Role of Dual-Acquisition Noninvasive Cardiac CT Imaging for the Detection of Vasospastic Angina
Abstract
1. Introduction
2. Methods
2.1. Study Design and Patient Population
2.2. Coronary Angiography and Spasm Provocation Test
2.3. CCTA Acquisition and Analysis
| CDI − CSA= [(CSA IV nitrate − CSA initial)/CSA IV nitrate] × 100% |
| or CDI − D= [(Diameter IV nitrate − Diameter initial)/Diameter IV nitrate] × 100% |
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
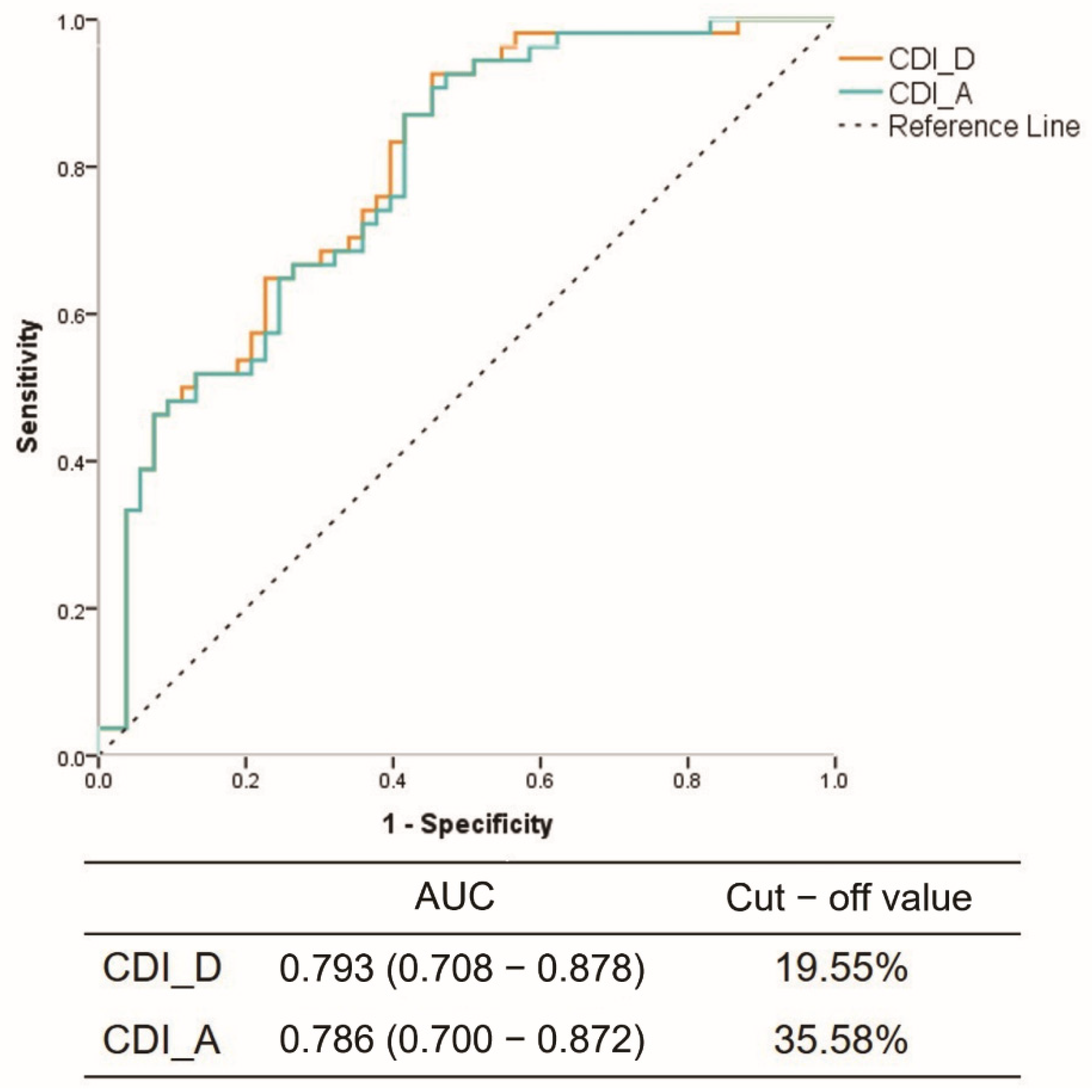
3.2. Reliability Prediction of CCTA Compared to Spasm Testing
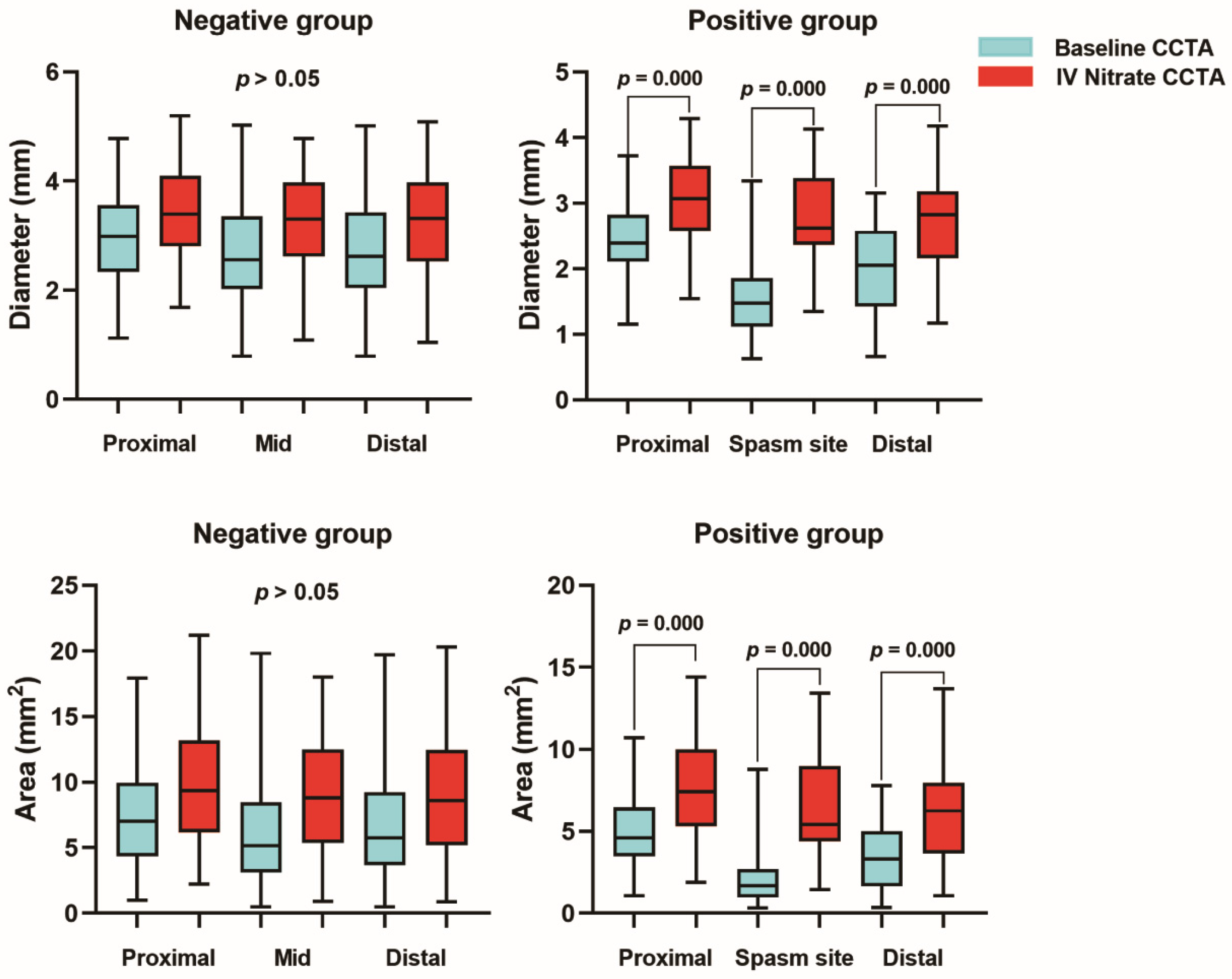
3.3. Quantitative Analysis for Coronary Arterial Distensibility
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, H.L.; Kim, J.; Kim, H.J.; Lim, W.H.; Lee, J.Y. Incidence and factors associated with mortality in 2476 patients with variant angina in Korea. Sci. Rep. 2017, 7, 46031. [Google Scholar] [CrossRef]
- JCS Joint Working Group. Guidelines for diagnosis and treatment of patients with vasospastic angina (Coronary Spastic Angina) (JCS 2013). Circ. J. 2014, 78, 2779–2801. [Google Scholar] [CrossRef] [PubMed]
- Tsujita, K.; Sakamoto, K.; Kojima, S.; Kojima, S.; Takaoka, N.; Nagayoshi, Y.; Sakamoto, T.; Tayama, S.; Kaikita, K.; Hokimoto, S.; et al. Coronary plaque component in patients with vasospastic angina: A virtual histology intravascular ultrasound study. Int. J. Cardiol. 2013, 168, 2411–2415. [Google Scholar] [CrossRef] [PubMed]
- Soeda, T.; Uemura, S.; Morikawa, Y.; Ishigami, K.; Okayama, S.; Hee, S.J.; Nishida, T.; Onoue, K.; Somekawa, S.; Takeda, Y.; et al. Diagnostic accuracy of dual-source computed tomography in the characterization of coronary atherosclerotic plaques: Comparison with intravascular optical coherence tomography. Int. J. Cardiol. 2011, 148, 313–318. [Google Scholar] [CrossRef]
- Kang, K.M.; Choi, S.I.; Chun, E.J.; Kim, J.A.; Youn, T.J.; Choi, D.J. Coronary vasospastic angina: Assessment by multidetector CT coronary angiography. Korean J. Radiol. 2012, 13, 27–33. [Google Scholar] [CrossRef]
- Ozaki, Y.; Keane, D.; Serruys, P.W. Relation of basal coronary tone and vasospastic activity in patients with variant angina. Heart (Br. Card. Soc.) 1996, 75, 267–273. [Google Scholar] [CrossRef]
- Kang, E.J.; Kim, M.H.; De Jin, C.; Seo, J.; Kim, D.W.; Yoon, S.K.; Park, T.H.; Lee, K.N.; Choi, S.I.; Yoon, Y.E. Noninvasive detection of coronary vasospastic angina using a double-acquisition coronary CT angiography protocol in the presence and absence of an intravenous nitrate: A pilot study. Eur. Radiol. 2017, 27, 1136–1147. [Google Scholar] [CrossRef]
- Jin, C.; Kim, M.H.; Kang, E.J.; Cho, Y.R.; Park, T.H.; Lee, K.N.; Serebruany, V. Assessing Vessel Tone during Coronary Artery Spasm by Dual-Acquisition Multidetector Computed Tomography Angiography. Cardiology 2018, 139, 25–32. [Google Scholar] [CrossRef]
- Beltrame, J.F.; Crea, F.; Kaski, J.C.; Ogawa, H.; Ong, P.; Sechtem, U.; Shimokawa, H.; Bairey Merz, C.N.; Coronary Vasomotion Disorders International Study, G. International standardization of diagnostic criteria for vasospastic angina. Eur. Heart J. 2017, 38, 2565–2568. [Google Scholar] [CrossRef]
- Takagi, Y.; Takahashi, J.; Yasuda, S.; Miyata, S.; Tsunoda, R.; Ogata, Y.; Seki, A.; Sumiyoshi, T.; Matsui, M.; Goto, T.; et al. Prognostic stratification of patients with vasospastic angina: A comprehensive clinical risk score developed by the Japanese Coronary Spasm Association. J. Am. Coll. Cardiol. 2013, 62, 1144–1153. [Google Scholar] [CrossRef]
- Park, J.; Kim, H.K.; Park, E.A.; Park, J.B.; Lee, S.P.; Lee, W.; Kim, Y.J.; Sohn, D.W. Coronary Computed Tomography Angiography for the Diagnosis of Vasospastic Angina: Comparison with Invasive Coronary Angiography and Ergonovine Provocation Test. Korean J. Radiol. 2019, 20, 719–728. [Google Scholar] [CrossRef]
- Kuga, T.; Egashira, K.; Inou, T.; Takeshita, A. Correlation of basal coronary artery tone with constrictive response to ergonovine in patients with variant angina. J. Am. Coll. Cardiol. 1993, 22, 144–150. [Google Scholar] [CrossRef]
- Yasue, H.; Nakagawa, H.; Itoh, T.; Harada, E.; Mizuno, Y. Coronary artery spasm--clinical features, diagnosis, pathogenesis, and treatment. J. Cardiol. 2008, 51, 2–17. [Google Scholar] [CrossRef]
- Hung, M.J.; Hu, P.; Hung, M.Y. Coronary artery spasm: Review and update. Int. J. Med. Sci. 2014, 11, 1161–1171. [Google Scholar] [CrossRef]
- Kim, M.H.; Park, E.H.; Yang, D.K.; Park, T.H.; Kim, S.G.; Yoon, J.H.; Cha, K.S.; Kum, D.S.; Kim, H.J.; Kim, J.S. Role of vasospasm in acute coronary syndrome: Insights from ergonovine stress echocardiography. Circ. J. 2005, 69, 39–43. [Google Scholar] [CrossRef]
- Song, J.-K.; Park, S.-W.; Kang, D.-H.; Hong, M.-K.; Kim, J.-J.; Lee, C.-W.; Park, S.-J. Safety and clinical impact of ergonovine stress echocardiography for diagnosis of coronary vasospasm. J. Am. Coll. Cardiol. 2000, 35, 1850–1856. [Google Scholar] [CrossRef]
- Song, J.-K.; Lee, S.J.-K.; Kang, D.-H.; Sang Sig, C.; Myeong Ki, H.; Kim, J.-J.; Park, S.-W.; Park, S.-J. Ergonovine echocardiography as a screening test for diagnosis of vasospastic angina before coronary angiography. J. Am. Coll. Cardiol. 1996, 27, 1156–1161. [Google Scholar] [CrossRef]
- Dwivedi, A.; Al’Aref, S.J.; Lin, F.Y.; Min, J.K. Evaluation of Atherosclerotic Plaque in Non-invasive Coronary Imaging. Korean Circ. J. 2018, 48, 124–133. [Google Scholar] [CrossRef]
- Park, T.H.; Kim, M.H.; Jang, S.J.; Kim, S.G.; Yang, D.K.; Oh, I.H.; Cha, K.S.; Kim, Y.D.; Kim, J.S. Heart Arrest Caused by Ergonovine Stress Echocardiography. Korean Circ. J. 2002, 32, 359–362. [Google Scholar] [CrossRef]
- Abdelrahman, K.M.; Chen, M.Y.; Dey, A.K.; Virmani, R.; Finn, A.V.; Khamis, R.Y.; Choi, A.D.; Min, J.K.; Williams, M.C.; Buckler, A.J.; et al. Coronary Computed Tomography Angiography From Clinical Uses to Emerging Technologies: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 1226–1243. [Google Scholar] [CrossRef]
- Bing, R.; Singh, T.; Dweck, M.R.; Mills, N.L.; Williams, M.C.; Adamson, P.D.; Newby, D.E. Validation of European Society of Cardiology pre-test probabilities for obstructive coronary artery disease in suspected stable angina. Eur. Heart J. Qual. Care Clin. Outcomes 2020, 6, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Delbaere, K.; Close, J.C.; Brodaty, H.; Sachdev, P.; Lord, S.R. Determinants of disparities between perceived and physiological risk of falling among elderly people: Cohort study. BMJ 2010, 341, c4165. [Google Scholar] [CrossRef]
- Chang, H.J.; Chung, N. Clinical perspective of coronary computed tomographic angiography in diagnosis of coronary artery disease. Circ. J. 2011, 75, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Ogawa, T.; Yoshimura, M. Severe coronary spasm occasionally detected by coronary computed tomography. Eur. Heart J. 2009, 30, 2768. [Google Scholar] [CrossRef]
- Tanabe, Y.; Itoh, E.; Suzuki, K.; Ito, M.; Hosaka, Y.; Nakagawa, I.; Kumakura, M. Limited role of coronary angioplasty and stenting in coronary spastic angina with organic stenosis. J. Am. Coll. Cardiol. 2002, 39, 1120–1126. [Google Scholar] [CrossRef]
- Ford, T.J.; Rocchiccioli, P.; Good, R.; McEntegart, M.; Eteiba, H.; Watkins, S.; Shaukat, A.; Lindsay, M.; Robertson, K.; Hood, S.; et al. Systemic microvascular dysfunction in microvascular and vasospastic angina. Eur. Heart J. 2018, 39, 4086–4097. [Google Scholar] [CrossRef]
- Suzuki, S.; Kaikita, K.; Yamamoto, E.; Jinnouchi, H.; Tsujita, K. Role of acetylcholine spasm provocation test as a pathophysiological assessment in nonobstructive coronary artery disease. Cardiovasc. Interv. 2021, 36, 39–51. [Google Scholar] [CrossRef]
- Jin, C.D.; Kim, M.H.; Jo, S.J.; Lim, K. Severe multivessel coronary artery spasm detected by computed tomography: A case report. Eur. Heart J. Case Rep. 2020, 4, 1–5. [Google Scholar] [CrossRef]
- Anderson, J.L.; Adams, C.D.; Antman, E.M.; Bridges, C.R.; Califf, R.M.; Casey, D.E., Jr.; Chavey, W.E., 2nd; Fesmire, F.M.; Hochman, J.S.; Levin, T.N.; et al. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 61, e179–e347. [Google Scholar] [CrossRef]
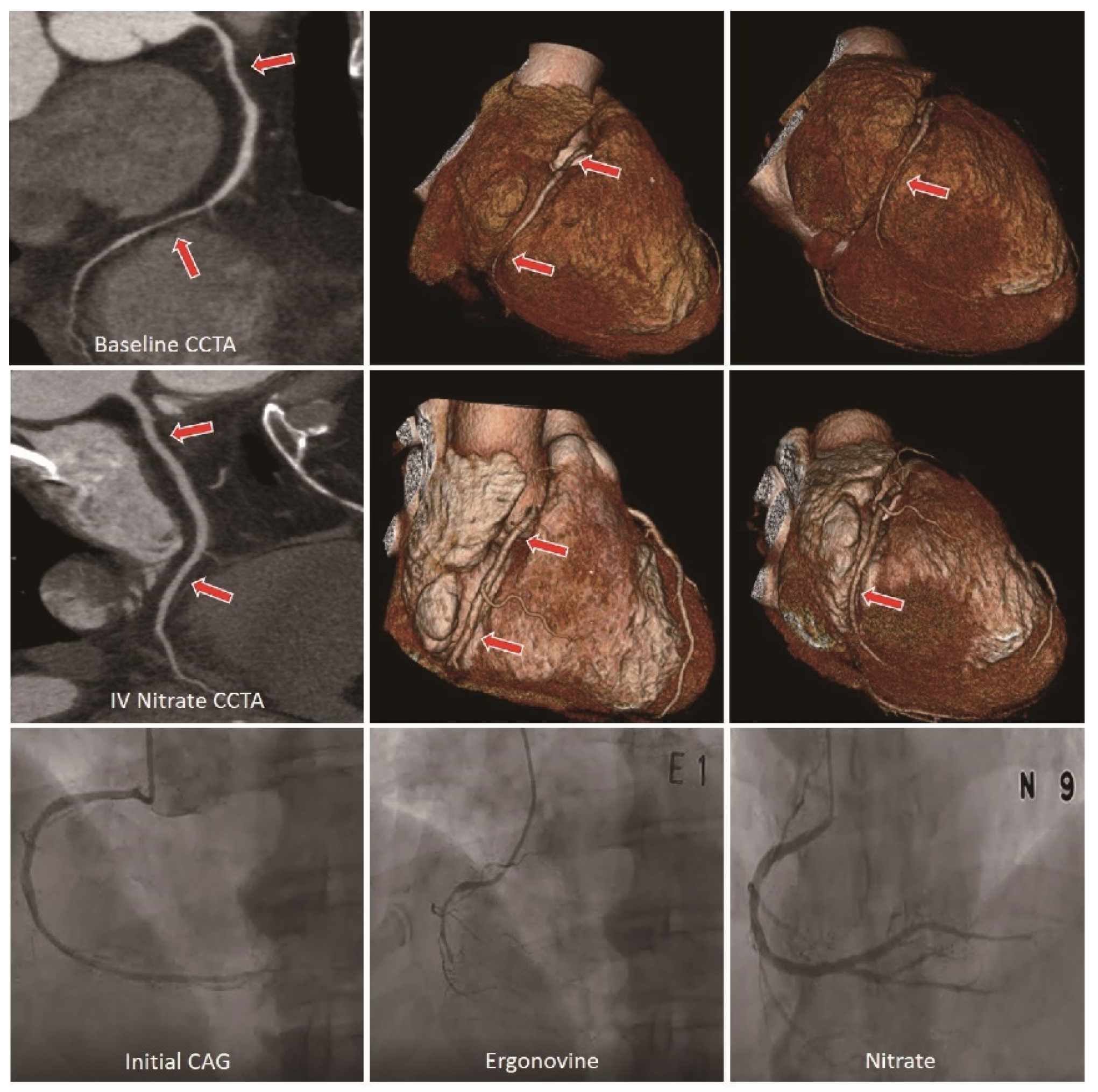
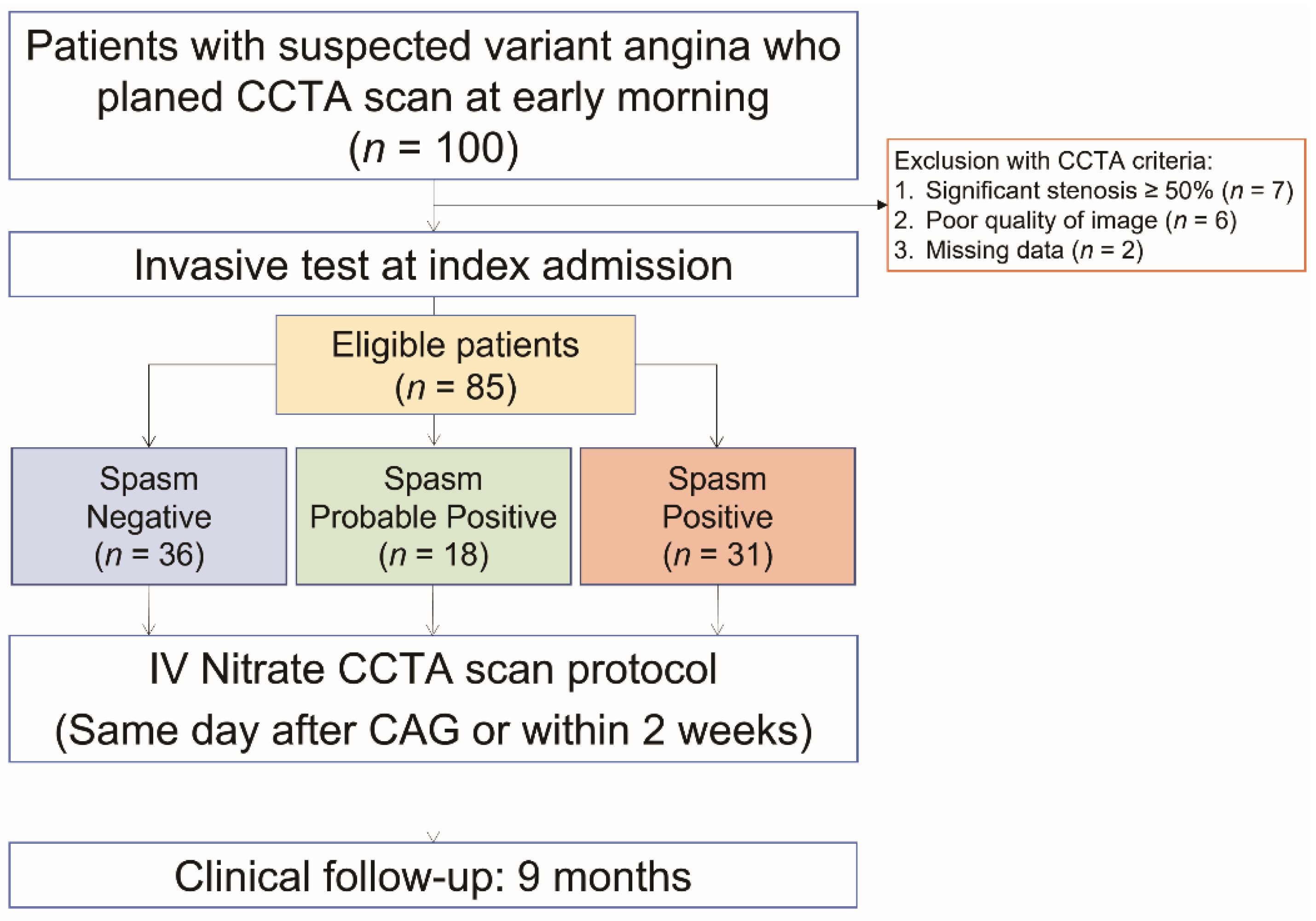
| Variables | Negative (n = 36) | Probable Positive (n = 18) | Positive (n = 31) | p |
|---|---|---|---|---|
| Age (years) | 59.6 ± 11.1 | 56.3 ± 9.1 | 60.0 ± 7.7 | 0.382 |
| Sex (male), % | 11 (30.6) | 10 (55.6) | 23 (74.2) | 0.002 |
| BMI (kg/cm2) | 24.6 ± 2.9 | 24.1 ± 3.0 | 24.2 ± 2.7 | 0.773 |
| LVEF (%) | 61.1 ± 3.9 | 61.0 ± 2.8 | 60.4 ± 4.4 | 0.809 |
| JCSA score | 1.1 ± 1.3 | 3.0 ± 1.0 | 4.3 ± 1.7 | <0.001 |
| Alcohol (%) | 6 (16.7) | 7 (38.9) | 12 (38.7) | 0.087 |
| Coronary risk factors (%) | ||||
| Smoking | 6 (16.7) | 3 (16.7) | 11 (35.5) | 0.144 |
| Hypertension | 12 (33.3) | 4 (22.2) | 7 (22.6) | 0.536 |
| Dyslipidemia | 16 (44.4) | 4 (22.2) | 9 (29.0) | 0.202 |
| Diabetes mellitus | 4 (11.1) | 3 (16.7) | 4 (12.9) | 0.848 |
| Laboratory assessment | ||||
| HbA1C | 5.6 ± 0.5 | 5.9 ± 1.0 | 5.8 ± 0.8 | 0.614 |
| Creatinine | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.9 ± 0.2 | 0.145 |
| Total cholesterol | 191.7 ± 49.2 | 165.9 ± 40.7 | 172.1 ± 28.3 | 0.053 |
| LDL-C | 116.8 ± 40.2 | 96.0 ± 29.0 | 102.4 ± 24.8 | 0.070 |
| HDL-C | 53.4 ± 12.3 | 47.6 ± 14.0 | 53.4 ± 12.8 | 0.248 |
| Triglyceride | 141.8 ± 106.3 | 167.4 ± 106.0 | 145.9 ± 79.3 | 0.653 |
| Troponin I peak | 0.02 ± 0.02 | 0.05 ± 0.08 | 0.14 ± 0.50 | 0.347 |
| Variables | Negative (n = 36) | Probable Positive (n = 18) | Positive (n = 31) | p |
|---|---|---|---|---|
| Organic stenosis (%) | 0.001 | |||
| No stenosis | 32 (88.9) | 16 (88.9) | 16 (51.6) | |
| Insignificant stenosis (25–50%) | 4 (11.1) | 2 (11.1) | 15 (48.4) | |
| Spasm artery (%) | <0.001 | |||
| LAD | 0 (0) | 9 (64.3) | 6 (23.1) | |
| LCX | 0 (0) | 1 (7.1) | 3 (11.5) | |
| RCA | 0 (0) | 4 (28.6) | 17 (65.4) | |
| Multi-vessel spasm | 0 (0) | 1 (5.6) | 7 (22.6) | 0.006 |
| 2 vessels | 1 (5.6) | 5 (16.1) | ||
| 3 vessels | 0 (0) | 2 (6.5) |
| Per Patient | Per Vessel | |
|---|---|---|
| Results of tests | For patients | For matching/total vessels |
| Ergonovine | ||
| Total No. | 85 | 197/197 |
| No. of positive + probable positive | 49 | 66/66 |
| No. of negatives | 36 | 131/131 |
| CCTA | ||
| Total No. | 85 | 197/255 |
| No. of positives | 31 | 29/58 |
| No. of negatives | 54 | 168/197 |
| Diagnostic performance of CT | For patients | For matching vessels |
| Total No. | 85 | 197 |
| No. of true negatives | 32 | 126 |
| No. of true positives | 27 | 24 |
| No. of false negatives | 22 | 42 |
| No. of false positives | 4 | 5 |
| Sensitivity, % | 55 (40–69) | 36 (25–49) |
| Specificity, % | 89 (74–97) | 96 (91–99) |
| PPV, % | 87 (72–95) | 83 (66–92) |
| NPV, % | 59 (51–67) | 75 (71–78) |
| Accuracy, % | 69 (58–79) | 76 (70–82) |
| Negative Group | Positive Group | p for CDI | |||||
|---|---|---|---|---|---|---|---|
| Variables | Baseline CT | IV Nitrate CT | CDI (%) | Baseline CT | IV Nitrate CT | CDI (%) | |
| Diameter (mm) | |||||||
| Proximal | 3.24 ± 0.65 | 3.74 ± 0.74 | 15.8 ± 14.9 | 2.23 ± 0.58 | 2.86 ± 0.63 | 21.4 ± 13.1 | 0.069 |
| Mid | 3.07 ± 0.79 | 3.53 ± 0.71 | 17.7 ± 15.4 | 1.48 ± 0.56 | 2.60 ± 0.70 | 42.3 ± 17.3 | <0.001 |
| Distal | 2.97 ± 0.75 | 3.47 ± 0.72 | 15.5 ± 17.0 | 1.88 ± 0.63 | 2.58 ± 0.71 | 26.1 ± 17.9 | 0.007 |
| Area (mm2) | |||||||
| Proximal | 8.61 ± 3.28 | 11.38 ± 4.31 | 27.0 ± 25.9 | 4.24 ± 2.07 | 6.77 ± 2.85 | 36.4 ± 20.5 | 0.066 |
| Mid | 7.80 ± 4.04 | 10.16 ± 3.81 | 31.5 ± 24.9 | 1.96 ± 1.53 | 5.66 ± 2.94 | 63.6 ± 21.0 | <0.001 |
| Distal | 7.50 ± 3.73 | 9.94 ± 3.83 | 24.9 ± 28.7 | 3.06 ± 1.84 | 5.59 ± 2.94 | 43.2 ± 23.5 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, X.; Kang, E.-J.; Jin, C.-D.; Lee, K.-M.; Lim, K.-H.; Rha, S.-W.; Choi, C.-U.; Yong, H.-S.; Yun, S.-C.; Budoff, M.J.; et al. Role of Dual-Acquisition Noninvasive Cardiac CT Imaging for the Detection of Vasospastic Angina. J. Clin. Med. 2023, 12, 3753. https://doi.org/10.3390/jcm12113753
Jin X, Kang E-J, Jin C-D, Lee K-M, Lim K-H, Rha S-W, Choi C-U, Yong H-S, Yun S-C, Budoff MJ, et al. Role of Dual-Acquisition Noninvasive Cardiac CT Imaging for the Detection of Vasospastic Angina. Journal of Clinical Medicine. 2023; 12(11):3753. https://doi.org/10.3390/jcm12113753
Chicago/Turabian StyleJin, Xuan, Eun-Ju Kang, Cai-De Jin, Kwang-Min Lee, Kyung-Hee Lim, Seung-Woon Rha, Cheol-Ung Choi, Hwan-Seok Yong, Sung-Cheol Yun, Matthew J. Budoff, and et al. 2023. "Role of Dual-Acquisition Noninvasive Cardiac CT Imaging for the Detection of Vasospastic Angina" Journal of Clinical Medicine 12, no. 11: 3753. https://doi.org/10.3390/jcm12113753
APA StyleJin, X., Kang, E.-J., Jin, C.-D., Lee, K.-M., Lim, K.-H., Rha, S.-W., Choi, C.-U., Yong, H.-S., Yun, S.-C., Budoff, M. J., Yu, L.-H., & Kim, M.-H. (2023). Role of Dual-Acquisition Noninvasive Cardiac CT Imaging for the Detection of Vasospastic Angina. Journal of Clinical Medicine, 12(11), 3753. https://doi.org/10.3390/jcm12113753








