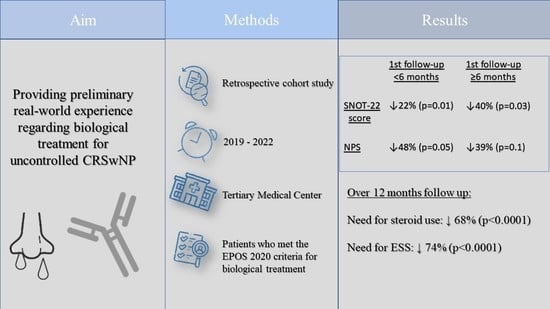
Biological Treatment for Uncontrolled Chronic Rhinosinusitis with Nasal Polyps: Preliminary Real-World Results from a Tertiary Medical Center
Abstract
1. Introduction
2. Materials and Methods
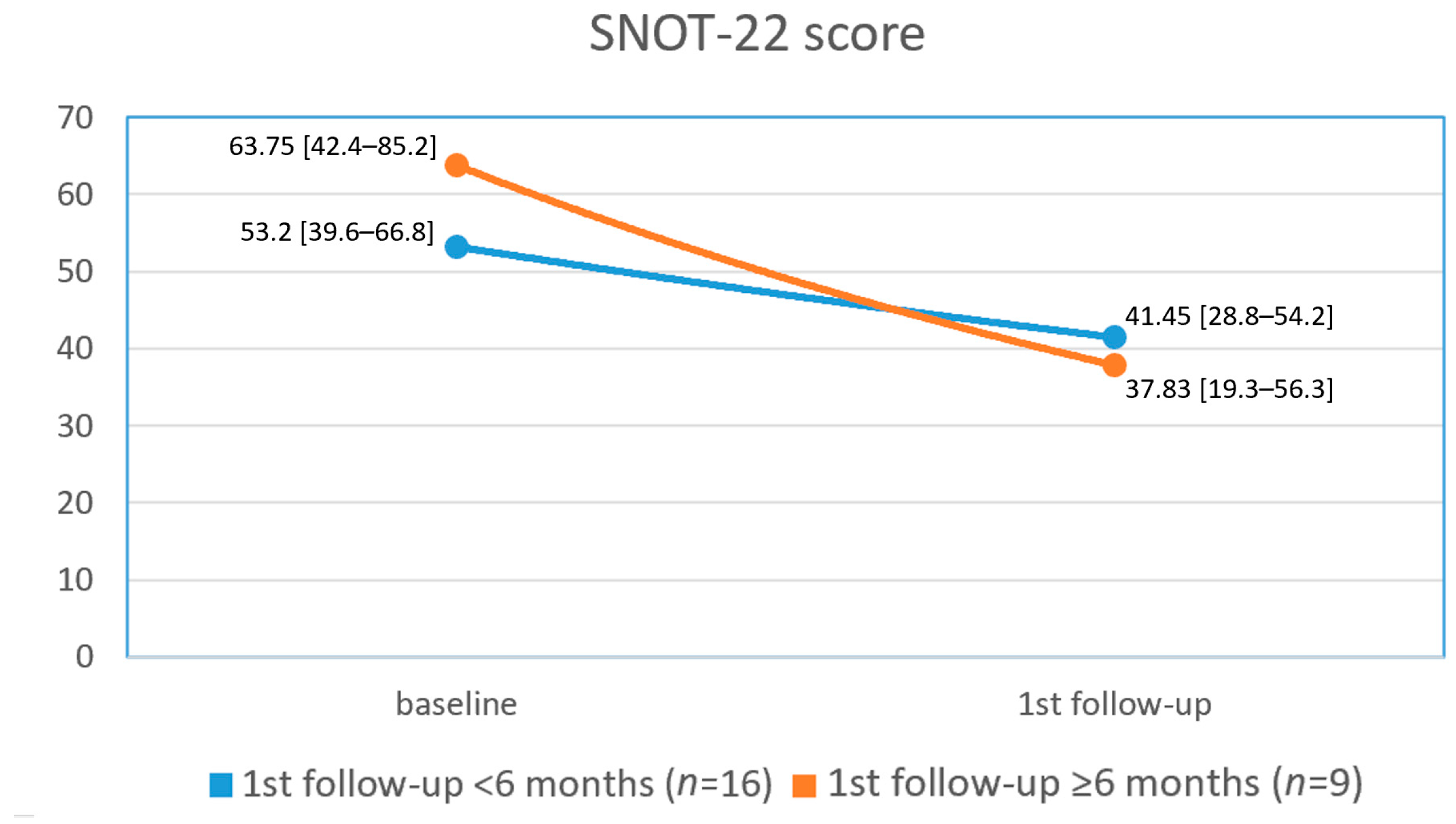
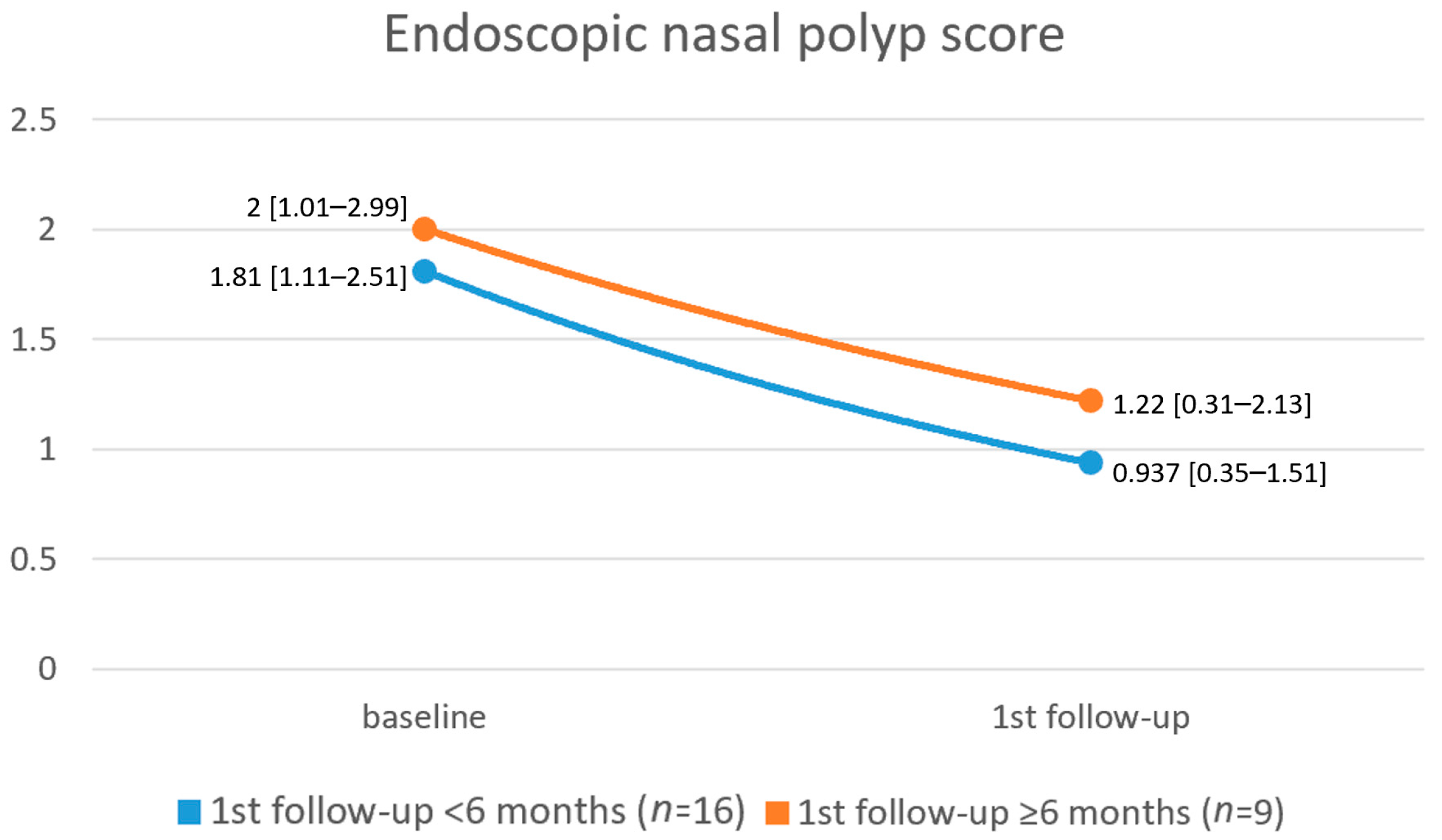
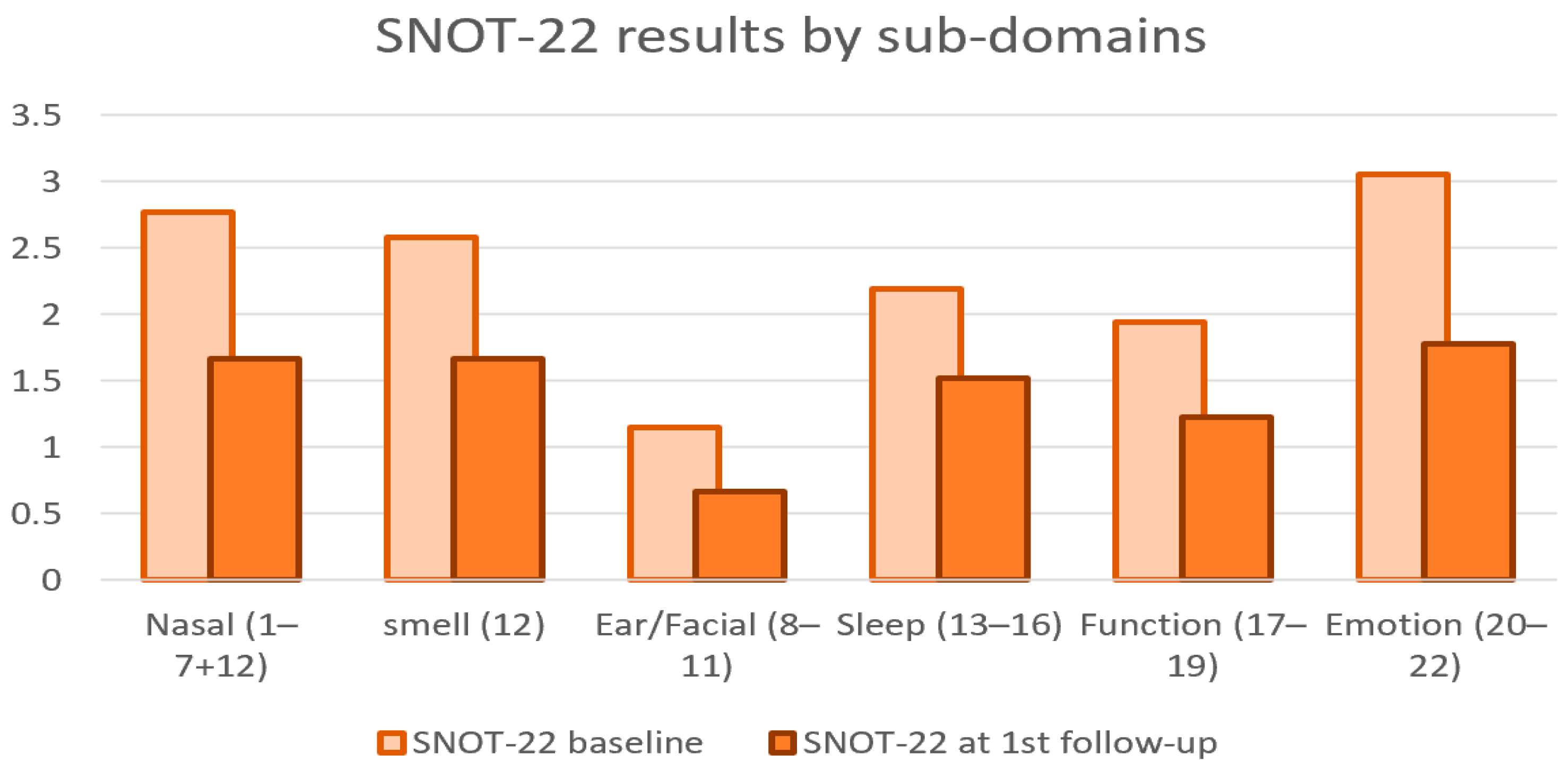
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, A.; Emmanuel, B.; Thomas, K.; Guiang, H. Systematic literature review of the epidemiology and clinical burden of chronic rhinosinusitis with nasal polyposis. Curr. Med. Res Opin. 2020, 36, 1897–1911. [Google Scholar] [CrossRef] [PubMed]
- Orlandi, R.R.; Kingdom, T.T.; Smith, T.L.; Bleier, B.; DeConde, A.; Luong, A.U.; Poetker, D.M.; Soler, Z.; Welch, K.C.; Wise, S.K.; et al. International consensus statement on allergy and rhinology: Rhinosinusitis 2021. Int. Forum Allergy Rhinol. 2021, 11, 213–739. [Google Scholar] [CrossRef] [PubMed]
- Adnane, C.; Adouly, T.; Khallouk, A.; Rouadi, S.; Abada, R.; Roubal, M.; Mahtar, M. Using preoperative unsupervised cluster analysis of chronic rhinosinusitis to inform patient decision and endoscopic sinus surgery outcome. Eur. Arch. Oto-Rhino-Laryngol. 2017, 274, 879–885. [Google Scholar] [CrossRef]
- DeConde, A.S.; Mace, J.C.; Levy, J.M.; Rudmik, L.; Alt, J.A.; Smith, T.L. Prevalence of polyp recurrence after endoscopic sinus surgery for chronic rhinosinusitis with nasal polyposis. Laryngoscope 2017, 127, 550–555. [Google Scholar] [CrossRef]
- Gevaert, P.; Omachi, T.A.; Corren, J.; Mullol, J.; Han, J.; Lee, S.E.; Kaufman, D.; Ligueros-Saylan, M.; Howard, M.; Zhu, R.; et al. Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J. Allergy Clin. Immunol. 2020, 146, 595–605. [Google Scholar] [CrossRef]
- De Corso, E.; Bellocchi, G.; De Benedetto, M.; Lombardo, N.; Macchi, A.; Malvezzi, L.; Motta, G.; Pagella, F.; Vicini, C.; Passali, D. Biologics for severe uncontrolled chronic rhinosinusitis with nasal polyps: A change management approach. Consensus of the Joint Committee of Italian Society of Otorhinolaryngology on biologics in rhinology. ACTA Otorhinolaryngol. Ital. 2022, 42, 1–16. [Google Scholar] [CrossRef]
- Bachert, C.; Han, J.K.; Desrosiers, M.; Hellings, P.W.; Amin, N.; Lee, S.E.; Mullol, J.; Greos, L.S.; Bosso, J.V.; Laidlaw, T.M.; et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): Results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019, 394, 1638–1650. [Google Scholar] [CrossRef]
- Kilty, S.J.; Lasso, A. Canadian real-world study of access and clinical results using dupilumab for chronic rhinosinusitis with polyps. J. Otolaryngol.-Head Neck Surg. 2022, 51, 17. [Google Scholar] [CrossRef]
- De Corso, E.; Settimi, S.; Montuori, C.; Corbò, M.; Passali, G.C.; Porru, D.P.; Lo Verde, S.; Spanu, C.; Penazzi, D.; Di Bella, G.A.; et al. Effectiveness of Dupilumab in the Treatment of Patients with Severe Uncontrolled CRSwNP: A “Real-Life” Observational Study in the First Year of Treatment. J. Clin. Med. 2022, 11, 2684. [Google Scholar] [CrossRef]
- Bachert, C.; Zhang, N.; Cavaliere, C.; Weiping, W.; Gevaert, E.; Krysko, O. Biologics for chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol. 2020, 145, 725–739. [Google Scholar] [CrossRef] [PubMed]
- Bachert, C.; Han, J.K.; Desrosiers, M.Y.; Gevaert, P.; Heffler, E.; Hopkins, C.; Tversky, J.R.; Barker, P.; Cohen, D.; Emson, C.; et al. Efficacy and safety of benralizumab in chronic rhinosinusitis with nasal polyps: A randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 2022, 149, 1309–1317.e12. [Google Scholar] [CrossRef] [PubMed]
- Han, J.K.; Bachert, C.; Fokkens, W.; Desrosiers, M.; Wagenmann, M.; Lee, S.E.; Smith, S.G.; Martin, N.; Mayer, B.; Yancey, S.W.; et al. Mepolizumab for chronic rhinosinusitis with nasal polyps (SYNAPSE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir. Med. 2021, 9, 1141–1153. [Google Scholar] [CrossRef] [PubMed]
- Kariyawasam, H.H.; James, L.K. Chronic Rhinosinusitis with Nasal Polyps: Targeting IgE with Anti-IgE Omalizumab Therapy. Drug Des. Dev. Ther. 2020, 14, 5483. [Google Scholar] [CrossRef]
- Bachert, C.; Hellings, P.W.; Mullol, J.; Hamilos, D.L.; Gevaert, P.; Naclerio, R.M.; Joish, V.N.; Chao, J.; Mannent, L.P.; Amin, N.; et al. Dupilumab improves health-related quality of life in patients with chronic rhinosinusitis with nasal polyposis. Allergy 2020, 75, 148–157. [Google Scholar] [CrossRef]
- Fujieda, S.; Matsune, S.; Takeno, S.; Ohta, N.; Asako, M.; Bachert, C.; Inoue, T.; Takahashi, Y.; Fujita, H.; Deniz, Y.; et al. Dupilumab efficacy in chronic rhinosinusitis with nasal polyps from SINUS-52 is unaffected by eosinophilic status. Allergy Eur. J. Allergy Clin. Immunol. 2022, 77, 186–196. [Google Scholar] [CrossRef]
- Soler, Z.M.; Sauer, D.A.; Mace, J.C.; Smith, T.L. Ethmoid Histopathology does not Predict Olfactory Outcomes after Endoscopic Sinus Surgery. Am. J. Rhinol. Allergy 2010, 24, 281–285. [Google Scholar] [CrossRef]
- Cantone, E.; De Corso, E.; Ricciardiello, F.; Di Nola, C.; Grimaldi, G.; Allocca, V.; Motta, G. Olfaction Recovery following Dupilumab Is Independent of Nasal Polyp Reduction in CRSwNP. J. Pers. Med. 2022, 12, 1215. [Google Scholar] [CrossRef]
- Mace, J.C.; Michael, Y.L.; Carlson, N.E.; Litvack, J.R.; Smith, T.L. Correlations between Endoscopy Score and Quality of Life Changes after Sinus Surgery. Arch. Otolaryngol. Neck Surg. 2010, 136, 340–346. [Google Scholar] [CrossRef]
- Schalek, P.; Hart, L.; Fuksa, J.; Guha, A. Quality of life in CRSwNP: Evaluation of ACCESS and Lund–Mackay computed tomography scores versus the QoL questionnaire. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 5721–5725. [Google Scholar] [CrossRef]
- Hopkins, C.; Gillett, S.; Slack, R.; Lund, V.J.; Browne, J.P. Psychometric validity of the 22-item Sinonasal Outcome Test. Clin. Otolaryngol. 2009, 34, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Galitz, Y.S.; Halperin, D.; Bavnik, Y.; Warman, M. Sino-Nasal Outcome Test–22. Otolaryngol.—Head Neck Surg. 2016, 154, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Lourijsen, E.S.; de Borgie, C.A.J.M.; Vleming, M.; Fokkens, W.J. Endoscopic sinus surgery in adult patients with chronic rhinosinusitis with nasal polyps (PolypESS): Study protocol for a randomised controlled trial. Trials 2017, 18, 39. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, N.I.; Mace, J.C.; Bodner, T.E.; Alt, J.A.; Deconde, A.S.; Levy, J.M.; Smith, T.L. Investigating the Minimal Clinically Important Difference for SNOT-22 Symptom Domains in Surgically Managed Chronic Rhinosinusitis. Int. Forum Allergy Rhinol. 2017, 7, 1149. [Google Scholar] [CrossRef]
- Hellings, P.W.; Akdis, C.A.; Bachert, C.; Bousquet, J.; Pugin, B.; Adriaensen, G.; Advani, R.; Agache, I.; Anjo, C.; Anmolsingh, R.; et al. EUFOREA Rhinology Research Forum 2016: Report of the brainstorming sessions on needs and priorities in rhinitis and rhinosinusitis. Rhinol. J. 2017, 55, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Bassiouni, A.; Wormald, P.J. Role of frontal sinus surgery in nasal polyp recurrence. Laryngoscope 2013, 123, 36–41. [Google Scholar] [CrossRef]
- Cai, S.; Xu, S.; Lou, H.; Zhang, L. Comparison of Different Biologics for Treating Chronic Rhinosinusitis with Nasal Polyps: A Network Analysis. J. Allergy Clin. Immunol. Pract. 2022, 10, 1876–1886.e7. [Google Scholar] [CrossRef]
- Haxel, B.R.; Hummel, T.; Fruth, K.; Lorenz, K.; Gunder, N.; Nahrath, P.; Cuevas, M. Real-world-effectiveness of biological treatment for severe chronic rhinosinusitis with nasal polyps. Rhinology 2022, 60, 435–443. [Google Scholar] [CrossRef]
- Dharmarajan, H.; Falade, O.; Lee, S.E.; Wang, E.W. Outcomes of dupilumab treatment versus endoscopic sinus surgery for chronic rhinosinusitis with nasal polyps. Int. Forum Allergy Rhinol. 2022, 12, 986–995. [Google Scholar] [CrossRef]
| Demographics, Laboratory Findings, Clinical Features and Background | Whole Sample (n = 38) | Attended the Follow-Up Visits (n = 25) | Did Not Attend Follow-Up Visits (n = 13) |
|---|---|---|---|
| Age, mean (range) | 51.55 (22–81) | 55.28 (22–81) | 44.38 (30–73) |
| Gender (male:female) | 20:18 | 13:12 | 7:6 |
| Samter’s triad | 9 (23%) | 8 (32%) | 1 (7%) |
| Asthma | 34 (89%) | 24 (96%) | 10 (76%) |
| Allergic status | 25 (65%) | 18 (72%) | 7 (53%) |
| Antihistamine/Montelukast usage | 18 (47%) | 13 (52%) | 5 (38%) |
| Steroid nasal spray usage | 27 (71%) | 20 (80%) | 7 (53%) |
| Steroid inhaler usage | 31 (82%) | 23 (92%) | 8 (61%) |
| Blood Eosinophils%, mean ± SD | 8.26 ± 4.53 | 8.33 ± 5.26 | 8.12 ± 2.92 |
| Blood Eosinophils#, mean ± SD | 0.76 ± 0.92 | 0.57 ± 0.45 | 1.14 ± 1.46 |
| Total IgE, mean ± SD | 310 ± 426 | 400 ± 545 | 158 ± 214 |
| Systemic steroid treatment | 38 (100%) | 25 (100%) | 13 (100%) |
| Number of prior Endoscopic Sinus Surgeries, Mean ± SD | 2.13 ± 1.33 | 2.12 ± 1.30 | 2.15 ± 1.46 |
| Months since last surgery, Mean ± SD | 29.6 ± 29.7 | 25.45 ± 20.50 | 37.9 ± 42.6 |
| Endoscopic nasal polyp score, Mean ± SD | 2.1 ± 1.3 | 1.87 ± 1.42 | 2.5 ± 0.9 |
| SNOT-22 score, Mean ± SD | 62.96 ± 25.42 | 56.21 ± 28.33 | 71.54 ± 19.04 |
| Variable | 95% Confidence Interval | Odds Ratio | p Value |
|---|---|---|---|
| Age | −0.3–0.16 | 0.93 | 0.32 |
| Gender | −2.26–4.4 | 2.9 | 0.3 |
| Samter’s triad | −2.71–2.69 | 0.98 | 0.98 |
| Asthma | −9.05–12.0 | 4.38 | 0.6 |
| Antihistamine/Montelukast usage | −3.3–2.89 | 0.81 | 0.8 |
| Steroid nasal spray usage | −2.24–3.84 | 2.21 | 0.37 |
| Steroid inhaler usage | −4.53–4.75 | 1.12 | 0.92 |
| Blood Eosinophils# | −1.39–2.27 | 1.55 | 0.4 |
| Number of prior endoscopic sinus surgeries | −1.54–1.86 | 1.17 | 0.72 |
| Variable | 95% Confidence Interval | Odds Ratio | p Value |
|---|---|---|---|
| Age | −0.13–0.02 | 0.94 | 0.09 |
| Gender | −0.42–1.97 | 2.16 | 0.1 |
| Samter’s triad | −1.07–0.98 | 0.95 | 0.87 |
| Asthma | −2.68–5.42 | 3.93 | 0.28 |
| Antihistamine/Montelukast usage | −1.28–0.99 | 0.86 | 0.64 |
| Steroid nasal spray usage | −0.46–1.71 | 1.86 | 0.13 |
| Steroid inhaler usage | −1.75–1.72 | 0.98 | 0.97 |
| Blood Eosinophils% | −0.01–0.09 | 1.04 | 0.09 |
| Number of prior endoscopic sinus surgeries | −0.55–0.77 | 1.11 | 0.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Book, R.; Eligal, S.; Tal, Y.; Eliashar, R. Biological Treatment for Uncontrolled Chronic Rhinosinusitis with Nasal Polyps: Preliminary Real-World Results from a Tertiary Medical Center. J. Clin. Med. 2023, 12, 3671. https://doi.org/10.3390/jcm12113671
Book R, Eligal S, Tal Y, Eliashar R. Biological Treatment for Uncontrolled Chronic Rhinosinusitis with Nasal Polyps: Preliminary Real-World Results from a Tertiary Medical Center. Journal of Clinical Medicine. 2023; 12(11):3671. https://doi.org/10.3390/jcm12113671
Chicago/Turabian StyleBook, Reut, Shalom Eligal, Yuval Tal, and Ron Eliashar. 2023. "Biological Treatment for Uncontrolled Chronic Rhinosinusitis with Nasal Polyps: Preliminary Real-World Results from a Tertiary Medical Center" Journal of Clinical Medicine 12, no. 11: 3671. https://doi.org/10.3390/jcm12113671
APA StyleBook, R., Eligal, S., Tal, Y., & Eliashar, R. (2023). Biological Treatment for Uncontrolled Chronic Rhinosinusitis with Nasal Polyps: Preliminary Real-World Results from a Tertiary Medical Center. Journal of Clinical Medicine, 12(11), 3671. https://doi.org/10.3390/jcm12113671