and Tools to Implants. Unfallchirurg 2019, 122, 278–285. [Google Scholar] [CrossRef]
- Merema, B.J.; Kraeima, J.; ten Duis, K.; Wendt, K.W.; Warta, R.; Vos, E.; Schepers, R.H.; Witjes, M.J.H.; IJpma, F.F.A. The Design, Production and Clinical Application of 3D Patient-Specific Implants with Drilling Guides for Acetabular Surgery. Injury 2017, 48, 2540–2547. [Google Scholar] [CrossRef]
- Krol, Z.; Skadlubowicz, P.; Hefti, F.; Krieg, A.H. Virtual Reconstruction of Pelvic Tumor Defects Based on a Gender-Specific Statistical Shape Model. Comput. Aided Surg. 2013, 18, 142–153. [Google Scholar] [CrossRef]
- Krishna, P.; Robinson, D.L.; Bucknill, A.; Lee, P.V.S. Generation of Hemipelvis Surface Geometry Based on Statistical Shape Modelling and Contralateral Mirroring. Biomech. Model. Mechanobiol. 2022, 21, 1317–1324. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal Component Analysis: A Review and Recent Developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Audenaert, E.A.; Pattyn, C.; Steenackers, G.; De Roeck, J.; Vandermeulen, D.; Claes, P. Statistical Shape Modeling of Skeletal Anatomy for Sex Discrimination: Their Training Size, Sexual Dimorphism, and Asymmetry. Front. Bioeng. Biotechnol. 2019, 7, 302. [Google Scholar] [CrossRef]
- Vancleef, S.; Herteleer, M.; Carette, Y.; Herijgers, P.; Duflou, J.R.; Nijs, S.; Vander Sloten, J. Why Off-the-Shelf Clavicle Plates Rarely Fit: Anatomic Analysis of the Clavicle through Statistical Shape Modeling. J. Shoulder Elb. Surg. 2019, 28, 631–638. [Google Scholar] [CrossRef]
- Quintens, L.; Herteleer, M.; Vancleef, S.; Carette, Y.; Duflou, J.; Nijs, S.; Sloten, J.V.; Hoekstra, H. Anatomical Variation of the Tibia—A Principal Component Analysis. Sci. Rep. 2019, 9, 7649. [Google Scholar] [CrossRef]
- Meynen, A.; Matthews, H.; Nauwelaers, N.; Claes, P.; Mulier, M.; Scheys, L. Accurate Reconstructions of Pelvic Defects and Discontinuities Using Statistical Shape Models. Comput. Methods Biomech. Biomed. Engin. 2020, 23, 1026–1033. [Google Scholar] [CrossRef]
- Meynen, A.; Vles, G.; Roussot, M.; Van Eemeren, A.; Wafa, H.; Mulier, M.; Scheys, L. Advanced Quantitative 3D Imaging Improves the Reliability of the Classification of Acetabular Defects. Arch. Orthop. Trauma Surg. 2022, 143, 1611–1617. [Google Scholar] [CrossRef]
- Ead, M.S.; Palizi, M.; Jaremko, J.L.; Westover, L.; Duke, K.K. Development and Application of the Average Pelvic Shape in Virtual Pelvic Fracture Reconstruction. Int. J. Med. Robot. Comput. Assist. Surg. 2021, 17, e2199. [Google Scholar] [CrossRef]
- Arand, C.; Noser, H.; Kamer, L.; Gehweiler, D.; Handrich, K.; Rommens, P.M.; Wagner, D. Is There a Correlation between Pelvic Incidence and Orientation of the Acetabulum? An Analysis Based on a three-dimensional Statistical Model of the Pelvic Ring. J. Anat. 2022, 241, 756–764. [Google Scholar] [CrossRef]
- Handrich, K.; Kamer, L.; Mayo, K.; Sawaguchi, T.; Noser, H.; Arand, C.; Wagner, D.; Rommens, P.M. Asymmetry of the Pelvic Ring Evaluated by CT-based 3D Statistical Modeling. J. Anat. 2021, 238, 1225–1232. [Google Scholar] [CrossRef]
- Vanden Berghe, P.; Demol, J.; Gelaude, F.; Vander Sloten, J. Virtual Anatomical Reconstruction of Large Acetabular Bone Defects Using a Statistical Shape Model. Comput. Methods Biomech. Biomed. Eng. 2017, 20, 577–586. [Google Scholar] [CrossRef]
- Ahrend, M.-D.; Noser, H.; Shanmugam, R.; Burr, F.; Kamer, L.; Kamarul, T.; Hügli, H.; Nagy, A.; Richards, R.G.; Gueorguiev-Rüegg, B. Development of Generic Asian Pelvic Bone Models Using CT-Based 3D Statistical Modelling. J. Orthop. Transl. 2020, 20, 100–106. [Google Scholar] [CrossRef]
- Arand, C.; Wagner, D.; Richards, R.G.; Noser, H.; Kamer, L.; Sawaguchi, T.; Rommens, P.M. 3D Statistical Model of the Pelvic Ring—A CT-based Statistical Evaluation of Anatomical Variation. J. Anat. 2019, 234, 376–383. [Google Scholar] [CrossRef]
- CBS Height Distribution Dutch Population. Available online: https://longreads.cbs.nl/nederland-in-cijfers-2022/hoe-lang-zijn-nederlanders/ (accessed on 6 July 2022).
- CBS Weight Distribution Dutch Population. Available online: https://www.cbs.nl/nl-nl/cijfers/detail/81565NED?q=gewicht (accessed on 6 July 2022).
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Ann. Intern. Med. 2007, 147, 573. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.-C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an Image Computing Platform for the Quantitative Imaging Network. Magn. Reson. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [PubMed]
- Manu Patch Remesher. Available online: https://nl.mathworks.com/matlabcentral/fileexchange/49691-patch-remesher (accessed on 15 August 2022).
- Manu NonrigidICP. Available online: https://www.mathworks.com/matlabcentral/fileexchange/41396-nonrigidicp (accessed on 11 August 2022).
- Audenaert, E.A.; Van Houcke, J.; Almeida, D.F.; Paelinck, L.; Peiffer, M.; Steenackers, G.; Vandermeulen, D. Cascaded Statistical Shape Model Based Segmentation of the Full Lower Limb in CT. Comput. Methods Biomech. Biomed. Eng. 2019, 22, 644–657. [Google Scholar] [CrossRef]
- Manu Shape Model Builder. Available online: https://nl.mathworks.com/matlabcentral/fileexchange/49940-shape-model-builder (accessed on 8 May 2023).
- Styner, M.A.; Rajamani, K.T.; Nolte, L.-P.; Zsemlye, G.; Székely, G.; Taylor, C.J.; Davies, R.H. Evaluation of 3D Correspondence Methods for Model Building; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar]
- Meesters, A.M.L.; Oldhoff, M.G.E.; Trouwborst, N.M.; Assink, N.; Kraeima, J.; Witjes, M.J.H.; de Vries, J.-P.P.M.; ten Duis, K.; IJpma, F.F.A. Quantitative Three-Dimensional Measurements of Acetabular Fracture Displacement Could Be Predictive for Native Hip Survivorship. J. Pers. Med. 2022, 12, 1464. [Google Scholar] [CrossRef]
- Kriechling, P.; Leoty, L.; Fürnstahl, P.; Rahbani, D.; Zingg, P.O.; Vlachopoulos, L. A Statistical Shape Model-Based Analysis of Periacetabular Osteotomies. J. Bone Jt. Surg. 2022, 104, 1107–1115. [Google Scholar] [CrossRef]
- Verbeek, D.O.; van der List, J.P.; Tissue, C.M.; Helfet, D.L. Predictors for Long-Term Hip Survivorship Following Acetabular Fracture Surgery. J. Bone Jt. Surg. 2018, 100, 922–929. [Google Scholar] [CrossRef]
- Meier, M.K.; Schmaranzer, F.; Kaim, T.; Tannast, M.; Novais, E.N.; Siebenrock, K.A.; Steppacher, S.D.; Lerch, T.D. Combined Femoral and Acetabular Version Is Sex-Related and Differs between Patients with Hip Dysplasia and Acetabular Retroversion. Eur. J. Radiol. 2023, 158, 110634. [Google Scholar] [CrossRef]
- Mandolini, M.; Brunzini, A.; Facco, G.; Mazzoli, A.; Forcellese, A.; Gigante, A. Comparison of Three 3D Segmentation Software Tools for Hip Surgical Planning. Sensors 2022, 22, 5242. [Google Scholar] [CrossRef]
- Ead, M.S.; Duke, K.K.; Jaremko, J.L.; Westover, L. Investigation of Pelvic Symmetry Using CAD Software. Med. Biol. Eng. Comput. 2020, 58, 75–82. [Google Scholar] [CrossRef]
- Bakhshayesh, P.; Zaghloul, A.; Sephton, B.M.; Enocson, A. A Novel 3D Technique to Assess Symmetry of Hemi Pelvises. Sci. Rep. 2020, 10, 18789. [Google Scholar] [CrossRef]
- Osterhoff, G.; Petersik, A.; Sprengel, K.; Pape, H.-C. Symmetry Matching of the Medial Acetabular Surface—A Quantitative Analysis in View of Patient-Specific Implants. J. Orthop. Trauma 2019, 33, e79–e83. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Liu, S.; Li, H.; Yang, D. Automatic and Hierarchical Segmentation of the Human Skeleton in CT Images. Phys. Med. Biol. 2017, 62, 2812–2833. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Yuan, Y.; Guo, C.; Wang, Y.; Cheng, Y.; Tamura, S. Accurate Pelvis and Femur Segmentation in Hip CT With a Novel Patch-Based Refinement. IEEE J. Biomed. Health Inform. 2019, 23, 1192–1204. [Google Scholar] [CrossRef] [PubMed]

| Total Group | Male Group (n = 100) | Female Group (n = 100) | p-Value | |
|---|---|---|---|---|
| Age, years | 56 ± 16 | 54 ± 17 | 57 ± 14 | 0.15 |
| Height, cm | 173 ± 10 | 180 ± 7 | 167 ± 7 | <0.001 |
| Weight, kg | 81 ± 19 | 88 ± 18 | 74 ± 17 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Veldhuizen, W.A.; van der Wel, H.; Kuipers, H.Y.; Kraeima, J.; ten Duis, K.; Wolterink, J.M.; de Vries, J.-P.P.M.; Schuurmann, R.C.L.; IJpma, F.F.A. Development of a Statistical Shape Model and Assessment of Anatomical Shape Variations in the Hemipelvis. J. Clin. Med. 2023, 12, 3767. https://doi.org/10.3390/jcm12113767
van Veldhuizen WA, van der Wel H, Kuipers HY, Kraeima J, ten Duis K, Wolterink JM, de Vries J-PPM, Schuurmann RCL, IJpma FFA. Development of a Statistical Shape Model and Assessment of Anatomical Shape Variations in the Hemipelvis. Journal of Clinical Medicine. 2023; 12(11):3767. https://doi.org/10.3390/jcm12113767
Chicago/Turabian Stylevan Veldhuizen, Willemina A., Hylke van der Wel, Hennie Y. Kuipers, Joep Kraeima, Kaj ten Duis, Jelmer M. Wolterink, Jean-Paul P. M. de Vries, Richte C. L. Schuurmann, and Frank F. A. IJpma. 2023. "Development of a Statistical Shape Model and Assessment of Anatomical Shape Variations in the Hemipelvis" Journal of Clinical Medicine 12, no. 11: 3767. https://doi.org/10.3390/jcm12113767
APA Stylevan Veldhuizen, W. A., van der Wel, H., Kuipers, H. Y., Kraeima, J., ten Duis, K., Wolterink, J. M., de Vries, J.-P. P. M., Schuurmann, R. C. L., & IJpma, F. F. A. (2023). Development of a Statistical Shape Model and Assessment of Anatomical Shape Variations in the Hemipelvis. Journal of Clinical Medicine, 12(11), 3767. https://doi.org/10.3390/jcm12113767








