Risk Factors for Medication-Related Osteonecrosis of the Jaw—A Binomial Analysis of Data of Cancer Patients from Craiova and Constanta Treated with Zoledronic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Outcome
- Current or previous treatment based on antiresorptive or antiangiogenic agents;
- The presence of exposed bone or an intraoral or extraoral fistula in the maxillofacial region that has persisted for more than eight weeks;
- Patients with no history of radiotherapy to the jaws or obvious metastatic disease of the jaws.
2.4. Data Acquisition
2.5. Statistical Analysis
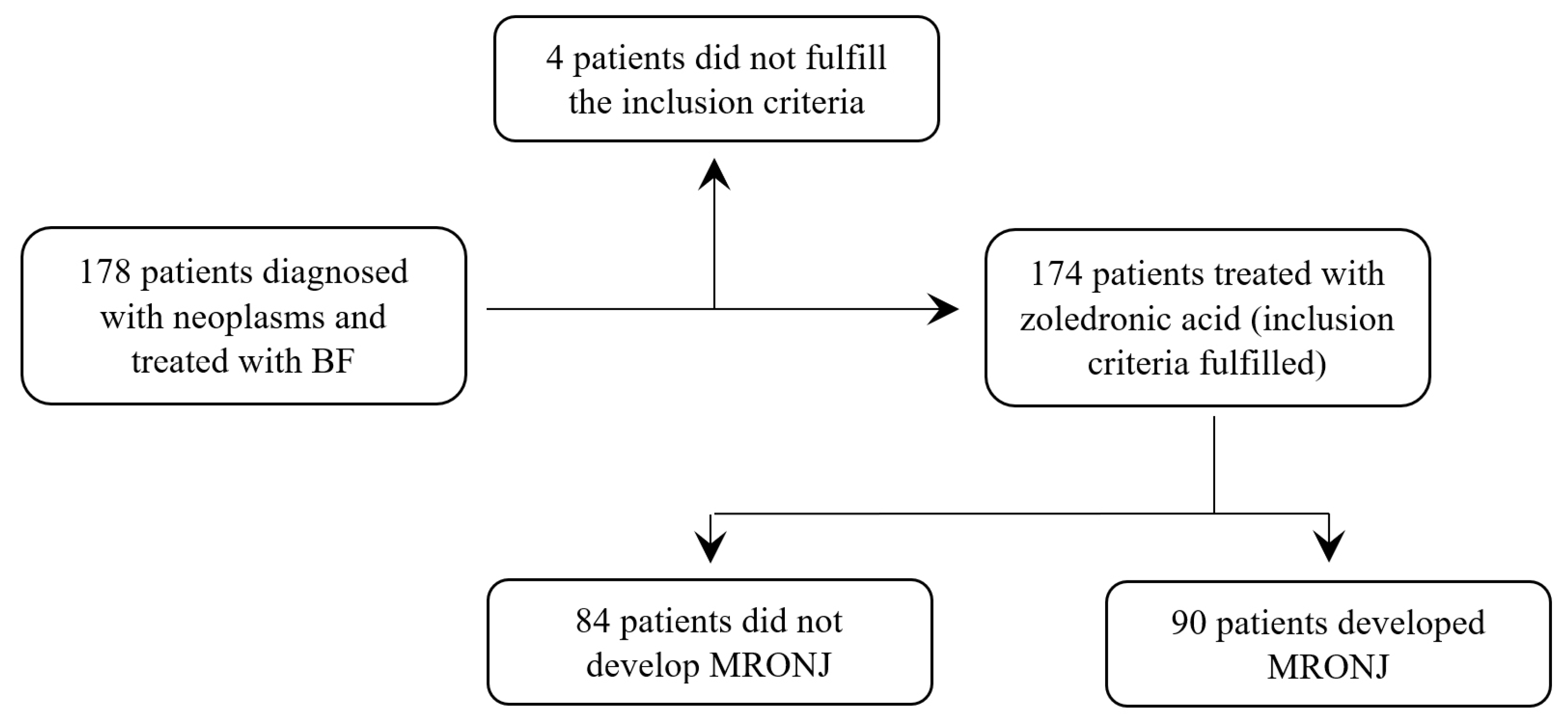
3. Results
3.1. Patients’ Characteristics
3.2. Bisphosphonate Treatment
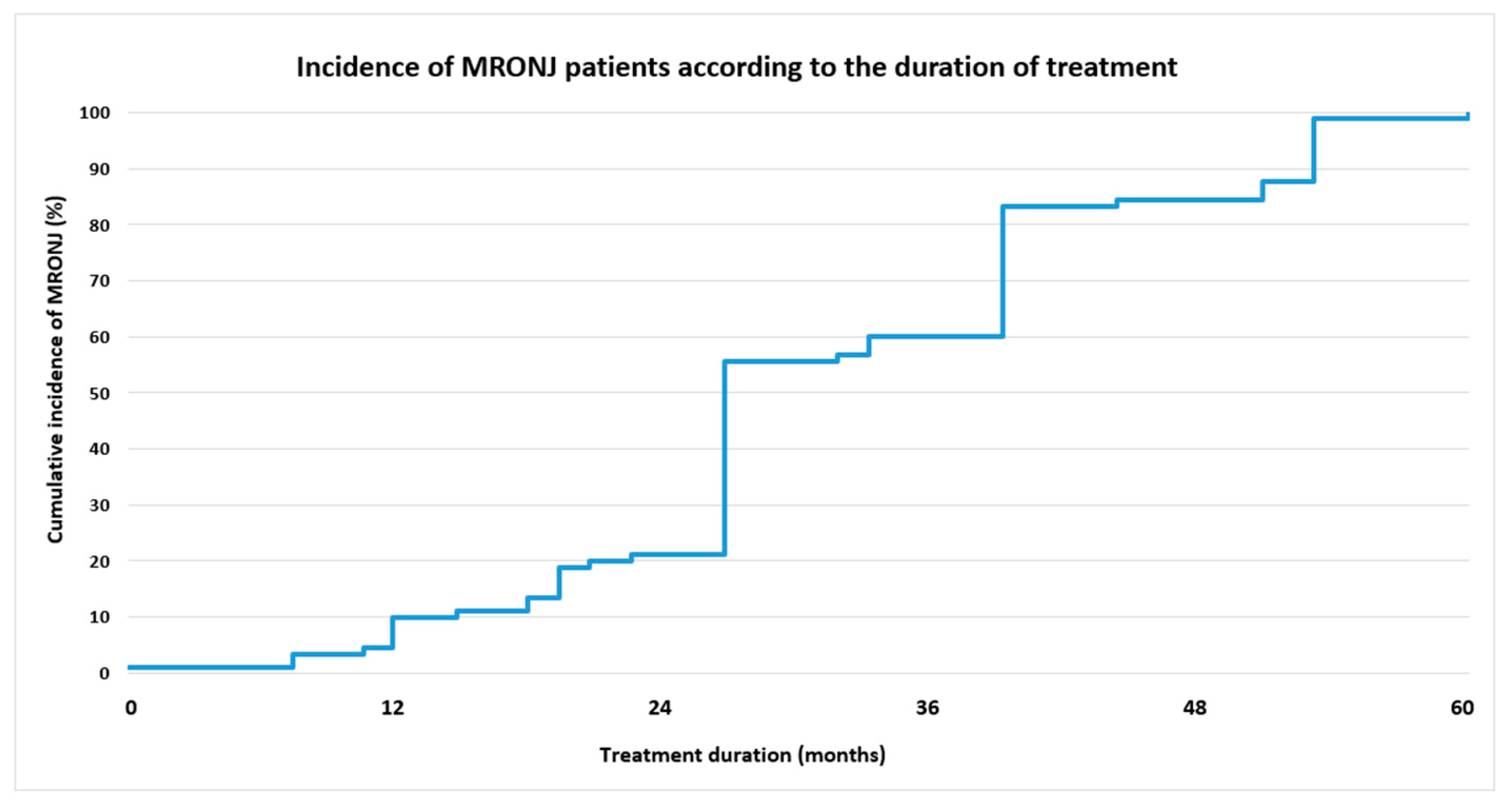
3.3. MRONJ Distribution Analysis
3.4. Binomial Logistic Regression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Chemotherapy Medication
| Therapy | Medication |
| CHEMOTHERAPY | DOCETAXEL |
| PEMETREXED (ALIMTA) | |
| PACLITAXEL | |
| NAVELBIN | |
| CYCLOPHOSPHAMIDE | |
| CARBOPLATIN | |
| ETOPOSID | |
| MELPHALAN (ALKERAN) | |
| GEMCITABINE (GEMZAR) | |
| DOXORUBICIN | |
| COMBINATION CHEMOTHERAPY | EC (EPIRUBICIN, CYCLOPHOSPHAMIDE) |
| PACLITAXEL + NAVELBINE | |
| DOXORUBICIN + CYCLOPHOSPHAMIDE | |
| ENDOCRINE THERAPY | GOSERELIN (ZOLADEX) |
| ABIRATERON | |
| LETROZOLE | |
| RIBOCICLIB | |
| FULVESTRANT | |
| LEUPROLIDE ACETATE (ELIGARD) | |
| TAMOXIFEN | |
| BICALUTAMIDE | |
| MOLECULAR-TARGETED THERAPY | PALBOCICLIB |
| BORTEZOMIB | |
| ABEMACICLIB | |
| PERTUZUMAB | |
| TRASTUZUMAB(HERCEPTIN) | |
| OSIMERTINIB | |
| SUNITINIB (SUTENT) | |
| CABOZANTINIB | |
| COMBINATION CHEMOTHERAPY/HORMONOTHERAPY AND TARGETED MOLECULAR THERAPY | ATEZOLIZUMAB + ETOPOSID + CRB |
| CYBORD (CYCLOPHOSPHAMIDE + BORTEZOMIB + DEXAMETHASONE) | |
| TAMOXIFEN + ABEMACICLIB + FULVESTRANT | |
| PALBOCICLIB + FULVESTRANT | |
| PALBOCICLIB + LETROZOLE | |
| AHT | ATEZOLIZUMAB |
| PEMBROLIZUMAB |
References
- Advisory Task Force on Bisphosphonate-Related Ostenonecrosis of the Jaws. American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws. J. Oral Maxillofac. Surg. 2007, 65, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Dodson, T.B.; Assael, L.A.; Landesberg, R.; Marx, R.E.; Mehrotra, B.; American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaws—2009 update. J. Oral Maxillofac. Surg. 2009, 67 (Suppl. S5), 2–12. [Google Scholar] [PubMed]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F.; American Association of Oral and Maxillofacial Surgeons. American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw—2014 update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956, Erratum in: J. Oral Maxillofac. Surg. 2015, 73, 1440. Erratum in: J. Oral Maxillofac. Surg. 2015, 73, 1879. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American Association of Oral and Maxillofacial Surgeons’ Position Paper on Medication-Related Osteonecrosis of the Jaws—2022 Update. J. Oral Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [CrossRef]
- Marx, R.E. Drug-Induced Osteonecrosis of the Jaws—How to Diagnose, Prevent, and Treat It; Quintessence Publishing: Batavia, IL, USA, 2022. [Google Scholar]
- Campisi, G.; Bedogni, A.; Fusco, V. Raccomandazioni Clinico-Terapeutiche Sull’Osteonecrosi delle Ossa Mascellari (ONJ) Farmaco-Relata e Sua Prevenzione; Palermo University Press: Palermo, Italy, 2020. [Google Scholar]
- Campisi, G.; Mauceri, R.; Bertoldo, F.; Bettini, G.; Biasotto, M.; Colella, G.; Consolo, U.; Di Fede, O.; Favia, G.; Fusco, V.; et al. Medication-Related Osteonecrosis of Jaws (MRONJ) Prevention and Diagnosis: Italian Consensus Update 2020. Int. J. Environ. Res. Public Health 2020, 17, 5998. [Google Scholar] [CrossRef]
- Anastasilakis, A.D.; Pepe, J.; Napoli, N.; Palermo, A.; Magopoulos, C.; Khan, A.A.; Zillikens, M.C.; Body, J.J. Osteonecrosis of the Jaw and Antiresorptive Agents in Benign and Malignant Diseases: A Critical Review Organized by the ECTS. J. Clin. Endocrinol. Metab. 2022, 107, 1441–1460. [Google Scholar] [CrossRef]
- Fleisch, H.; Russell, R.G.; Straumann, F. Effect of pyrophosphate on hydroxyapatite and its implications in calcium homeostasis. Nature 1966, 212, 901–903. [Google Scholar] [CrossRef]
- Clarke, B.L.; Khosla, S. Bisphosphonates: Mechanism of action and role in clinical practice. Mayo Clin. Proc. 2008, 83, 1032–1045. [Google Scholar]
- Farrell, K.B.; Karpeisky, A.; Thamm, D.H.; Zinnen, S. Bisphosphonate conjugation for bone specific drug targeting. Bone Rep. 2018, 3, 47–60. [Google Scholar] [CrossRef]
- Miller, K.; Steger, G.G.; Niepel, D.; Lüftner, D. Harnessing the potential of therapeutic agents to safeguard bone health in prostate cancer. Prostate Cancer Prostatic Dis. 2018, 21, 461–472. [Google Scholar] [CrossRef]
- Otto, S.; Pautke, C.; Van den Wyngaert, T.; Niepel, D.; Schiødt, M. Medication-related osteonecrosis of the jaw: Prevention, diagnosis and management in patients with cancer and bone metastases. Cancer Treat. Rev. 2018, 69, 177–187. [Google Scholar] [CrossRef]
- Voss, P.J.; Poxleitner, P.; Schmelzeisen, R.; Stricker, A.; Semper-Hogg, W. Update MRONJ and perspectives of its treatment. J. Stomatol. Oral Maxillofac. Surg. 2017, 118, 232–235. [Google Scholar] [CrossRef]
- InoMed-IMAS. Percepții și Atitudini cu Privire la Prevenirea, Diagnosticarea și Tratarea Cancerului Asociația Centrul pentru Inovație în Medicină. 2020. Available online: www.imas-inc.com (accessed on 2 April 2023).
- InoMed. A New Vision for Cancer in European Union. Data, Technology and Human Touch Position Paper Published by Centre for Innovation in Medicine in the Context of Romanian Presidency of Council of European Union. 2019. Available online: https://www.who.int/cancer/PRGlobocanFinal.pdf (accessed on 2 April 2023).
- Yang, L.; Du, S. Efficacy and Safety of Zoledronic Acid and Pamidronate Disodium in the Treatment of Malignant Skeletal Metastasis: A Meta-Analysis. Medicine 2015, 94, e1822. [Google Scholar] [CrossRef]
- Henry, D.; Vadhan-Raj, S.; Hirsh, V.; Von Moos, R.; Hungria, V.; Costa, L.; Woll, P.J.; Scagliotti, G.; Smith, G.; Feng, A.; et al. Delaying skeletal-related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: An analysis of data from patients with solid tumors. Support. Care Cancer 2014, 22, 679–687. [Google Scholar] [CrossRef]
- Limones, A.; Sáez-Alcaide, L.M.; Díaz-Parreño, S.A.; Helm, A.; Bornstein, M.M.; Molinero-Mourelle, P. Medication-related osteonecrosis of the jaws (MRONJ) in cancer patients treated with denosumab VS. zoledronic acid: A systematic review and meta-analysis. Med. Oral Patol. Oral Cir. Bucal 2020, 25, e326–e336. [Google Scholar] [CrossRef]
- Ng, T.L.; Tu, M.M.; Ibrahim, M.F.K.; Basulaiman, B.; McGee, S.F.; Srikanthan, A.; Fernandes, R.; Vandermeer, L.; Stober, C.; Sienkiewicz, M.; et al. Long-term impact of bone-modifying agents for the treatment of bone metastases: A systematic review. Support. Care Cancer 2021, 29, 925–943. [Google Scholar] [CrossRef]
- Van Poznak, C.H.; Unger, J.M.; Darke, A.K.; Moinpour, C.; Bagramian, R.A.; Schubert, M.M.; Hansen, L.K.; Floyd, J.D.; Dakhil, S.R.; Lew, D.L.; et al. Association of Osteonecrosis of the Jaw with Zoledronic Acid Treatment for Bone Metastases in Patients with Cancer. JAMA Oncol. 2021, 7, 246–254. [Google Scholar] [CrossRef]
- Hallmer, F.; Andersson, G.; Götrick, B.; Warfvinge, G.; Anderud, J.; Bjørnland, T. Prevalence, initiating factor, and treatment outcome of medication-related osteonecrosis of the jaw-a 4-year prospective study. Oral Surg Oral Med. Oral Pathol. Oral Radiol. 2018, 126, 477–485. [Google Scholar] [CrossRef]
- Jeong, H.G.; Hwang, J.J.; Lee, J.H.; Kim, Y.H.; Na, J.Y.; Han, S.S. Risk factors of osteonecrosis of the jaw after tooth extraction in osteoporotic patients on oral bisphosphonates. Imaging Sci. Dent. 2017, 47, 45. [Google Scholar] [CrossRef]
- Kuroshima, S.; Sasaki, M.; Sawase, T. Medication-related osteonecrosis of the jaw: A literature review. J. Oral Biosci. 2019, 61, 99–104. [Google Scholar] [CrossRef]
- AlRowis, R.; Aldawood, A.; AlOtaibi, M.; Alnasser, E.; AlSaif, I.; Aljaber, A.; Natto, Z. Medication-Related Osteonecrosis of the Jaw (MRONJ): A Review of Pathophysiology, Risk Factors, Preventive Measures and Treatment Strategies. Saudi Dent. J. 2022, 34, 202–210. [Google Scholar] [CrossRef] [PubMed]
- McGowan, K.; McGowan, T.; Ivanovski, S. Risk factors for medication-related osteonecrosis of the jaws: A systematic review. Oral Dis. 2018, 24, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Marcianò, A.; Ingrasciotta, Y.; Isgrò, V.; L’Abbate, L.; Foti, S.S.; Picone, A.; Peditto, M.; Guzzo, G.M.; Alibrandi, A.; Oteri, G. Cancer Patients at Risk for Medication-Related Osteonecrosis of the Jaw. A Case and Control Study Analyzing Predictors of MRONJ Onset. J. Clin. Med. 2021, 10, 4762. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.A.M.; Nielsen, C.E.N.; Schiodt, M. Medication related osteonecrosis of the jaws associated with targeted therapy as monotherapy and in combination with antiresorptives. A report of 7 cases from the Copenhagen Cohort. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2018, 125, 157–163. [Google Scholar] [CrossRef]
- Nicolatou-Galitis, O.; Schiødt, M.; Mendes, R.A.; Ripamonti, C.; Hope, S.; Drudge-Coates, L.; Niepel, D.; Van den Wyngaert, T. Medication-related osteonecrosis of the jaw: Definition and best practice for prevention, diagnosis, and treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 117–135. [Google Scholar] [CrossRef]
- Hasegawa, T.; Hayashida, S.; Kondo, E.; Takeda, Y.; Miyamoto, H.; Kawaoka, Y.; Ueda, N.; Iwata, E.; Nakahara, H.; Kobayashi, M.; et al. Medication-related osteonecrosis of the jaw after tooth extraction in cancer patients: A multicenter retrospective study. Osteoporos. Int. 2019, 30, 231–239. [Google Scholar] [CrossRef]
- Veszelyné Kotán, E.; Bartha-Lieb, T.; Parisek, Z.; Meskó, A.; Vaszilkó, M.; Hankó, B. Database analysis of the risk factors of bisphosphonate-related osteonecrosis of the jaw in Hungarian patients. BMJ Open 2019, 9, e025600. [Google Scholar] [CrossRef]
- Ishimaru, M.; Ono, S.; Morita, K.; Matsui, H.; Hagiwara, Y.; Yasunaga, H. Prevalence, Incidence Rate, and Risk Factors of Medication-Related Osteonecrosis of the Jaw in Patients with Osteoporosis and Cancer: A Nationwide Population-Based Study in Japan. J. Oral Maxillofac. Surg. 2022, 80, 714–727. [Google Scholar] [CrossRef]
- Kim, J.Y.; Song, H.C.; Jee, H.G. Refractory healing after surgical therapy of osteonecrosis of the jaw: Associated risk factors in aged patients. Clin. Interv. Aging 2019, 14, 797–804. [Google Scholar] [CrossRef]
- Sánchez-Gallego Albertos, C.; Del Castillo Pardo de Vera, J.L.; Viejo Llorente, A.; Cebrián Carretero, J.L. Medication related osteonecrosis of the jaws (MRONJ): Factors related to recurrence after treatment with surgery and platelet rich plasma (PRP) placement. Med. Oral Patol. Oral Cir. Bucal 2021, 26, e684–e690. [Google Scholar] [CrossRef]
- Mirelli, C.; Marino, S.; Bovio, A.; Pederielli, S.; Dall’Agnola, C.; Gianni, A.B.; Biagi, R. Medication-Related Osteonecrosis of the Jaw in Dental Practice: A Retrospective Analysis of Data from the Milan Cohort. Dent. J. 2022, 10, 89. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Wróbel, K.; Sikora, M.; Chęciński, M.; Jas, M.; Chlubek, D. Medication-Related Osteonecrosis of the Jaw—A Continuing Issue. Appl. Sci. 2021, 11, 7781. [Google Scholar] [CrossRef]
- Ikesue, H.; Doi, K.; Morimoto, M.; Hirabatake, M.; Muroi, N.; Yamamoto, S.; Takenobu, T.; Hashida, T. Risk evaluation of denosumab and zoledronic acid for medication-related osteonecrosis of the jaw in patients with bone metastases: A propensity score-matched analysis. Support. Care Cancer 2022, 30, 2341–2348. [Google Scholar] [CrossRef]
- Kemp, A.P.T.; Ferreira, V.H.C.; Mobile, R.Z.; Brandão, T.B.; Sassi, L.M.; Zarpellon, A.; Braz-Silva, P.H.; Schussel, J.L. Risk factors for medication-related osteonecrosis of the jaw and salivary IL-6 IN cancer patients. Braz. J. Otorhinolaryngol. 2022, 88, 683–690. [Google Scholar] [CrossRef]
- Gómez-Salgado, J.; Fagundo-Rivera, J.; Ortega-Moreno, M.; Allande-Cussó, R.; Ayuso-Murillo, D.; Ruiz-Frutos, C. Night Work and Breast Cancer Risk in Nurses: Multif Multifactorial Risk Analysis. Cancers 2021, 13, 1470. [Google Scholar] [CrossRef]
- Arnaud, M.P.; Talibi, S.; Lejeune-Cairon, S. Knowledge and attitudes of French dentists on bone resorption inhibitors (bisphosphonates and denosumab): A cross-sectional study. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, 163–170. [Google Scholar] [CrossRef]
- Rosendahl Huber, A.; Van Hoeck, A.; Van Boxtel, R. The Mutagenic Impact of Environmental Exposures in Human Cells and Cancer: Imprints Through Time. Front. Genet. 2021, 12, 760039. [Google Scholar] [CrossRef]
- Linee Guida AIOM (Associazione Italiana Oncologia Medica) sul Trattamento Delle Metastasi Ossee. 2019. Available online: https://www.aiom.it/linee-guida-aiom-trattamento-delle-metastasi-ossee-2019/ (accessed on 2 April 2023).
- The Economist. Country Profile: Cancer Medicine Shortages. Available online: https://www.economist.com/ (accessed on 2 April 2023).
- State of Health in the EU. România. Profilul de Țară din 2019 în Ceea ce Privește Sănătatea. Available online: https://ec.europa.eu/health/sites/health/files/state/docs/2019_chp_romania_romanian.pdf (accessed on 29 January 2023).
- Raportul Național al Stării de Sănătate al Populației. 2019; pp. 125–131. Available online: https://insp.gov.ro/wpfb-file/raport-starea-de-sanatate-2019-pdf/ (accessed on 2 April 2023).
- Centrul Național de Statistică și Informatică în Sănătate Publică (CNSISP), Mortalitatea Generală, 2019; pp. 10–12. Available online: https://cnsisp.insp.gov.ro/wp-content/uploads/2021/01/MORTALITATEA-GENERALA-2019.pdf (accessed on 2 April 2023).
- Petrescu, O.; Surlin, V.; Munteanu, C.; Camen, A.; Petrescu, G.S.; Andrei, E.; Ciobanu, A. Incidence of osteonecrosis of the jaw due to bisphosphonate treatment in the city of Craiova. Arch. Euromedica 2020, 10, 12–20. [Google Scholar]
- Rodriguez-Archilla, A.; Rodriguez-Soriano, I. Predictive risk factors of medication-related osteonecrosis of the jaw. Int. Dent. Med. J. Adv. Res. 2019, 5, 1–6. [Google Scholar]
- Ciobanu, G.A.; Camen, A.; Ionescu, M.; Vlad, D.; Mercuț, V.; Staicu, I.E.; Petrescu, G.S.; Asan, A.A.; Popescu, S.M. Biphosphonates related osteonecrosis of the jaw in cancer patients—Epidemiological study. Rom. J. Oral Rehabil. 2022, 14, 56–66. [Google Scholar]
- Romania: Breast Cancer. Available online: https://www.worldlifeexpectancy.com/romania-breast-cancer (accessed on 2 April 2023).
- Insider. (2019). Statistics: 140 Romanians Die of Cancer Daily, Disease Is Diagnosed Late Romania Insider. Available online: https://www.romania-insider.com/140-romanians-die-of-cancer-daily (accessed on 2 April 2023).
- Hata, H.; Imamachi, K.; Ueda, M.; Matsuzaka, M.; Hiraga, H.; Osanai, T.; Harabayashi, T.; Fujimoto, K.; Oizumi, S.; Takahashi, M.; et al. Prognosis by cancer type and incidence of zoledronic acid-related osteonecrosis of the jaw: A single-center retrospective study. Support. Care Cancer 2022, 30, 4505–4514. [Google Scholar] [CrossRef] [PubMed]
- European Commisssion, Eurostat Database. Available online: http://ec.europa.eu/eurostat/data/database (accessed on 2 April 2023).
- Gaiţă, D.; Moşteoru, S. Cardiometabolic prevention in Romania: A midsummer night’s dream? Cardiovasc. Endocrinol. 2017, 6, 45–47. [Google Scholar] [CrossRef] [PubMed]
- Gherbon, A.M.C.; Gherbon, A.; Frandes, M.; Bercea, V.; Timar, B.; Timar, R. IDF21-0033 Risk factors of cardiovascular disease in Romanian elderly patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2022, 186 (Suppl. S1), 109241. [Google Scholar] [CrossRef]
- Pop, C.; Gheorghe Fronea, O.F.; Branea, I.A.; Itu, L.M.; Darabont, R.; Parepa, I.; Benedek, T.; Dorobantu, M. Prevalence and Predictors of Renal Disease in a National Representative Sample of the Romanian Adult Population: Data from the SEPHAR IV Survey. Diagnostics 2022, 12, 3199. [Google Scholar] [CrossRef]
- Berbec, N.M.; Stanculeanu, D.L.; Badelita, N.S.; Vasilica, M.; Popovici, D.I.; Colita, A.; Neacsu, C.; Iordan, A. APPLY: A prospective observational study of clinical practice patterns of darbepoetin alfa use in patients with chemotherapy-induced anemia in Romania. Memo 2018, 11, 144–151. [Google Scholar] [CrossRef]
- Kawahara, M.; Kuroshima, S.; Sawase, T. Clinical considerations for medication-related osteonecrosis of the jaw: A comprehensive literature review. Int. J. Implant Dent. 2021, 7, 47. [Google Scholar] [CrossRef]
- Popa, A.E. The national cancer programme in Romania: Challenges in a low-resource healthcare system. J. Cancer Policy 2017, 14, 33–38. [Google Scholar] [CrossRef]
- Feng, Z.; An, J.; Zhang, Y. Factors Influencing Severity of Medication-Related Osteonecrosis of the Jaw: A Retrospective Study. J. Oral Maxillofac. Surg. 2021, 79, 1683–1688. [Google Scholar] [CrossRef]
- Chen, S.; Ren, H.; He, Y.; An, J.; Zhang, Y. Recurrence-Related Factors of Medication-Related Osteonecrosis of the Jaw: A Five-Year Experience. J. Oral Maxillofac. Surg. 2021, 79, 2472–2481. [Google Scholar] [CrossRef]
- Coropciuc, R.; Coopman, R.; Garip, M.; Gielen, E.; Politis, C.; Van den Wyngaert, T.; Beuselinck, B. Risk of medication-related osteonecrosis of the jaw after dental extractions in patients receiving antiresorptive agents—A retrospective study of 240 patients. Bone 2023, 170, 116722. [Google Scholar] [CrossRef]
- Loyson, T.; Van Cann, T.; Schöffski, P.; Clement, P.M.; Bechter, O.; Spriet, I.; Coropciuc, R.; Politis, C.; Vandeweyer, R.O.; Schoenaers, J.; et al. Incidence of osteonecrosis of the jaw in patients with bone metastases treated sequentially with bisphosphonates and denosumab. Acta Clin. Belg. 2018, 73, 100–109. [Google Scholar] [CrossRef]
- Wick, A.; Bankosegger, P.; Otto, S.; Hohlweg-Majert, B.; Steiner, T.; Probst, F.; Ristow, O.; Pautke, C. Risk factors associated with onset of medication-related osteonecrosis of the jaw in patients treated with denosumab. Clin. Oral Investig. 2022, 26, 2839–2852. [Google Scholar] [CrossRef]
- Aguirre, J.I.; Castillo, E.J.; Kimmel, D.B. Biologic and pathologic aspects of osteocytes in the setting of medication-related osteonecrosis of the jaw (MRONJ). Bone 2021, 153, 116168. [Google Scholar] [CrossRef]
- AlDhalaan, N.A.; BaQais, A.; Al-Omar, A. Medication-related Osteonecrosis of the Jaw: A Review. Cureus 2020, 12, e6944. [Google Scholar] [CrossRef]
- Lončar Brzak, B.; Horvat Aleksijević, L.; Vindiš, E.; Kordić, I.; Granić, M.; Vidović Juras, D.; Andabak Rogulj, A. Osteonecrosis of the Jaw. Dent. J. 2023, 11, 23. [Google Scholar] [CrossRef]
- Sacco, R.; Umar, G.; Guerra, R.C.; Akintola, O. Evaluation of segmental mandibular resection without microvascular reconstruction in patients affected by medication-related osteonecrosis of the jaw: A systematic review. Br J. Oral Maxillofac. Surg. 2021, 59, 648–660. [Google Scholar] [CrossRef]
- Aljohani, S.; Troeltzsch, M.; Hafner, S.; Kaeppler, G.; Mast, G.; Otto, S. Surgical treatment of medication-related osteonecrosis of the upper jaw: Case series. Oral Dis. 2019, 25, 497–507. [Google Scholar] [CrossRef]
- Ghidul de Practică în Medicina Dentară: Algoritmul Terapeutic la Pacienții sub Terapie Antiresorbtivă/Antiangiogenică. Available online: https://cmdr.ro/document/ghid-de-practica-algoritmul-terapeutic-la-pacientii-sub-terapie-antiresorbtiva_antiangiogenica/ (accessed on 2 April 2023).
- Ciobanu, G.A.; Gheorghiţă, M.I.; Petrescu, O.M.; Popescu, S.M.; Staicu, I.E. Mandibulectomy Reconstruction with Pectoralis Major Island Flap Associated with Primary Reconstruction Plate for Mandibular Medication-Related Osteonecrosis. Curr. Health Sci. J. 2021, 47, 117–122. [Google Scholar]
- Kün-Darbois, J.D.; Fauvel, F. Medication-related osteonecrosis and osteoradionecrosis of the jaws: Update and current management. Morphologie 2021, 105, 170–187. [Google Scholar] [CrossRef]
- Favia, G.; Tempesta, A.; Limongelli, L.; Crincoli, V.; Maiorano, E. Medication-related osteonecrosis of the jaw: Surgical or non-surgical treatment? Oral Dis. 2018, 24, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, K.; Hayashida, S.; Rokutanda, S.; Kawakita, A.; Soutome, S.; Sawada, S.; Yanamoto, S.; Kojima, Y.; Umeda, M. Surgical strategy for medication-related osteonecrosis of the jaw (MRONJ) on maxilla: A multicenter retrospective study. J. Dent. Sci. 2021, 16, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.; Aljohani, S.; Fliefel, R.; Ecke, S.; Ristow, O.; Burian, E.; Troeltzsch, M.; Pautke, C.; Ehrenfeld, M. Infection as an Important Factor in Medication-Related Osteonecrosis of the Jaw (MRONJ). Medicina 2021, 57, 463. [Google Scholar] [CrossRef] [PubMed]
| Category | Parameter | Value | N (%)/Mean ± SD/Interval |
|---|---|---|---|
| Demographic factors | Gender | Female | 109 (62.6%) |
| Male | 65 (37.4%) | ||
| Age (years old) | 22–54 | 30 (17.4%)/46.9 ± 6.5 | |
| 55–64 | 46 (26.3%)/60.3 ± 2.7 | ||
| 65–71 | 50 (28.7%)/67.8 ± 2.1 | ||
| 72–84 | 48 (27.6%)/76.6 ± 3.7 | ||
| Medical center | Craiova | 73 (41.9%) | |
| Constanta | 101 (58.1%) | ||
| Residency | Urban | 125 (71.8%) | |
| Rural | 49 (28.2%) | ||
| Smoking status | No | 100 (57.5%) | |
| Yes | 74 (42.5%) | ||
| Cancer type | Primary diagnostic (neoplasm) | Breast | 82 (47.2%) |
| Prostate | 46 (26.4%) | ||
| Other neoplasms | 46 (26.4%) | ||
| Pulmonary | 14 (30.4%) | ||
| Myeloma | 9 (19.6%) | ||
| Genital | 7 (15.2%) | ||
| Digestive | 6 (13.0%) | ||
| Renal | 5 (10.8%) | ||
| Cerebral | 1 (2.2%) | ||
| Bladder | 1 (2.2%) | ||
| Spinal cord | 1 (2.2%) | ||
| Pharynx | 1 (2.2%) | ||
| Thyroid | 1 (2.2%) | ||
| Metastasis | Bone metastasis | No | 20 (11.5%) |
| Yes | 154 (88.5%) | ||
| Cancer therapy | Chemotherapy | No | 23 (13.2%) |
| Yes | 151 (86.8%) | ||
| Endocrine therapy | No | 138 (79.3%) | |
| Yes | 36 (20.7%) | ||
| Immunotherapy | No | 172 (98.8%) | |
| Yes | 2 (1.2%) | ||
| Radiotherapy | No | 106 (60.9%) | |
| Yes | 68 (39.1%) | ||
| Corticotherapy | No | 164 (94.2%) | |
| Yes | 10 (5.8%) | ||
| BF | BF type | Zoledronic acid | 174 (100%) |
| BF | IV Zoledronic acid | 169 (97.1%) | |
| IV Zoledronic acid administered after a treatment with oral BF | 5 (2.9%) | ||
| Treatment duration (months) | <12 | 52 (29.9%)/8.2 ± 3.2 | |
| 13–24 | 60 (34.5%)/21.0 ± 3.8 | ||
| 25–36 | 34 (19.5%)/34.1 ± 3.1 | ||
| >36 | 28 (16.1%)/50.3 ± 12.1 | ||
| Comorbidities | Cardiovascular diseases | No | 93 (53.5%) |
| Yes | 81 (46.5%) | ||
| Hypertension | No | 110 (63.2%) | |
| Yes | 64 (36.8%) | ||
| Nutritional diseases | No | 118 (67.8%) | |
| Yes | 56 (32.2%) | ||
| Diabetes mellitus | No | 156 (89.6%) | |
| Yes | 18 (10.4%) | ||
| Obesity | No | 158 (90.8%) | |
| Yes | 16 (9.2%) | ||
| Anemia | No | 156 (89.6%) | |
| Yes | 18 (10.4%) | ||
| Renal diseases | No | 143 (82.2%) | |
| Yes | 31 (17.8%) |
| Comorbidities | Total N | Age Groups/Gender—Number of Patients (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 22–54 | 55–64 | 65–71 | 72–84 | ||||||
| F | M | F | M | F | M | F | M | ||
| Cardiovasc. diseases | 81 | 4 (4.9%) | 4 (4.9%) | 11 (13.6%) | 7 (8.6%) | 18 (22.2%) | 10 (12.4%) | 17 (21.0%) | 10 (12.4%) |
| Hypertension | 64 | - | 2 (3.1%) | 10 (15.6%) | 6 (9.4%) | 15 (23.4%) | 8 (12.5%) | 14 (21.9%) | 9 (14.1%) |
| Nutritional diseases | 56 | 7 (12.5%) | 2 (3.6%) | 10 (17.9%) | 5 (8.8%) | 10 (17.9%) | 7 (12.5%) | 7 (12.5%) | 8 (14.3%) |
| Diabetes mellitus | 18 | - | 1 (5.6%) | 2 (11.1%) | 4 (22.2%) | 2 (11.1%) | 2 (11.1%) | 4 (22.2%) | 3 (16.7%) |
| Obesity | 16 | 3 (18.7%) | - | 3 (18.7%) | 1 (6.3%) | 5 (31.3%) | - | 1 (6.3%) | 3 (18.7%) |
| Renal diseases | 31 | 1 (3.2%) | 1 (3.2%) | 3 (9.7%) | 1 (3.2%) | 1 (3.2%) | 7 (22.6%) | 6 (19.4%) | 11 (35.5%) |
| Anemia | 18 | 3 (16.7%) | 1 (5.6%) | 3 (16.7%) | - | 1 (5.6%) | 4 (22.2%) | 4 (22.2%) | 2 (11.0%) |
| Total | 18 | 11 | 42 | 24 | 52 | 38 | 53 | 36 | |
| Therapy | Total N | Age Groups/Gender—Number of Patients (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 22–54 | 55–64 | 65–71 | 72–84 | ||||||
| F | M | F | M | F | M | F | M | ||
| Chemotherapy | 151 | 22 (14.6%) | 4 (2.6%) | 32 (21.2%) | 13 (8.6%) | 25 (16.6%) | 18 (11.9%) | 22 (14.6%) | 15 (9.9%) |
| Endocrine therapy | 36 | 5 (13.8%) | 1 (2.8%) | 6 (16.7%) | 4 (11.1%) | - | 6 (16.7%) | 1 (2.8%) | 13 (36.1%) |
| Immuno-therapy | 2 | - | - | - | - | 2 (100%) | - | - | - |
| Radiotherapy | 68 | 10 (14.7%) | 2 (2.9%) | 13 (19.2%) | 3 (4.4%) | 8 (11.8%) | 11 (16.2%) | 9 (13.2%) | 12 (17.6%) |
| Cortico-therapy | 10 | 3 (30. 0%) | - | 3 (30.0%) | 1 (10.0%) | 1 (10.0%) | 1 (10. 0%) | - | 1 (10.0%) |
| Total | 33 | 7 | 38 | 18 | 35 | 33 | 56 | 47 | |
| Medical Center/Neoplasm | Overall Mean ± SD | Age Groups—Years Old (Mean ± SD) | |||
|---|---|---|---|---|---|
| 22–54 Years Old | 55–64 Years Old | 65–71 Years Old | 72–84 Years Old | ||
| Craiova | 25.3 ± 17.7 | 15.8 ± 9.3 | 30.4 ± 20.9 | 23.6 ± 14.5 | 26.3 ± 18.9 |
| Constanta | 23.8 ± 14.4 | 18.4 ± 14.1 | 26.3 ± 13.7 | 25.2 ± 13.7 | 23.8 ± 15.9 |
| Breast | 24.7 ± 15.7 | 18.4 ± 12.5 | 30.5 ± 20.6 | 21.8 ± 9.6 | 28.4 ± 13.7 |
| Prostate | 26.1 ± 16.5 | 11.0 ± 0.0 | 31.1 ± 10.5 | 26.6 ± 14.9 | 24.2 ± 19.6 |
| Others | 22.2 ± 15.6 | 16.0 ± 14.2 | 20.4 ± 11.5 | 26.2 ± 17.5 | 22.1 ± 17.2 |
| Mean ± SD | 24.4 ± 15.8 | 17.6 ± 12.5 | 28.2 ± 17.2 | 24.6 ± 13.9 | 25.0 ± 17.2 |
| Category | Parameter | Value | N (%)/Mean ± SD | p | |
|---|---|---|---|---|---|
| Non-MRONJ | MRONJ | ||||
| Demographic factors | Gender | Female | 51 (60.7%) | 58 (64.4%) | 0.611 * |
| Male | 33 (39.3%) | 32 (35.6%) | |||
| Age (years old) | 22–54 | 20 (23.8%)/47.1 ± 7.6 | 10 (11.1%)/46.4 ± 3.9 | 0.173 * | |
| 55–64 | 20 (23.8%)/60.3 ± 3.0 | 26 (28.9%)/60.2 ± 2.5 | |||
| 65–71 | 23 (27.4%)/68.4 ± 2.1 | 27 (30.0%)/67.3 ± 2.1 | |||
| 72–84 | 21 (25.0%)/77.4 ± 4.1 | 27 (30.0%)/76.0 ± 3.2 | |||
| Medical center | Craiova | 20 (23.8%) | 53 (58.9%) | <0.0005 * | |
| Constanta | 64 (76.2%) | 37 (41.1%) | |||
| Residency | Urban | 64 (76.2%) | 61 (67.8%) | 0.218 * | |
| Rural | 20 (23.8%) | 29 (32.2%) | |||
| Smoking status | No | 47 (56.0%) | 53 (58.9%) | 0.695 * | |
| Yes | 37 (44.0%) | 37 (41.1%) | |||
| Cancer type | Primary diagnostic (neoplasm) | Breast | 39 (46.4%) | 43 (47.8%) | 0.963 * |
| Prostate | 22 (26.2%) | 24 (26.7%) | |||
| Other neoplasms | 23 (27.4%) | 23 (25.5%) | |||
| Pulmonary | 11 (47.9%) | 3 (13.0%) | - | ||
| Myeloma | 7 (30.6%) | 2 (8.8%) | - | ||
| Genital | 0 (0.0%) | 7 (30.5%) | - | ||
| Digestive | 1 (4.3%) | 5 (21.7%) | - | ||
| Renal | 1 (4.3%) | 4 (17.4%) | - | ||
| Cerebral | 1 (4.3%) | 0 (0.0%) | - | ||
| Bladder | 1 (4.3%) | 0 (0.0%) | - | ||
| Spinal cord | 0 (0.0%) | 1 (4.3%) | - | ||
| Pharynx | 1 (4.3%) | 0 (0.0%) | - | ||
| Thyroid | 0 (0.0%) | 1 (4.3%) | - | ||
| Metastasis | Bone metastasis | No | 19 (22.6%) | 1 (1.1%) | <0.0005 * |
| Yes | 65 (77.4%) | 89 (98.9%) | |||
| Therapy | Chemotherapy | No | 18 (21.4%) | 5 (5.6%) | 0.002 * |
| Yes | 66 (78.6%) | 85 (94.4%) | |||
| Endocrine therapy | No | 57 (67.9%) | 81 (90.0%) | <0.0005 * | |
| Yes | 27 (32.1%) | 9 (10.0%) | |||
| Immunotherapy | No | 83 (98.8%) | 89 (98.9%) | 0.734 ** | |
| Yes | 1 (1.2%) | 1 (1.1%) | |||
| Radiotherapy | No | 51 (60.7%) | 55 (61.1%) | 0.957 * | |
| Yes | 33 (39.3%) | 35 (38.9%) | |||
| Corticotherapy | No | 74 (88.1%) | 90 (100%) | 0.001 ** | |
| Yes | 10 (11.9%) | 0 (0%) | |||
| BF | BF type | Zoledronic acid | 84 (100%) | 90 (100%) | - |
| BF | Only Zoledronic acid | 79 (94.0%) | 90 (100%) | 0.025 ** | |
| Oral BF followed by Zoledronic acid | 5 (6.0%) | 0 (0.0%) | |||
| Comorbidities | Cardiovascular diseases | No | 55 (65.5%) | 38 (42.2%) | 0.420 |
| Yes | 29 (34.5%) | 52 (57.8%) | |||
| Hypertension | No | 62 (73.8%) | 48 (53.3%) | 0.005 * | |
| Yes | 22 (26.2%) | 42 (46.7%) | |||
| Nutritional diseases | No | 54 (64.3%) | 64 (71.1%) | 0.336 | |
| Yes | 30 (35.7%) | 26 (28.9%) | |||
| Diabetes mellitus | No | 77 (91.7%) | 79 (87.8%) | 0.400 | |
| Yes | 7 (8.3%) | 11 (12.2%) | |||
| Obesity | No | 72 (85.7%) | 86 (95.6%) | 0.025 * | |
| Yes | 12 (14.3%) | 4 (4.4%) | |||
| Anemia | No | 67 (79.8%) | 89 (98.9%) | <0.0005 * | |
| Yes | 17 (20.2%) | 1 (1.1%) | |||
| Renal diseases | No | 67 (79.8%) | 76 (84.4%) | 0.420 | |
| Yes | 17 (20.2%) | 14 (15.6%) | |||
| Parameter | Value | N (%) | p | ||
|---|---|---|---|---|---|
| Overall | Craiova | Constanta | |||
| MRONJ stage | 2 | 71 (78.9%) | 43 (82.0%) | 28 (75.7%) | 0.604 * |
| 3 | 19 (21.1%) | 10 (18.0%) | 9 (24.3%) | ||
| Trigger factor | Periodontal disease | 13 (14.4%) | 10 (18.9%) | 3 (8.1%) | 0.351 * |
| Periapical disease | 26 (28.9%) | 15 (28.3%) | 11 (29.7%) | ||
| Extraction | 51 (56.7%) | 28 (52.8%) | 23 (62.2%) | ||
| Overall location | Upper jaw | 28 (31.2%) | 16 (30.2%) | 12 (32.4%) | 0.897 ** |
| Lower jaw | 58 (64.4%) | 35 (66.0%) | 23 (62.2%) | ||
| Both jaws | 4 (4.4%) | 2 (3.8%) | 2 (5.4%) | ||
| Maxillary | Anterior | 8 (25.0%) | 3 (16.7%) | 5 (35.7%) | - |
| Posterior | 24 (75.0%) | 15 (83.3%) | 9 (64.3%) | ||
| Mandible | Anterior | 13 (21.0%) | 8 (21.6%) | 5 (20.0%) | - |
| Posterior | 49 (79.0 %) | 29 (78.4%) | 20 (80.0%) | ||
| Denudated bone | No | 3 (3.3%) | 1 (1.9%) | 2 (5.4%) | 0.360 ** |
| Yes | 87 (96.7%) | 52 (98.1%) | 35 (94.6%) | ||
| Hypoesthesia | No | 74 (82.2%) | 44 (83.0%) | 30 (81.1%) | 1.000 * |
| Yes | 16 (17.8%) | 9 (17.0%) | 7 (18.9%) | ||
| Surgical treatment | No | 6 (6.7%) | 3 (5.7%) | 3 (8.1%) | 0.886 ** |
| Surgical intervention | 76 (84.4%) | 45 (84.9%) | 31 (83.8%) | ||
| Relapse followed by another surgical intervention | 8 (8.9%) | 5 (9.4%) | 3 (8.1%) | ||
| Resection | No | 69 (76.7%) | 36 (67.9%) | 33 (89.2%) | 0.023 * |
| Yes | 21 (23.3%) | 17 (32.1%) | 4 (10.8%) | ||
| Sequestrectomy and Curettage | No | 19 (21.1%) | 15 (28.3%) | 4 (10.8%) | 0.045 * |
| Yes | 71 (78.9%) | 38 (71.7%) | 33 (89.2%) | ||
| Parameter | Value (Months) | N (%)/Mean ± SD | Total | p | |
|---|---|---|---|---|---|
| Non-MRONJ | MRONJ | ||||
| Treatment duration | 1–12 | 43 (82.7%)/8.0 ± 3.0 | 9 (17.3%)/9.2 ± 4.3 | 52 (100%) | <0.0005 * |
| 13–24 | 19 (31.7%)/17.7 ± 3.7 | 41 (68.3%)/22.5 ± 2.8 | 60 (100%) | ||
| 25–36 | 9 (26.5%)/31.7 ± 3.7 | 25 (73.5%)/35.0 ± 2.3 | 34 (100%) | ||
| >36 | 13 (46.4%)/52.4 ± 19.9 | 15 (53.6%)/48.5 ± 6.3 | 28 (100%) | ||
| Parameter | p | Odds Ratio | 95% CI for Odds Ratio * | |
|---|---|---|---|---|
| Lower | Upper | |||
| Gender | 0.515 | 1.380 | 0.524 | 3.637 |
| Age | 0.572 | 1.012 | 0.972 | 1.053 |
| Smoking status | 0.754 | 1.148 | 0.484 | 2.724 |
| Duration of treatment | <0.005 | 1.701 | 1.319 | 2.194 |
| Chemotherapy | 0.007 | 7.529 | 1.744 | 32.507 |
| Endocrine therapy | 0.001 | 0.174 | 0.061 | 0.496 |
| Radiotherapy | 0.299 | 1.550 | 0.678 | 3.542 |
| DM | 0.660 | 1.350 | 0.354 | 5.145 |
| Obesity | 0.024 | 0.182 | 0.041 | 0.800 |
| Hypertension | 0.002 | 3.793 | 1.613 | 8.916 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciobanu, G.A.; Camen, A.; Ionescu, M.; Vlad, D.; Munteanu, C.M.; Gheorghiță, M.I.; Lungulescu, C.V.; Staicu, I.E.; Sin, E.C.; Chivu, L.; et al. Risk Factors for Medication-Related Osteonecrosis of the Jaw—A Binomial Analysis of Data of Cancer Patients from Craiova and Constanta Treated with Zoledronic Acid. J. Clin. Med. 2023, 12, 3747. https://doi.org/10.3390/jcm12113747
Ciobanu GA, Camen A, Ionescu M, Vlad D, Munteanu CM, Gheorghiță MI, Lungulescu CV, Staicu IE, Sin EC, Chivu L, et al. Risk Factors for Medication-Related Osteonecrosis of the Jaw—A Binomial Analysis of Data of Cancer Patients from Craiova and Constanta Treated with Zoledronic Acid. Journal of Clinical Medicine. 2023; 12(11):3747. https://doi.org/10.3390/jcm12113747
Chicago/Turabian StyleCiobanu, George Adrian, Adrian Camen, Mihaela Ionescu, Daniel Vlad, Cristina Maria Munteanu, Mircea Ionuț Gheorghiță, Cristian Virgil Lungulescu, Ionela Elisabeta Staicu, Elena Claudia Sin, Luminița Chivu, and et al. 2023. "Risk Factors for Medication-Related Osteonecrosis of the Jaw—A Binomial Analysis of Data of Cancer Patients from Craiova and Constanta Treated with Zoledronic Acid" Journal of Clinical Medicine 12, no. 11: 3747. https://doi.org/10.3390/jcm12113747
APA StyleCiobanu, G. A., Camen, A., Ionescu, M., Vlad, D., Munteanu, C. M., Gheorghiță, M. I., Lungulescu, C. V., Staicu, I. E., Sin, E. C., Chivu, L., Mercuț, R., & Popescu, S. M. (2023). Risk Factors for Medication-Related Osteonecrosis of the Jaw—A Binomial Analysis of Data of Cancer Patients from Craiova and Constanta Treated with Zoledronic Acid. Journal of Clinical Medicine, 12(11), 3747. https://doi.org/10.3390/jcm12113747






