Cardiovascular Subphenotypes in ARDS: Diagnostic and Therapeutic Implications and Overlap with Other ARDS Subphenotypes
Abstract
:1. Introduction
2. Literature Search
3. The Problem
- Utilising a limited number of variables that can be difficult to accurately measure given the complexity in RV geometry [38];
- Using arbitrary, unvalidated cut-off values for these variables in a binary manner, which means that patients at the border of this cut-off may frequently change groups;
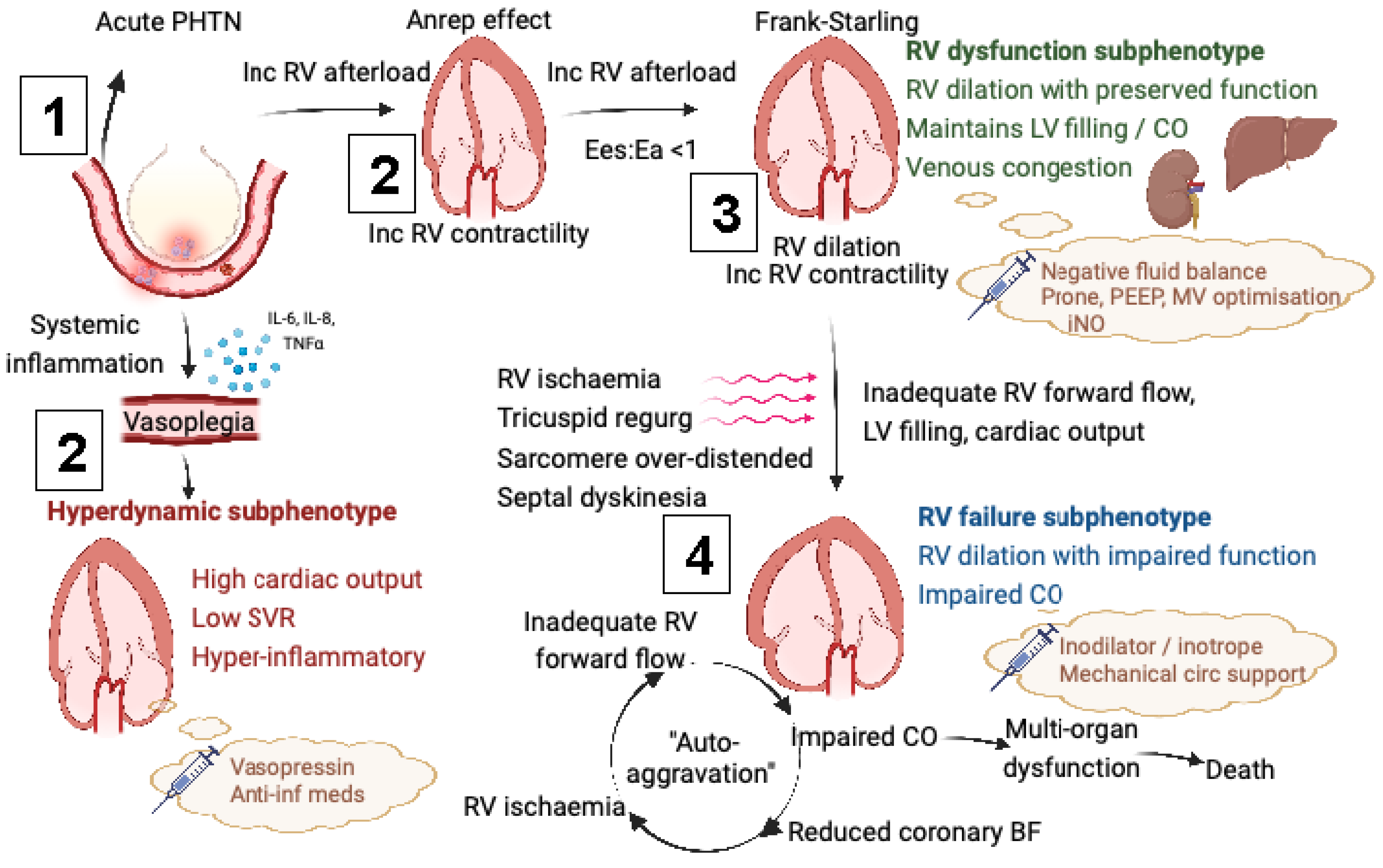
- Neglecting the effect of RVD on LV function, cardiac output, despite the RV being connected in series to the LV, and ventricular interdependence, meaning that RVD can negatively impact LV filling [39];
- Overlooking the predominant LV pathology that may precipitate shock in ARDS [40] by solely focusing on the right ventricle; this surprising oversight occurs despite ARDS sharing notable overlap with sepsis, a condition where septic cardiomyopathy [41] and hyperdynamic LV ejection fraction (HDLVEF; [42,43]) are frequent and associated with poor outcomes.
4. Clustering Analysis: A Possible Solution
- It is data driven and, therefore, unbiased;
- It can incorporate multiple haemodynamic variables that assess global cardiovascular function;
- It identifies the states of CV dysfunction that commonly occur and are, therefore, more likely to describe genuine pathophysiology [52];
- Trials of therapies in these subphenotypes will benefit from predictive and prognostic enrichment, by using treatments targeting the aberrant pathophysiological process in a subgroup with a high-risk of mortality [18].
5. Pathophysiological Implications
- Raised RV end-diastolic pressure precipitating subendocardial ischaemia by only allowing coronary blood flow in systole [58];
- Marked septal dyskinesia impairing LV filling and output (ventricular interdependence) [59];
- Excessive RV dilation decreasing RV stroke volume by
- Stretching the tricuspid annulus precipitating tricuspid regurgitation [60];
- Lengthening sarcomeres above their optimal interactive capacity.
6. Diagnostic Implications for RVD
7. Therapeutic Implications
- Reducing RV afterload, either by
- Combatting the deleterious effects of venous congestion by achieving negative fluid balances with either diuretics or renal replacement therapy [70].
8. Left Ventricular Function in ARDS
9. Limitations
- (i)
- Employing a clustering approach that incorporates multiple parameters to delineate subphenotype class, rather than focusing on one or two parameters per the existing definitions of RVD;
- (ii)
- Excluding patients with inadequate TTE views from the study;
- (iii)
- Ensuring TTEs were performed by practitioners with advanced accreditation only.
10. Potential Overlap with Other ARDS Subphenotypes
| Subphenotype Classification: | Systemic Inflammation [49,82] | Cause of Lung Injury [88,89] | Lung Pathophysiology [83] | Lung Morphology [84,85] | Cardiovascular [50,51] | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hyper-inf/Reactive | Hypo-inf/Uninflamed | Indirect | Direct | Recruitable | Non-Recruitable | Diffuse “Non-Focal” | Focal | RV Failure | HDLVEF | Normal | |
| Prevalence | 35% | 65% | 27% | 73% | 55% | 45% | 50% | 50% * | 13% | 21% | 43% |
| Characteristics | |||||||||||
| Respiratory | |||||||||||
| P/F ratio | 121 | 132 | 136 | 128 | 144 | 193 | 240 | 217 | 188 | 210 | 210 |
| Direct lung injury (%) | 55% | 85% | N/A | N/A | 67% | 43% | ? | ? | 68% | 42% | 64% |
| Driving pressure/elastance/compliance | Lower Cstat, higher DP | Higher Cstat, Lower DP | Lower lung elastance | Higher lung elastance | Lung elastance 28 DP 17 | Lung elastance 22 DP 12 | Cstat 34 DP 12 | Cstat 40 DP 11 | Cdyn 30 | Cdyn 32 | Cdyn 31 |
| Lung morphology on imaging | ? | ? | Diffuse, ground glass opacification | Focal, consoli-dation | Diffuse “inhomo-geneous” | Focal “homo-geneous” | Diffuse | Focal | Greater opacification score | Lower opacification score | Lower opacification score |
| Extra-organ dysfunction | |||||||||||
| On vasopressor | 81% | 58% | 49% | 30% | 58% | 52% | No different | No different | 83% | 72% | 55% |
| Scoring system | ? | ? | APACHE III 103 | APACHE III 94 | SAPS II 43 | SAPS II 41 | ? | ? | SOFA 10 (7–12) | SOFA 9 (7–12) | SOFA 6 (4–9) |
| pH/bicarbonate | Lower Lower | Higher Higher | No difference | No difference | Lower/higher | Higher/lower | Lower Lower | Higher Higher | Lower Lower | Lower Lower | Higher Higher |
| Creatinine, bilirubin, platelet | Higher Higher Lower | Lower Lower Higher | ? | ? | ? | ? | ? | ? | Higher Higher Normal | Higher Higher Lower | Lower Lower Higher |
| Systemic inflammatory (vascular) markers, e.g., IL-6, IL-8, TNF, RAGE Ang-2 and vWF | Higher | Lower | Higher | Lower | Higher sRAGE | Lower sRAGE | Higher | Lower | Higher WBC and PMN | Higher WBC, PMN and CRP | Lower WBC, PMN and CRP |
| Alveolar inflammatory markers | No difference | No difference | Lower | Higher | ? | ? | ? | ? | ? | ? | ? |
| Mortality | 40–60% | 15–25% | 90-day 35% | 90-day 29% | ICU 52% | ICU 23% | 90-day 28% | 90-day 16% | 90-day 78% | 90-day 59% | 90-day 19% |
| Treatment response | |||||||||||
| Recruitment manoeuvre | ? | ? | Beneficial, including resp compliance | Harmful, decrease in resp compliance | Beneficial (including ventilated tissue, compliance and P/F) | No difference | Decrease in mortality (if correctly classified) | Increase in mortality | ? | ? | ? |
| High PEEP | Beneficial | No difference | Beneficial (recruitment and dec elastance) | No difference | ? | ? | Decrease in mortality if correctly classified | Increase in mortality | ? | ? | ? |
| Prone positioning | ? | ? | Beneficial (recruitment and dec elastance) | No difference | ? | ? | Harm if mis-classified | Mortality benefit if correctly classified | Greater improvement in blood gas parameters | ? | Less improvement in blood gas parameters |
11. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thompson, B.T.; Chambers, R.C.; Liu, K.D. Acute respiratory distress syndrome. N. Engl. J. Med. 2017, 377, 562–572. [Google Scholar] [CrossRef]
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.M.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Prim. 2019, 5, 18. [Google Scholar] [CrossRef]
- Bos, L.D.; Ware, L.B. Acute respiratory distress syndrome: Causes, pathophysiology, and phenotypes. Lancet 2022, 400, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Gorman, E.A.; O’Kane, C.M.; McAuley, D.F. Acute respiratory distress syndrome in adults: Diagnosis, outcomes, long-term sequelae, and management. Lancet 2022, 400, 1157–1170. [Google Scholar] [CrossRef] [PubMed]
- Force, A.D.; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.; Ferguson, N.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute respiratory distress syndrome. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; Van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef]
- Stapleton, R.D.; Wang, B.M.; Hudson, L.D.; Rubenfeld, G.D.; Caldwell, E.S.; Steinberg, K.P. Causes and timing of death in patients with ARDS. Chest 2005, 128, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Ketcham, S.W.; Sedhai, Y.R.; Miller, H.C.; Bolig, T.C.; Ludwig, A.; Co, I.; Claar, D.; McSparron, J.I.; Prescott, H.C.; Sjoding, M.W. Causes and characteristics of death in patients with acute hypoxemic respiratory failure and acute respiratory distress syndrome: A retrospective cohort study. Crit. Care 2020, 24, 391. [Google Scholar] [CrossRef]
- Zochios, V.; Parhar, K.; Tunnicliffe, W.; Roscoe, A.; Gao, F. The right ventricle in ARDS. Chest 2017, 152, 181–193. [Google Scholar] [CrossRef]
- Vieillard-Baron, A.; Price, L.C.; Matthay, M. Acute cor pulmonale in ARDS. Intensive Care Med. 2013, 39, 1836–1838. [Google Scholar] [CrossRef]
- Mekontso Dessap, A.; Boissier, F.; Charron, C.; Bégot, E.; Repessé, X.; Legras, A.; Brun-Buisson, C.; Vignon, P.; Vieillard-Baron, A. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: Prevalence, predictors, and clinical impact. Intensive Care Med. 2016, 42, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Boissier, F.; Katsahian, S.; Razazi, K.; Thille, A.W.; Roche-Campo, F.; Leon, R.; Vivier, E.; Brochard, L.; Vieillard-Baron, A.; Brun-Buisson, C.; et al. Prevalence and prognosis of cor pulmonale during protective ventilation for acute respiratory distress syndrome. Intensive Care Med. 2013, 39, 1725–1733. [Google Scholar] [CrossRef]
- Ryan, D.; Frohlich, S.; McLoughlin, P. Pulmonary vascular dysfunction in ARDS. Ann. Intensive Care 2014, 4, 28. [Google Scholar] [CrossRef]
- Mebazaa, A.; Karpati, P.; Renaud, E.; Algotsson, L. Acute right ventricular failure—From pathophysiology to new treatments. In Applied Physiology in Intensive Care Medicine; Springer: Berlin/Heidelberg, Germany, 2009; pp. 261–272. [Google Scholar]
- Vieillard-Baron, A.; Charron, C.; Caille, V.; Belliard, G.; Page, B.; Jardin, F. Prone positioning unloads the right ventricle in severe ARDS. Chest 2007, 132, 1440–1446. [Google Scholar] [CrossRef]
- Bouferrache, K.; Vieillard-Baron, A. Acute respiratory distress syndrome, mechanical ventilation, and right ventricular function. Current opinion in critical care. Curr. Opin. Crit. Care 2011, 17, 30–35. [Google Scholar] [CrossRef]
- Morelli, A.; Teboul, J.L.; Maggiore, S.M.; Vieillard-Baron, A.; Rocco, M.; Conti, G.; De Gaetano, A.; Picchini, U.; Orecchioni, A.; Carbone, I.; et al. Effects of levosimendan on right ventricular afterload in patients with acute respiratory distress syndrome: A pilot study. Crit. Care Med. 2006, 34, 2287–2293. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.G.; Calfee, C.S. ARDS subphenotypes: Understanding a heterogeneous syndrome. Annu. Update Intensive Care Emerg. Med. 2020, 2020, 67–79. [Google Scholar]
- Sinha, P.; Calfee, C.S. Phenotypes in ARDS: Moving towards precision medicine. Curr. Opin. Crit. Care 2019, 25, 12. [Google Scholar] [CrossRef] [PubMed]
- Ashbaugh, D.; Bigelow, D.B.; Petty, T.; Levine, B. Acute respiratory distress in adults. Lancet 1967, 290, 319–323. [Google Scholar] [CrossRef]
- Khan, Y.A.; Fan, E.; Ferguson, N.D. Precision medicine and heterogeneity of treatment effect in therapies for ARDS. Chest 2021, 160, 1729–1738. [Google Scholar] [CrossRef]
- Boyle, A.J.; Sweeney, R.M.; McAuley, D.F. Pharmacological treatments in ARDS; a state-of-the-art update. BMC Med. 2013, 11, 166. [Google Scholar] [CrossRef]
- Evrard, B.; Goudelin, M.; Giraudeau, B.; François, B.; Vignon, P. Right ventricular failure is strongly associated with mortality in patients with moderate-to-severe COVID-19-related ARDS and appears related to respiratory worsening. Intensive Care Med. 2022, 48, 765–767. [Google Scholar] [CrossRef]
- Dugar, S.; Sato, R.; Zochios, V.; Duggal, A.; Vallabhajosyula, S. Defining right ventricular dysfunction in acute respiratory distress syndrome. J. Cardiothorac. Vasc. Anesth. 2022, 36, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, A.; Knight, D.S.; Augustine, D.X.; Harkness, A.; Oxborough, D.; Pearce, K.; Ring, L.; Robinson, S.; Stout, M.; Willis, J.; et al. Echocardiographic assessment of the right heart in adults: A practical guideline from the British Society of Echocardiography. Echo Res. Pract. 2020, 7, G19–G41. [Google Scholar] [CrossRef]
- Harkness, A.; Ring, L.; Augustine, D.X.; Oxborough, D.; Robinson, S.; Sharma, V. Normal reference intervals for cardiac dimensions and function for use in echocardiographic practice: A guideline from the British Society of Echocardiography. Echo Res. Pract. 2020, 7, G1–G8. [Google Scholar] [CrossRef]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for performing a comprehensive transthoracic echocardiographic examination in adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef]
- Vieillard-Baron, A.; Naeije, R.; Haddad, F.; Bogaard, H.J.; Bull, T.M.; Fletcher, N.; Lahm, T.; Magder, S.; Orde, S.; Schmidt, G.; et al. Diagnostic workup, etiologies and management of acute right ventricle failure. Intensive Care Med. 2018, 44, 774–790. [Google Scholar] [CrossRef] [PubMed]
- Lahm, T.; Douglas, I.S.; Archer, S.L.; Bogaard, H.J.; Chesler, N.C.; Haddad, F.; Hemnes, A.R.; Kawut, S.M.; Kline, J.A.; Kolb, T.M.; et al. Assessment of right ventricular function in the research setting: Knowledge gaps and pathways forward. An Official American Thoracic Society Research Statement. Am. J. Respir. Crit. Care Med. 2018, 198, e15–e43. [Google Scholar] [CrossRef] [PubMed]
- Jardin, F.; Vieillard-Baron, A. Acute cor pulmonale. Curr. Opin. Crit. Care 2009, 15, 67–70. [Google Scholar] [CrossRef]
- Cavaleiro, P.; Masi, P.; Bagate, F.; d’Humières, T.; Mekontso Dessap, A. Acute cor pulmonale in COVID-19 related acute respiratory distress syndrome. Crit. Care 2021, 25, 346. [Google Scholar] [CrossRef]
- Vieillard-Baron, A.; Prin, S.; Chergui, K.; Dubourg, O.; Jardin, F. Echo–Doppler demonstration of acute cor pulmonale at the bedside in the medical intensive care unit. Am. J. Respir. Crit. Care Med. 2002, 166, 1310–1319. [Google Scholar] [CrossRef]
- Huang, S.; Vignon, P.; Mekontso-Dessap, A.; Tran, S.; Prat, G.; Chew, M.; Balik, M.; Sanfilippo, F.; Banauch, G.; Clau-Terre, F.; et al. Echocardiography findings in COVID-19 patients admitted to intensive care units: A multi-national observational study (the ECHO-COVID study). Intensive Care Med. 2022, 48, 667–678. [Google Scholar] [CrossRef]
- Vieillard-Baron, A.; Schmitt, J.M.; Augarde, R.; Fellahi, J.L.; Prin, S.; Page, B.; Beauchet, A.; Jardin, F. Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: Incidence, clinical implications, and prognosis. Crit. Care Med. 2001, 29, 1551–1555. [Google Scholar] [CrossRef]
- McCall, P.J.; Willder, J.M.; Stanley, B.L.; Messow, C.M.; Allan, J.; Gemmell, L.; Puxty, A.; Strachan, D.; Berry, C.; Shelley, B.G.; et al. Right ventricular dysfunction in patients with COVID-19 pneumonitis whose lungs are mechanically ventilated: A multicentre prospective cohort study. Anaesthesia 2022, 77, 772–784. [Google Scholar] [CrossRef]
- McErlane, J.; McCall, P.; Willder, J.; Berry, C.; Shelley, B. Right ventricular free wall longitudinal strain is independently associated with mortality in mechanically ventilated patients with COVID-19. Ann. Intensive Care 2022, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Bleakley, C.; Singh, S.; Garfield, B.; Morosin, M.; Surkova, E.; Mandalia, M.S.; Dias, B.; Androulakis, E.; Price, L.C.; McCabe, C.; et al. Right ventricular dysfunction in critically ill COVID-19 ARDS. Int. J. Cardiol. 2021, 327, 251–258. [Google Scholar] [CrossRef]
- Badano, L.P.; Addetia, K.; Pontone, G.; Torlasco, C.; Lang, R.M.; Parati, G.; Muraru, D. Advanced imaging of right ventricular anatomy and function. Heart 2020, 106, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Haddad, F.; Doyle, R.; Murphy, D.J.; Hunt, S.A. Right ventricular function in cardiovascular disease, part II: Pathophysiology, clinical importance, and management of right ventricular failure. Circulation 2008, 117, 1717–1731. [Google Scholar] [CrossRef]
- Formenti, P.; Coppola, S.; Massironi, L.; Annibali, G.; Mazza, F.; Gilardi, L.; Pozzi, T.; Chiumello, D. Left Ventricular Diastolic Dysfunction in ARDS Patients. J. Clin. Med. 2022, 11, 5998. [Google Scholar] [CrossRef] [PubMed]
- Beesley, S.J.; Weber, G.; Sarge, T.; Nikravan, S.; Grissom, C.K.; Lanspa, M.J.; Shahul, S.; Brown, S.M. Septic cardiomyopathy. Crit. Care Med. 2018, 46, 625–634. [Google Scholar] [CrossRef]
- Paonessa, J.R.; Brennan, T.; Pimentel, M.; Steinhaus, D.; Feng, M.; Celi, L.A. Hyperdynamic left ventricular ejection fraction in the intensive care unit. Crit. Care 2015, 19, 288. [Google Scholar] [CrossRef]
- Chotalia, M.; Ali, M.; Hebballi, R.; Singh, H.; Parekh, D.; Bangash, M.N.; Patel, J.M. Hyperdynamic left ventricular ejection fraction in ICU patients with sepsis. Crit. Care Med. 2021, 50, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Vieillard-Baron, A.; Pinsky, M.R. The difficulty in defining right ventricular failure at the bedside and its clinical significance. Ann. Intensive Care 2021, 11, 122. [Google Scholar] [CrossRef]
- Sinha, P.; Calfee, C.S.; Delucchi, K.L. Practitioner’s Guide to Latent Class Analysis: Methodological Considerations and Common Pitfalls. Crit. Care Med. 2021, 49, e63. [Google Scholar] [CrossRef]
- Calfee, C.S.; Delucchi, K.; Parsons, P.E.; Thompson, B.T.; Ware, L.B.; Matthay, M.A.; Nhlbi Ards Network. Subphenotypes in acute respiratory distress syndrome: Latent class analysis of data from two randomised controlled trials. Lancet Respir. Med. 2014, 2, 611–620. [Google Scholar] [CrossRef]
- Sinha, P.; Delucchi, K.L.; Thompson, B.T.; McAuley, D.F.; Matthay, M.A.; Calfee, C.S. Latent class analysis of ARDS subphenotypes: A secondary analysis of the statins for acutely injured lungs from sepsis (SAILS) study. Intensive Care Med. 2018, 44, 1859–1869. [Google Scholar] [CrossRef] [PubMed]
- Famous, K.R.; Delucchi, K.; Ware, L.B.; Kangelaris, K.N.; Liu, K.D.; Thompson, B.T.; Calfee, C.S. Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am. J. Respir. Crit. Care Med. 2017, 195, 331–338. [Google Scholar] [CrossRef]
- Calfee, C.S.; Delucchi, K.L.; Sinha, P.; Matthay, M.A.; Hackett, J.; Shankar-Hari, M.; McDowell, C.; Laffey, J.G.; O’Kane, C.M.; McAuley, D.F.; et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: Secondary analysis of a randomised controlled trial. Lancet Respir. Med. 2018, 6, 691–698. [Google Scholar] [CrossRef]
- Chotalia, M.; Ali, M.; Alderman, J.E.; Patel, J.M.; Parekh, D.; Bangash, M.N. Cardiovascular subphenotypes in patients with COVID-19 pneumonitis whose lungs are mechanically ventilated: A single-centre retrospective observational study. Anaesthesia 2022, 77, 763–771. [Google Scholar] [CrossRef]
- Chotalia, M.; Ali, M.; Bansal, S.; Alderman, J.E.; Patel, J.M.; Bangash, M.N.; Parekh, D. Cardiovascular subphenotypes in acute respiratory distress syndrome. Crit. Care Med. 2022; accepted. [Google Scholar] [CrossRef] [PubMed]
- Geri, G.; Vignon, P.; Aubry, A.; Fedou, A.L.; Charron, C.; Silva, S.; Repessé, X.; Vieillard-Baron, A. Cardiovascular clusters in septic shock combining clinical and echocardiographic parameters: A post hoc analysis. Intensive Care Med. 2019, 45, 657–667. [Google Scholar] [CrossRef]
- Zochios, V.; Yusuff, H.; Schmidt, M. Acute right ventricular injury phenotyping in ARDS. Intensive Care Med. 2022, 49, 99–102. [Google Scholar] [CrossRef]
- Milani-Nejad, N.; Canan, B.D.; Elnakish, M.T.; Davis, J.P.; Chung, J.H.; Fedorov, V.V.; Binkley, P.F.; Higgins, R.S.; Kilic, A.; Mohler, P.J.; et al. The Frank-Starling mechanism involves deceleration of cross-bridge kinetics and is preserved in failing human right ventricular myocardium. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H2077–H2086. [Google Scholar] [CrossRef]
- Rola, P.; Miralles-Aguiar, F.; Argaiz, E.; Beaubien-Souligny, W.; Haycock, K.; Karimov, T.; Dinh, V.A.; Spiegel, R. Clinical applications of the venous excess ultrasound (VExUS) score: Conceptual review and case series. Ultrasound J. 2021, 13, 32. [Google Scholar] [CrossRef]
- Chen, C.; Lee, J.; Johnson, A.E.; Mark, R.G.; Celi, L.A.; Danziger, J. Right ventricular function, peripheral edema, and acute kidney injury in critical illness. Kidney Int. Rep. 2017, 2, 1059–1065. [Google Scholar] [CrossRef]
- Haines, R.; Crichton, S.; Wilson, J.; Treacher, D.; Ostermann, M. Cardiac biomarkers are associated with maximum stage of acute kidney injury in critically ill patients: A prospective analysis. Crit. Care 2017, 21, 88. [Google Scholar] [CrossRef]
- Gold, F.L.; Bache, R.J. Transmural right ventricular blood flow during acute pulmonary artery hypertension in the sedated dog. Evidence for subendocardial ischemia despite residual vasodilator reserve. Circ. Res. 1982, 51, 196–204. [Google Scholar] [CrossRef]
- Jardin, F. Ventricular interdependence: How does it impact on hemodynamic evaluation in clinical practice? Intensive Care Med. 2003, 29, 361–363. [Google Scholar] [CrossRef]
- Medvedofsky, D.; Aronson, D.; Gomberg-Maitland, M.; Thomeas, V.; Rich, S.; Spencer, K.; Mor-Avi, V.; Addetia, K.; Lang, R.M.; Shiran, A. Tricuspid regurgitation progression and regression in pulmonary arterial hypertension: Implications for right ventricular and tricuspid valve apparatus geometry and patients outcome. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 86–94. [Google Scholar] [CrossRef]
- Chotalia, M.; Ali, M.; Alderman, J.E.; Kalla, M.; Parekh, D.; Bangash, M.N.; Patel, J.M. Right ventricular dysfunction and its association with mortality in coronavirus disease 2019 acute respiratory distress syndrome. Crit. Care Med. 2021, 49, 1757. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, W.; Zhang, Q.; Chen, X.; Wang, X.; Liu, D. Prevalence and prognostic value of various types of right ventricular dysfunction in mechanically ventilated septic patients. Ann. Intensive Care 2021, 11, 108. [Google Scholar] [CrossRef]
- Blancas, R.; Martínez-González, Ó.; Ballesteros, D.; Núñez, A.; Luján, J.; Rodríguez-Serrano, D.; Hernández, A.; Martínez-Díaz, C.; Parra, C.M.; Matamala, B.L.; et al. Lack of correlation between left ventricular outflow tract velocity time integral and stroke volume index in mechanically ventilated patients. Med. Intensiv. 2019, 43, 73–78. [Google Scholar] [CrossRef]
- D’Alto, M.; Marra, A.M.; Severino, S.; Salzano, A.; Romeo, E.; De Rosa, R.; Stagnaro, F.M.; Pagnano, G.; Verde, R.; Murino, P.; et al. Right ventricular-arterial uncoupling independently predicts survival in COVID-19 ARDS. Crit. Care 2020, 24, 670. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.H.; Ren, X.; Suffredini, G.; Dodd-o, J.M.; Gao, W.D. Right ventricular diastolic dysfunction and failure: A review. Heart Fail. Rev. 2021, 27, 1077–1090. [Google Scholar] [CrossRef] [PubMed]
- Jury, D.; Shaw, A.D. Utility of bedside ultrasound derived hepatic and renal parenchymal flow patterns to guide management of acute kidney injury. Curr. Opin. Crit. Care 2021, 27, 587–592. [Google Scholar] [CrossRef]
- Vieillard-Baron, A.; Jardin, F. Why protect the right ventricle in patients with acute respiratory distress syndrome? Curr. Opin. Crit. Care 2003, 9, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Fierobe, L.; Brunet, F.; Dhainaut, J.F.; Monchi, M.; Belghith, M.; Mira, J.P.; Dall’ava-Santucci, J.; Dinh-Xuan, A.T. Effect of inhaled nitric oxide on right ventricular function in adult respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1995, 151, 1414–1419. [Google Scholar] [CrossRef]
- Grant, C., Jr.; Richards, J.B.; Frakes, M.; Cohen, J.; Wilcox, S.R. ECMO and right ventricular failure: Review of the literature. J. Intensive Care Med. 2021, 36, 352–360. [Google Scholar] [CrossRef]
- Hua, Z.; Xin, D.; Xiaoting, W.; Dawei, L. High Central Venous Pressure and Right Ventricle Size Are Related to Non-decreased Left Ventricle Stroke Volume after Negative Fluid Balance in Critically Ill Patients: A Single Prospective Observational Study. Front. Med. 2021, 8, 715099. [Google Scholar] [CrossRef]
- Guérin, C.; Reignier, J.; Richard, J.C.; Beuret, P.; Gacouin, A.; Boulain, T.; Mercier, E.; Badet, M.; Mercat, A.; Baudin, O.; et al. Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 2013, 368, 2159–2168. [Google Scholar] [CrossRef]
- Paternot, A.; Repessé, X.; Vieillard-Baron, A. Rationale and description of right ventricle-protective ventilation in ARDS. Respir. Care 2016, 61, 1391–1396. [Google Scholar] [CrossRef]
- Petit, M.; Mekontso-Dessap, A.; Masi, P.; Legras, A.; Vignon, P.; Vieillard-Baron, A. Evaluation of right ventricular function and driving pressure with blood gas analysis could better select patients eligible for VV ECMO in severe ARDS. Crit. Care 2021, 25, 220. [Google Scholar] [CrossRef]
- Shah, F.A.; Meyer, N.J.; Angus, D.C.; Awdish, R.; Azoulay, É.; Calfee, C.S.; Clermont, G.; Gordon, A.C.; Kwizera, A.; Leligdowicz, A.; et al. A research agenda for precision medicine in sepsis and acute respiratory distress syndrome: An official American Thoracic Society Research Statement. Am. J. Respir. Crit. Care Med. 2021, 204, 891–901. [Google Scholar] [CrossRef]
- Lambden, S.; Creagh-Brown, B.C.; Hunt, J.; Summers, C.; Forni, L.G. Definitions and pathophysiology of vasoplegic shock. Crit. Care 2018, 22, 174. [Google Scholar] [CrossRef]
- Gordon, A.C.; Mason, A.J.; Thirunavukkarasu, N.; Perkins, G.D.; Cecconi, M.; Cepkova, M.; Pogson, D.G.; Aya, H.D.; Anjum, A.; Frazier, G.J.; et al. Effect of early vasopressin vs norepinephrine on kidney failure in patients with septic shock: The VANISH randomized clinical trial. JAMA 2016, 316, 509–518. [Google Scholar] [CrossRef]
- Morelli, A.; Ertmer, C.; Westphal, M.; Rehberg, S.; Kampmeier, T.; Ligges, S.; Orecchioni, A.; D’Egidio, A.; D’Ippoliti, F.; Raffone, C.; et al. Effect of heart rate control with esmolol on hemodynamic and clinical outcomes in patients with septic shock: A randomized clinical trial. JAMA 2013, 310, 1683–1691. [Google Scholar] [CrossRef]
- Levy, B.; Fritz, C.; Piona, C.; Duarte, K.; Morelli, A.; Guerci, P.; Kimmoun, A.; Girerd, N. Hemodynamic and anti-inflammatory effects of early esmolol use in hyperkinetic septic shock: A pilot study. Crit. Care 2021, 25, 21. [Google Scholar] [CrossRef]
- Sinha, P.; Delucchi, K.L.; Chen, Y.; Zhuo, H.; Abbott, J.; Wang, C.; Wickersham, N.; McNeil, J.B.; Jauregui, A.; Ke, S.; et al. Latent class analysis-derived subphenotypes are generalisable to observational cohorts of acute respiratory distress syndrome: A prospective study. Thorax 2022, 77, 13–21. [Google Scholar] [CrossRef]
- Maddali, M.V.; Churpek, M.; Pham, T.; Rezoagli, E.; Zhuo, H.; Zhao, W.; He, J.; Delucchi, K.L.; Wang, C.; Wickersham, N.; et al. Validation and utility of ARDS subphenotypes identified by machine-learning models using clinical data: An observational, multicohort, retrospective analysis. Lancet Respir. Med. 2022, 10, 367–377. [Google Scholar] [CrossRef]
- Millar, J.E.; Wildi, K.; Bartnikowski, N.; Bouquet, M.; Hyslop, K.; Passmore, M.R.; Ki, K.K.; See Hoe, L.E.; Obonyo, N.G.; Neyton, L.; et al. Characterizing preclinical sub-phenotypic models of acute respiratory distress syndrome: An experimental ovine study. Physiol. Rep. 2021, 9, e15048. [Google Scholar] [CrossRef]
- Bos, L.D.; Scicluna, B.P.; Ong, D.S.; Cremer, O.; van Der Poll, T.; Schultz, M.J. Understanding heterogeneity in biologic phenotypes of acute respiratory distress syndrome by leukocyte expression profiles. Am. J. Respir. Crit. Care Med. 2019, 200, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Wendel Garcia, P.D.; Caccioppola, A.; Coppola, S.; Pozzi, T.; Ciabattoni, A.; Cenci, S.; Chiumello, D. Latent class analysis to predict intensive care outcomes in acute respiratory distress syndrome: A proposal of two pulmonary phenotypes. Crit. Care 2021, 25, 154. [Google Scholar] [CrossRef]
- Constantin, J.M.; Jabaudon, M.; Lefrant, J.Y.; Jaber, S.; Quenot, J.P.; Langeron, O.; Ferrandiere, M.; Grelon, F.; Seguin, P.; Ichai, C.; et al. Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): A multicentre, single-blind, randomised controlled trial. Lancet Respir. Med. 2019, 7, 870–880. [Google Scholar] [CrossRef]
- Constantin, J.M.; Grasso, S.; Chanques, G.; Aufort, S.; Futier, E.; Sebbane, M.; Jung, B.; Gallix, B.; Bazin, J.E.; Rouby, J.J.; et al. Lung morphology predicts response to recruitment maneuver in patients with acute respiratory distress syndrome. Crit. Care Med. 2010, 38, 1108–1117. [Google Scholar] [CrossRef]
- Alhamdi, Y.; Zi, M.; Abrams, S.T.; Liu, T.; Su, D.; Welters, I.; Dutt, T.; Cartwright, E.J.; Wang, G.; Toh, C.H. Circulating histone concentrations differentially affect the predominance of left or right ventricular dysfunction in critical illness. Crit. Care Med. 2016, 44, e278–e288. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Qin, G.; Cao, Y.; Yang, Y.; Dai, S.; Wang, L.; Wang, E. Regulation of the Immune Microenvironment by an NLRP3 Inhibitor Contributes to Attenuation of Acute Right Ventricular Failure in Rats with Pulmonary Arterial Hypertension. J. Inflamm. Res. 2021, 14, 5699. [Google Scholar] [CrossRef] [PubMed]
- Calfee, C.S.; Janz, D.R.; Bernard, G.R.; May, A.K.; Kangelaris, K.N.; Matthay, M.A.; Ware, L.B. Distinct molecular phenotypes of direct vs indirect ARDS in single-center and multicenter studies. Chest 2015, 147, 1539–1548. [Google Scholar] [CrossRef]
- Gattinoni, L.; Pelosi, P.; Suter, P.M.; Pedoto, A.; Vercesi, P.; Lissoni, A. Acute respiratory distress syndrome caused by pulmonary and extrapulmonary disease: Different syndromes? Am. J. Respir. Crit. Care Med. 1998, 158, 3–11. [Google Scholar] [CrossRef]
- Heijnen, N.F.; Hagens, L.A.; Smit, M.R.; Schultz, M.J.; van der Poll, T.; Schnabel, R.M.; van der Horst, I.C.; Dickson, R.P.; Bergmans, D.C.; Bos, L.D.; et al. Biological subphenotypes of acute respiratory distress syndrome may not reflect differences in alveolar inflammation. Physiol. Rep. 2021, 9, e14693. [Google Scholar] [CrossRef]
- Sathe, N.A.; Morrell, E.D.; Bhatraju, P.K.; Fessler, M.B.; Stapleton, R.D.; Wurfel, M.M.; Mikacenic, C. Alveolar Biomarker Profiles in Subphenotypes of the Acute Respiratory Distress Syndrome. Crit. Care Med. 2022, 51, e13–e18. [Google Scholar] [CrossRef]
- Heijnen, N.F.; Hagens, L.A.; Smit, M.R.; Cremer, O.L.; Ong, D.S.; van der Poll, T.; van Vught, L.A.; Scicluna, B.P.; Schnabel, R.M.; van der Horst, I.C.; et al. Biological subphenotypes of acute respiratory distress syndrome show prognostic enrichment in mechanically ventilated patients without acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2021, 203, 1503–1511. [Google Scholar] [CrossRef]
- Bhatraju, P.K.; Zelnick, L.R.; Herting, J.; Katz, R.; Mikacenic, C.; Kosamo, S.; Morrell, E.D.; Robinson-Cohen, C.; Calfee, C.S.; Christie, J.D.; et al. Identification of acute kidney injury subphenotypes with differing molecular signatures and responses to vasopressin therapy. Am. J. Respir. Crit. Care Med. 2019, 199, 863–872. [Google Scholar] [CrossRef]
- Wiersema, R.; Jukarainen, S.; Vaara, S.T.; Poukkanen, M.; Lakkisto, P.; Wong, H.; Linder, A.; Van Der Horst, I.C.; Pettilä, V. Two subphenotypes of septic acute kidney injury are associated with different 90-day mortality and renal recovery. Crit. Care 2020, 24, 150. [Google Scholar] [CrossRef]
- Neyton, L.; Zheng, X.; Skouras, C.; Doeschl-Wilson, A.; Gutmann, M.U.; Uings, I.; Rao, F.V.; Nicolas, A.; Marshall, C.; Wilson, L.M.; et al. Molecular patterns in acute pancreatitis reflect generalizable endotypes of the host response to systemic injury in humans. Ann. Surg. 2022, 275, e453–e462. [Google Scholar] [CrossRef]
- Hashem, M.D.; Hopkins, R.O.; Colantuoni, E.; Dinglas, V.D.; Sinha, P.; Friedman, L.A.; Morris, P.E.; Jackson, J.C.; Hough, C.L.; Calfee, C.S.; et al. Six-month and 12-month patient outcomes based on inflammatory subphenotypes in sepsis-associated ARDS: Secondary analysis of SAILS-ALTOS trial. Thorax 2022, 77, 22–30. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chotalia, M.; Patel, J.M.; Bangash, M.N.; Parekh, D. Cardiovascular Subphenotypes in ARDS: Diagnostic and Therapeutic Implications and Overlap with Other ARDS Subphenotypes. J. Clin. Med. 2023, 12, 3695. https://doi.org/10.3390/jcm12113695
Chotalia M, Patel JM, Bangash MN, Parekh D. Cardiovascular Subphenotypes in ARDS: Diagnostic and Therapeutic Implications and Overlap with Other ARDS Subphenotypes. Journal of Clinical Medicine. 2023; 12(11):3695. https://doi.org/10.3390/jcm12113695
Chicago/Turabian StyleChotalia, Minesh, Jaimin M. Patel, Mansoor N. Bangash, and Dhruv Parekh. 2023. "Cardiovascular Subphenotypes in ARDS: Diagnostic and Therapeutic Implications and Overlap with Other ARDS Subphenotypes" Journal of Clinical Medicine 12, no. 11: 3695. https://doi.org/10.3390/jcm12113695
APA StyleChotalia, M., Patel, J. M., Bangash, M. N., & Parekh, D. (2023). Cardiovascular Subphenotypes in ARDS: Diagnostic and Therapeutic Implications and Overlap with Other ARDS Subphenotypes. Journal of Clinical Medicine, 12(11), 3695. https://doi.org/10.3390/jcm12113695






