Physical Function of RA patients Tapering Treatment—A Post Hoc Analysis of the Randomized Controlled RETRO Trial
Abstract
1. Introduction
2. Methods
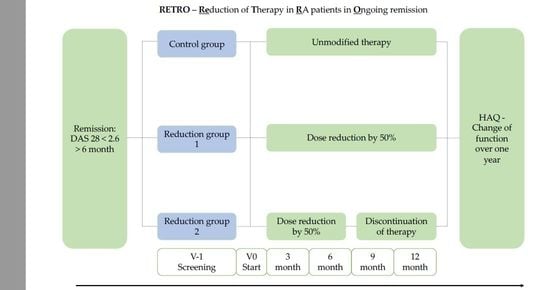
2.1. Study Design and Patients
2.2. Outcome
2.3. Statistical Analysis
3. Results
3.1. Patients
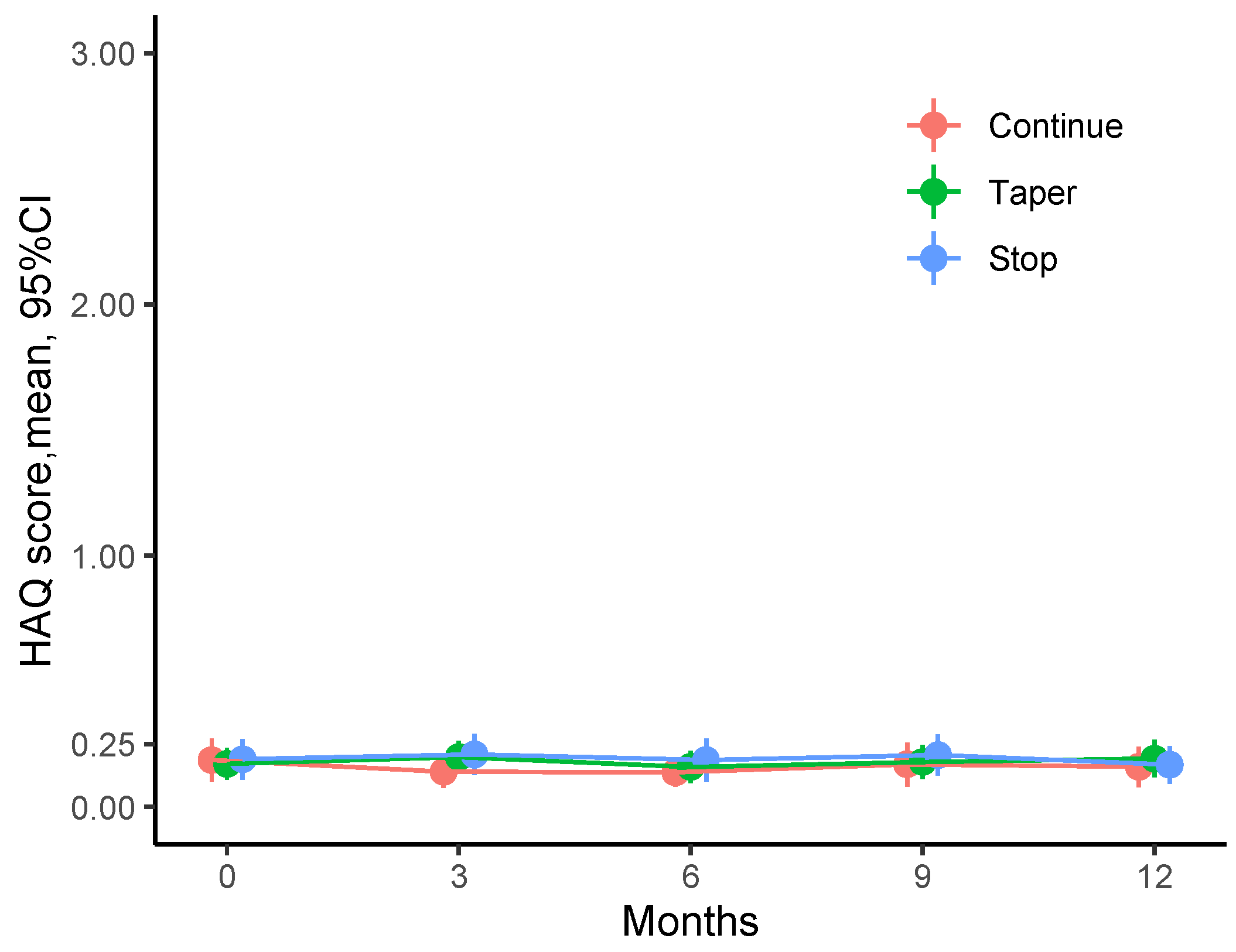
3.2. Physical Function over Time
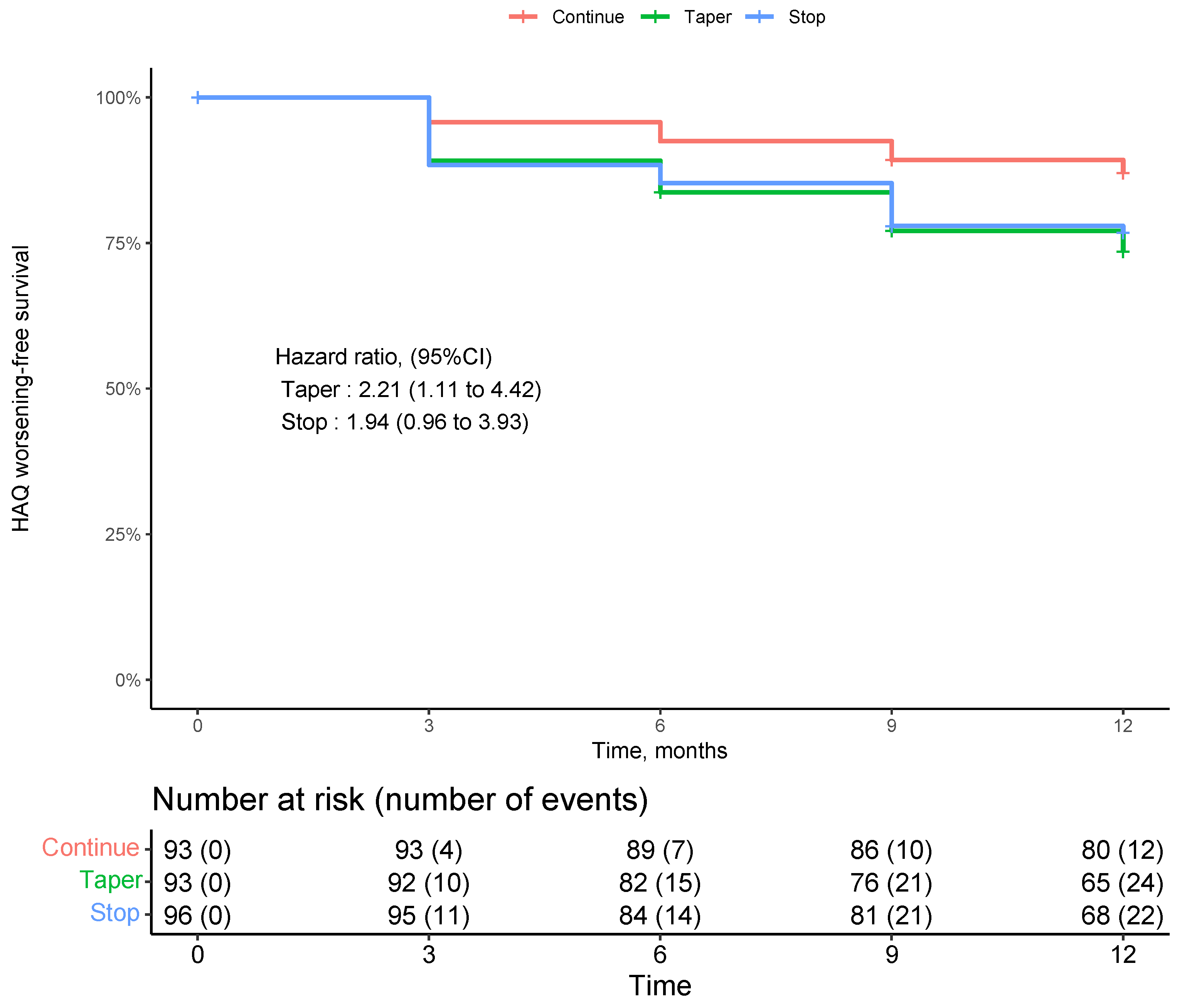
3.3. Incident Decline in Physical Function
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McInnes, I.B.; Schett, G. The Pathogenesis of Rheumatoid Arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid Arthritis Classification Criteria: An American College of Rheumatology/European League Against Rheumatism Collaborative Initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Tascilar, K.; Hagen, M.; Kleyer, A.; Simon, D.; Reiser, M.; Hueber, A.J.; Manger, B.; Englbrecht, M.; Finzel, S.; Tony, H.-P.; et al. Treatment Tapering and Stopping in Patients with Rheumatoid Arthritis in Stable Remission (RETRO): A Multicentre, Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet Rheumatol. 2021, 3, e767–e777. [Google Scholar] [CrossRef]
- Hagen, M.; Englbrecht, M.; Haschka, J.; Reiser, M.; Kleyer, A.; Hueber, A.; Manger, B.; Figueiredo, C.; Cobra, J.F.; Tony, H.-P.; et al. Cost-Effective Tapering Algorithm in Patients with Rheumatoid Arthritis: Combination of Multibiomarker Disease Activity Score and Autoantibody Status. J. Rheumatol. 2019, 46, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, L.M.; Tweehuysen, L.; Hulscher, M.E.; Fautrel, B.; den Broeder, A.A. BDMARD Dose Reduction in Rheumatoid Arthritis: A Narrative Review with Systematic Literature Search. Rheumatol. Ther. 2017, 4, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Bechman, K.; Tweehuysen, L.; Garrood, T.; Scott, D.L.; Cope, A.P.; Galloway, J.B.; Ma, M.H.Y. Flares in Rheumatoid Arthritis Patients with Low Disease Activity: Predictability and Association with Worse Clinical Outcomes. J. Rheumatol. 2018, 45, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Markusse, I.M.; Dirven, L.; Gerards, A.H.; van Groenendael, J.H.L.M.; Ronday, H.K.; Kerstens, P.J.S.M.; Lems, W.F.; Huizinga, T.W.J.; Allaart, C.F. Disease Flares in Rheumatoid Arthritis Are Associated with Joint Damage Progression and Disability: 10-Year Results from the BeSt Study. Arthritis Res. Ther. 2015, 17, 232. [Google Scholar] [CrossRef] [PubMed]
- Haschka, J.; Englbrecht, M.; Hueber, A.J.; Manger, B.; Kleyer, A.; Reiser, M.; Finzel, S.; Tony, H.-P.; Kleinert, S.; Feuchtenberger, M.; et al. Relapse Rates in Patients with Rheumatoid Arthritis in Stable Remission Tapering or Stopping Antirheumatic Therapy: Interim Results from the Prospective Randomised Controlled RETRO Study. Ann. Rheum. Dis. 2016, 75, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Li, N.; Peterson, S. Minimal Important Difference in HAQ: A Validation from Health Economic Perspectives in Patient with Rheumatoid Arthritis Using Real-World Data from Adelphi Database. Ann. Rheum. Dis. 2016, 75 (Suppl 2), 193. [Google Scholar] [CrossRef]
- Heckman, M.G.; Davis, J.M., 3rd; Crowson, C.S. Post Hoc Power Calculations: An Inappropriate Method for Interpreting the Findings of a Research Study. J. Rheumatol. 2022, 49, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.A.; Saag, K.G.; Bridges, S.L.; Akl, E.A.; Bannuru, R.R.; Sullivan, M.C.; Vaysbrot, E.; McNaughton, C.; Osani, M.; Shmerling, R.H.; et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2016, 68, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Klarenbeek, N.B.; van der Kooij, S.M.; Güler-Yüksel, M.; van Groenendael, J.H.L.M.; Han, K.H.; Kerstens, P.J.S.M.; Huizinga, T.W.J.; Dijkmans, B.A.C.; Allaart, C.F. Discontinuing Treatment in Patients with Rheumatoid Arthritis in Sustained Clinical Remission: Exploratory Analyses from the BeSt Study. Ann. Rheum. Dis. 2011, 70, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Emery, P.; Tanaka, Y.; Burmester, G.; Pisetsky, D.S.; Naredo, E.; Fautrel, B.; van Vollenhoven, R. Tapering Biologic and Conventional DMARD Therapy in Rheumatoid Arthritis: Current Evidence and Future Directions. Ann. Rheum. Dis. 2016, 75, 1428–1437. [Google Scholar] [CrossRef] [PubMed]
- Rech, J.; Hueber, A.J.; Finzel, S.; Englbrecht, M.; Haschka, J.; Manger, B.; Kleyer, A.; Reiser, M.; Cobra, J.F.; Figueiredo, C.; et al. Prediction of Disease Relapses by Multibiomarker Disease Activity and Autoantibody Status in Patients with Rheumatoid Arthritis on Tapering DMARD Treatment. Ann. Rheum. Dis. 2016, 75, 1637–1644. [Google Scholar] [CrossRef] [PubMed]
- van der Woude, D.; Young, A.; Jayakumar, K.; Mertens, B.J.; Toes, R.E.M.; van der Heijde, D.; Huizinga, T.W.J.; van der Helm-van Mil, A.H.M. Prevalence of and Predictive Factors for Sustained Disease-Modifying Antirheumatic Drug-Free Remission in Rheumatoid Arthritis: Results from Two Large Early Arthritis Cohorts. Arthritis Rheum. 2009, 60, 2262–2271. [Google Scholar] [CrossRef] [PubMed]
- Higgins, S.C.; Adams, J.; Hughes. R. Measuring hand grip strength in rheumatoid arthritis. Rheumatol. Int. 2018, 38, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Patanè, M.; Carmisciano, L.; Hysa, E.; Gotelli, E.; Sulli, A.; Paolino, S.; Smith, V.; Cutolo, M. Engineered glove to evaluate hand disability in rheumatoid arthritis: A pilot-study. Jt. Bone Spine 2022, 89, 105272. [Google Scholar] [CrossRef] [PubMed]
- Ørnbjerg, L.M.; Østergaard, M. Assessment of structural damage progression in established rheumatoid arthritis by conventional radiography, computed tomography, and magnetic resonance imaging. Best Pract. Res. Clin. Rheumatol. 2019, 33, 101481. [Google Scholar] [CrossRef] [PubMed]
| Group | ||||
|---|---|---|---|---|
| Continue | Taper | Stop | Overall | |
| N | 93 | 93 | 96 | 282 |
| Age, mean Standard Deviation (SD) | 55.9 (12.7) | 56.9 (13.0) | 56.5 (13.3) | 56.5 (13.0) |
| Female, n (%) | 53 (57.0) | 57 (62.0) | 57 (59.4) | 167 (59.4) |
| Rheumatoid Factor (RF), n (%) | 52 (55.9) | 58 (62.4) | 52 (54.2) | 162 (57.4) |
| Anti-citrullinated protein antibodies (ACPA`s), n (%) | 53 (57.0) | 50 (54.9) | 55 (57.3) | 158 (56.4) |
| Disease duration, years, mean (SD) | 7.6 (6.9) | 7.8 (6.9) | 6.8 (8.1) | 7.4 (7.3) |
| Remission duration, months, mean (SD) | 20.6 (18.0) | 16.5 (15.9) | 22.7 (30.4) | 20.0 (22.6) |
| Health Assessment Questionnaire (HAQ), standard, mean (SD) | 0.2 (0.4) | 0.2 (0.3) | 0.2 (0.4) | 0.2 (0.4) |
| DAS28, mean (SD) | 1.7 (0.7) | 1.7 (0.6) | 1.7 (0.6) | 1.7 (0.6) |
| Glucocorticoids, n (%) | 27 (29.0) | 23 (24.7) | 17 (17.7) | 67 (23.8) |
| csDMARD (conventional synthetic disease - modifying antirheumatic drug —total, n (%) | 85 (91.4) | 81 (87.1) | 84 (87.5) | 250 (88.7) |
| csDMARD—solo, n (%) | 47 (50.5) | 46 (49.5) | 52 (54.2) | 145 (51.4) |
| csDMARD—combination, n (%) | 6 (6.5) | 3 (3.2) | 5 (5.2) | 14 (5.0) |
| Methotrexate, n (%) | 72 (77.4) | 67 (72.0) | 75 (78.1) | 214 (75.9) |
| Antimalarials | 14 (15.1) | 10 (10.8) | 6 (6.2) | 30 (10.6) |
| Leflunomide | 5 (5.4) | 5 (5.4) | 8 (8.3) | 18 (6.4) |
| Sulfasalazine | 4 (4.3) | 4 (4.3) | 2 (2.1) | 10 (3.5) |
| Azathioprine | 1 (1.1) | 1 (1.1) | 0 (0.0) | 2 (0.7) |
| Biologics—total, n (%) | 40 (43.0) | 44 (47.3) | 39 (40.6) | 123 (43.6) |
| Biologics—solo, n (%) | 8 (8.6) | 12 (12.9) | 12 (12.5) | 32 (11.3) |
| Biologics—combination, n (%) | 32 (34.4) | 32 (34.4) | 27 (28.1) | 91 (32.3) |
| anti-TNF, n (%) | 28 (30.1) | 33 (35.5) | 29 (30.2) | 90 (31.9) |
| Adalimumab | 8 (8.6) | 13 (14.0) | 11 (11.5) | 32 (11.3) |
| Certolizumab | 7 (7.5) | 10 (10.8) | 8 (8.3) | 25 (8.9) |
| Etanercept | 7 (7.5) | 4 (4.3) | 7 (7.3) | 18 (6.4) |
| Infliximab | 6 (6.5) | 4 (4.3) | 3 (3.1) | 13 (4.6) |
| Golimumab | 1 (1.1) | 2 (2.2) | 0 (0.0) | 3 (1.1) |
| Other biologics, n (%) | 11 (11.8) | 11 (11.8) | 10 (10.4) | 32 (11.3) |
| Tocilizumab | 10 (10.8) | 9 (9.7) | 8 (8.3) | 27 (9.6) |
| Abatacept | 1 (1.1) | 2 (2.2) | 2 (2.1) | 5 (1.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stephan, M.; Tascilar, K.; Yalcin-Mutlu, M.; Hagen, M.; Haschka, J.; Reiser, M.; Hartmann, F.; Kleyer, A.; Hueber, A.J.; Manger, B.; et al. Physical Function of RA patients Tapering Treatment—A Post Hoc Analysis of the Randomized Controlled RETRO Trial. J. Clin. Med. 2023, 12, 3723. https://doi.org/10.3390/jcm12113723
Stephan M, Tascilar K, Yalcin-Mutlu M, Hagen M, Haschka J, Reiser M, Hartmann F, Kleyer A, Hueber AJ, Manger B, et al. Physical Function of RA patients Tapering Treatment—A Post Hoc Analysis of the Randomized Controlled RETRO Trial. Journal of Clinical Medicine. 2023; 12(11):3723. https://doi.org/10.3390/jcm12113723
Chicago/Turabian StyleStephan, Marlene, Koray Tascilar, Melek Yalcin-Mutlu, Melanie Hagen, Judith Haschka, Michaela Reiser, Fabian Hartmann, Arnd Kleyer, Axel J. Hueber, Bernhard Manger, and et al. 2023. "Physical Function of RA patients Tapering Treatment—A Post Hoc Analysis of the Randomized Controlled RETRO Trial" Journal of Clinical Medicine 12, no. 11: 3723. https://doi.org/10.3390/jcm12113723
APA StyleStephan, M., Tascilar, K., Yalcin-Mutlu, M., Hagen, M., Haschka, J., Reiser, M., Hartmann, F., Kleyer, A., Hueber, A. J., Manger, B., Figueiredo, C., Cobra, J. F., Tony, H.-P., Finzel, S., Kleinert, S., Wendler, J., Schuch, F., Ronneberger, M., Feuchtenberger, M., ... Rech, J. (2023). Physical Function of RA patients Tapering Treatment—A Post Hoc Analysis of the Randomized Controlled RETRO Trial. Journal of Clinical Medicine, 12(11), 3723. https://doi.org/10.3390/jcm12113723