Local Anesthesia Onset and Pain Perception in Hemophilic and Thalassemic Conditions
Abstract
1. Introduction
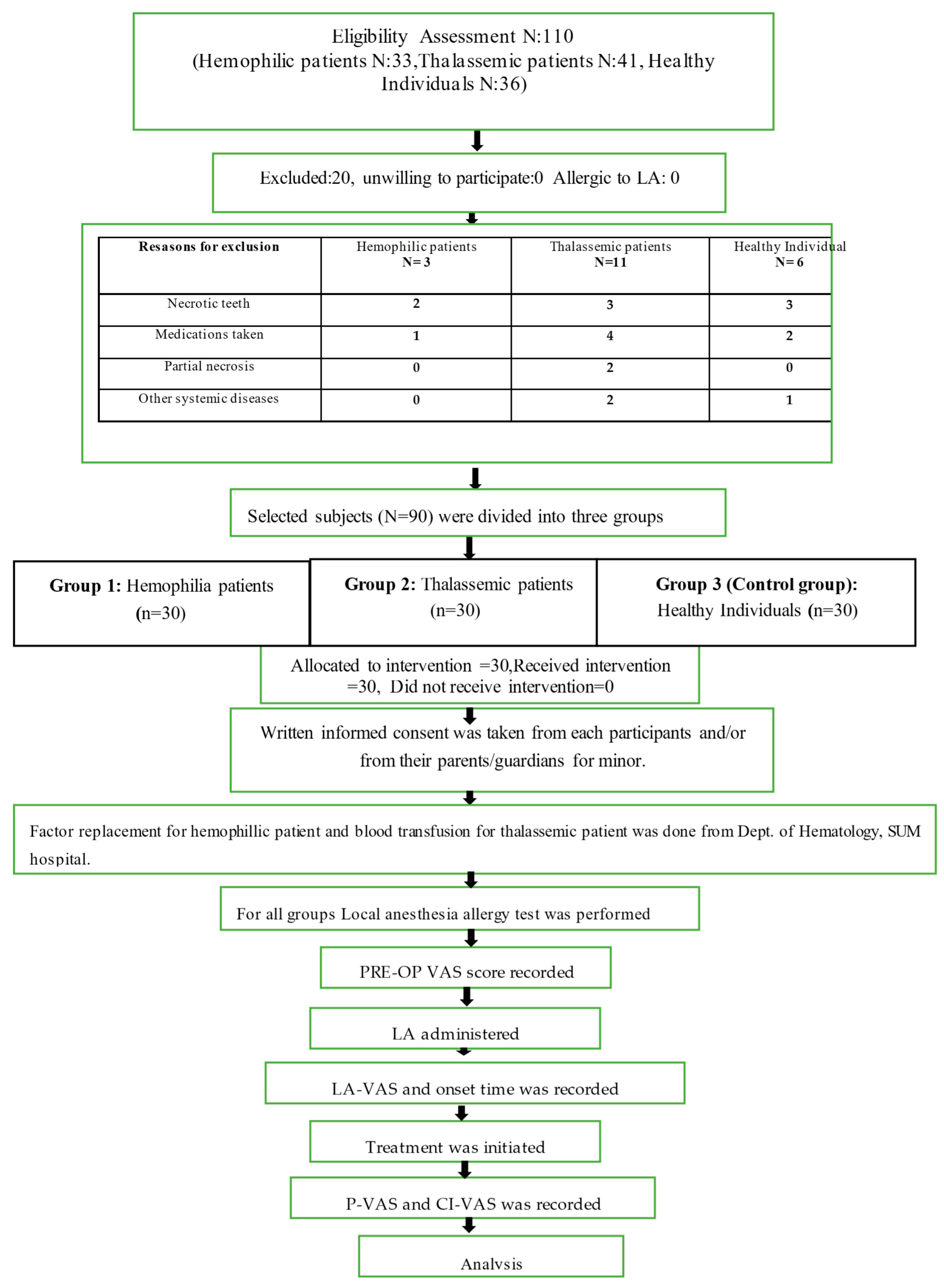
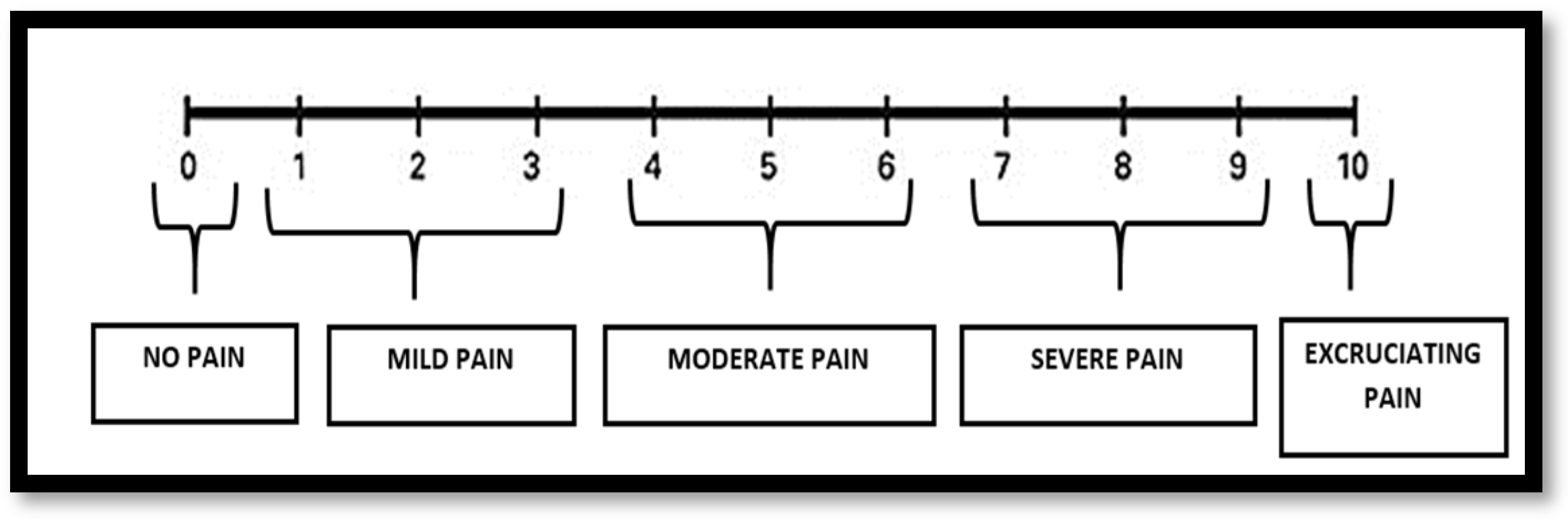
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shastry, S.P.; Kaul, R.; Baroudi, K.; Umar, D. Hemophilia A: Dental Considerations and Management. J. Int. Soc. Prev. Community Dent. 2014, 4, S147. [Google Scholar] [PubMed]
- Marengo-Rowe, A.J. The Thalassemias and Related Disorders. Bayl. Univ. Med. Cent. Proc. 2007, 20, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Gosein, M.; Maharaj, P.; Balkaransingh, P.; Banfield, R.; Greene, C.; Latchman, S.; Sinanan, A. Imaging Features of Thalassaemia. Br. J. Radiol. 2019, 92, 20180658. [Google Scholar] [CrossRef]
- Madhok, D.S.; Madhok, D.S. Dental Considerations in Thalassemic Patients. IOSR J. Dent. Med. Sci. 2014, 13, 57–62. [Google Scholar] [CrossRef]
- Dudeja, P.G.; Dudeja, K.K.; Lakhanpal, M.; Ali, S. Endodontic Management of a Haemophilic Patient—A Clinical Perspective. J. Clin. Diagn. Res. JCDR 2014, 8, ZD17. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, N.M.; Moussa, S.A. Endodontic Treatment in the Patients with Bleeding Disorders—Short Review. Int. J. Oral Health Dent. Manag. 2018, 2, 1–3. [Google Scholar] [CrossRef]
- Ricerca, B.M.; Di Girolamo, A.; Rund, D. Infections in Thalassemia and Hemoglobinopathies: Focus on Therapy-Related Complications. Mediterr. J. Hematol. Infect. Dis. 2009, 1, e2009028. [Google Scholar] [CrossRef]
- Helmi, N.; Bashir, M.; Shireen, A.; Ahmed, I.M. Thalassemia Review: Features, Dental Considerations and Management. Electron. Physician 2017, 9, 4003–4008. [Google Scholar] [CrossRef]
- Ong, C.K.S.; Lirk, P.; Tan, C.H.; Seymour, R.A. An Evidence-Based Update on Nonsteroidal Anti-Inflammatory Drugs. Clin. Med. Res. 2007, 5, 19–34. [Google Scholar] [CrossRef]
- Dolan, G. The Challenge of an Ageing Haemophilic Population. Haemoph. Off. J. World Fed. Hemoph. 2010, 16 (Suppl. S5), 11–16. [Google Scholar] [CrossRef]
- Arachchillage, D.R.J.; Makris, M. Choosing and Using Non-Steroidal Anti-Inflammatory Drugs in Haemophilia. Haemophilia 2016, 22, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-Y.; Wu, Z.-F.; Lin, Y.-T.; Cheng, K.-I.; Huang, Y.-T.; Huang, S.-T.; Hargono, A.; Li, C.-Y. Association between General Anesthesia and Root Canal Treatment Outcomes in Patients with Mental Disability: A Retrospective Cohort Study. J. Pers. Med. 2022, 12, 213. [Google Scholar] [CrossRef] [PubMed]
- Nusstein, J.M.; Reader, A.; Drum, M. Local Anesthesia Strategies for the Patient with a “Hot” Tooth. Dent. Clin. N. Am. 2010, 54, 237–247. [Google Scholar] [CrossRef]
- Oosterink, F.M.; De Jongh, A.; Hoogstraten, J. Prevalence of Dental Fear and Phobia Relative to Other Fear and Phobia Subtypes. Eur. J. Oral Sci. 2009, 117, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, N.B.; Mantri, S.P.; Dube, K.A.; Jaiswal, N.U.; Singh, V.J. Pulpal-Anesthesia of a Mandibular First Molar with Irreversible Pulpitis by Inferior Alveolar Nerve Block plus Buccal Infiltration Using Articaine or Lignocaine. J. Conserv. Dent. JCD 2020, 23, 201–205. [Google Scholar] [CrossRef]
- Nair, M.; Gurunathan, D. Comparative Evaluation of the Efficacy of Two Anesthetic Gels (2% Lignocaine and 20% Benzocaine) in Reducing Pain during Administration of Local Anesthesia—A Randomized Controlled Trial. J. Anaesthesiol. Clin. Pharmacol. 2019, 35, 65. [Google Scholar]
- Malamed, S.F. Handbook of Local Anesthesia—E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Kambalimath, D.H.; Dolas, R.S.; Kambalimath, H.V.; Agrawal, S.M. Efficacy of 4% Articaine and 2% Lidocaine: A Clinical Study. J. Maxillofac. Oral Surg. 2013, 12, 3–10. [Google Scholar] [CrossRef]
- Tortamano, I.P.; Siviero, M.; Lee, S.; Sampaio, R.M.; Simone, J.L.; Rocha, R.G. Onset and Duration Period of Pulpal Anesthesia of Articaine and Lidocaine in Inferior Alveolar Nerve Block. Braz. Dent. J. 2013, 24, 371–374. [Google Scholar] [CrossRef]
- Pain Intensity Threshold—Pain Management Collaboratory. Available online: https://painmanagementcollaboratory.org/pain-intensity-threshold/ (accessed on 15 April 2023).
- Ahmad, Z.H.; Ravikumar, H.; Karale, R.; Preethanath, R.S.; Sukumaran, A. Study of the Anesthetic Efficacy of Inferior Alveolar Nerve Block Using Articaine in Irreversible Pulpitis. J. Contemp. Dent. Pract. 2014, 15, 71–74. [Google Scholar] [CrossRef]
- Lee, J.; Lee, J.-Y.; Kim, H.J.; Seo, K.-S. Dental Anesthesia for Patients with Allergic Reactions to Lidocaine: Two Case Reports. J. Dent. Anesth. Pain Med. 2016, 16, 209–212. [Google Scholar] [CrossRef]
- Sui, H.; Lv, Y.; Xiao, M.; Zhou, L.; Qiao, F.; Zheng, J.; Sun, C.; Fu, J.; Chen, Y.; Liu, Y.; et al. Relationship between the Difference in Electric Pulp Test Values and the Diagnostic Type of Pulpitis. BMC Oral Health 2021, 21, 339. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.; Reader, A.; Beck, M.; Nusstein, J. A Prospective, Randomized, Double-Blind Comparison of Bupivacaine and Lidocaine for Inferior Alveolar Nerve Blocks. J. Endod. 2005, 31, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Bertamino, M.; Riccardi, F.; Banov, L.; Svahn, J.; Molinari, A.C. Hemophilia Care in the Pediatric Age. J. Clin. Med. 2017, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Vij, R.; Machado, R.F. Pulmonary Complications of Hemoglobinopathies. Chest 2010, 138, 973–983. [Google Scholar] [CrossRef]
- Joly, P.; Pondarre, C.; Badens, C. Beta-Thalassemias: Molecular, Epidemiological, Diagnostical and Clinical Aspects. Ann. Biol. Clin. 2014, 72, 639–668. [Google Scholar] [CrossRef]
- Boström, E.A.; Lira-Junior, R. Non-Malignant Blood Disorders and Their Impact on Oral Health: An Overview. Curr. Oral Health Rep. 2019, 6, 161–168. [Google Scholar] [CrossRef]
- Kumar, M.; Pai, K.M.; Kurien, A.; Vineetha, R. Oral Hygiene and Dentition Status in Children and Adults with Hemophilia: A Case–Control Study. Spec. Care Dent. 2018, 38, 391–394. [Google Scholar] [CrossRef]
- Nayak, S.; Govind, S.; Jena, A.; Samal, P.; Sahoo, N.K.; Rath, S. Evaluation of Oral Hygiene Status, Salivary Fluoride Concentration and Microbial Level in Thalassemic and Hemophilic Patients. Siriraj Med. J. 2022, 74, 314–322. [Google Scholar] [CrossRef]
- Gringeri, A.; Mantovani, L.; Mackensen, S.V. Quality of Life Assessment in Clinical Practice in Haemophilia Treatment. Haemoph. Off. J. World Fed. Hemoph. 2006, 12 (Suppl. S3), 22–29. [Google Scholar] [CrossRef]
- Logan, W.H.G.; Kronfeld, R. Development of the Human Jaws and Surrounding Structures from Birth to the Age of Fifteen Years. J. Am. Dent. Assoc. 1933, 20, 379–428. [Google Scholar] [CrossRef]
- Motta, I.; Mancarella, M.; Marcon, A.; Vicenzi, M.; Cappellini, M.D. Management of Age-Associated Medical Complications in Patients with β-Thalassemia. Expert Rev. Hematol. 2020, 13, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Tagliaferri, A.; Mannucci, P.M. The Management of Hemophilia in Elderly Patients. Clin. Interv. Aging 2007, 2, 361–368. [Google Scholar] [PubMed]
- Stromer, W.; Pabinger, I.; Ay, C.; Crevenna, R.; Donnerer, J.; Feistritzer, C.; Hemberger, S.; Likar, R.; Sevelda, F.; Thom, K.; et al. Pain Management in Hemophilia: Expert Recommendations. Wien. Klin. Wochenschr. 2021, 133, 1042–1056. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.; Martin, M.; Carson, S.; Oliveros, O.; Green, S.; Coates, T.; Eile, J.; Schilling, L.; Dinu, B.; Mendoza, T.; et al. Pain in Thalassaemia: The Effects of Age on Pain Frequency and Severity. Br. J. Haematol. 2013, 160, 680–687. [Google Scholar] [CrossRef]
- Auerswald, G.; Dolan, G.; Duffy, A.; Hermans, C.; Jiménez-Yuste, V.; Ljung, R.; Morfini, M.; Lambert, T.; Šalek, S.Z. Pain and Pain Management in Haemophilia. Blood Coagul. Fibrinolysis 2016, 27, 845. [Google Scholar] [CrossRef]
- Glossary of Endodontic Terms—American Association of Endodontists. Available online: https://www.aae.org/specialty/clinical-resources/glossary-endodontic-terms/ (accessed on 16 April 2023).
- Santos, J.M.; Pereira, J.F.; Marques, A.; Sequeira, D.B.; Friedman, S. Vital Pulp Therapy in Permanent Mature Posterior Teeth with Symptomatic Irreversible Pulpitis: A Systematic Review of Treatment Outcomes. Medicina 2021, 57, 573. [Google Scholar] [CrossRef]
- Boopathi, T.; Sebeena, M.; Sivakumar, K.; Harikaran, J.; Karthick, K.; Raj, A. Supplemental Pulpal Anesthesia for Mandibular Teeth. J. Pharm. Bioallied Sci. 2013, 5, S103–S108. [Google Scholar] [CrossRef]
- Anderson, J.A.M.; Brewer, A.; Creagh, D.; Hook, S.; Mainwaring, J.; McKernan, A.; Yee, T.T.; Yeung, C.A. Guidance on the Dental Management of Patients with Haemophilia and Congenital Bleeding Disorders. Br. Dent. J. 2013, 215, 497–504. [Google Scholar] [CrossRef]
- Srivastava, A.; Brewer, A.K.; Mauser-Bunschoten, E.P.; Key, N.S.; Kitchen, S.; Llinas, A.; Ludlam, C.A.; Mahlangu, J.N.; Mulder, K.; Poon, M.C.; et al. Guidelines for the Management of Hemophilia. Haemoph. Off. J. World Fed. Hemoph. 2013, 19, e1–e47. [Google Scholar] [CrossRef]
- Brewer, A.; Correa, M.E. Guidelines for Dental Treatment of Patients with Inherited Bleeding Disorders. Haemophilia 2005, 11, 504–509. [Google Scholar]
- Kar, A.; Phadnis, S.; Dharmarajan, S.; Nakade, J. Epidemiology & Social Costs of Haemophilia in India. Indian J. Med. Res. 2014, 140, 19. [Google Scholar] [PubMed]
- Ahn, J.; Pogrel, M.A. The Effects of 2% Lidocaine with 1:100,000 Epinephrine on Pulpal and Gingival Blood Flow. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 1998, 85, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Berman, L.H.; Hargreaves, K.M. Cohen’s Pathways of the Pulp Expert Consult—E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Turk, D.C.; Dworkin, R.H.; Revicki, D.; Harding, G.; Burke, L.B.; Cella, D.; Cleeland, C.S.; Cowan, P.; Farrar, J.T.; Hertz, S.; et al. Identifying Important Outcome Domains for Chronic Pain Clinical Trials: An IMMPACT Survey of People with Pain. Pain 2008, 137, 276–285. [Google Scholar] [CrossRef]
- Lee, C.; Yang, H. Alternative Techniques for Failure of Conventional Inferior Alveolar Nerve Block. J. Dent. Anesth. Pain Med. 2019, 19, 125. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.A.; Michanowicz, A.E.; Mundell, R.D.; Wilson, E.G. A Pilot Study of the Clinical Problem of Regionally Anesthetizing the Pulp of an Acutely Inflamed Mandibular Molar. Oral Surg. Oral Med. Oral Pathol. 1985, 59, 517–521. [Google Scholar] [CrossRef]
- Byers, M.R.; Taylor, P.E.; Khayat, B.G.; Kimberly, C.L. Effects of Injury and Inflammation on Pulpal and Periapical Nerves. J. Endod. 1990, 16, 78–84. [Google Scholar] [CrossRef]
- Roy, M.L.; Narahashi, T. Differential Properties of Tetrodotoxin-Sensitive and Tetrodotoxin-Resistant Sodium Channels in Rat Dorsal Root Ganglion Neurons. J. Neurosci. 1992, 12, 2104–2111. [Google Scholar] [CrossRef]
- Kjeldsen, H.B.; Klausen, T.W.; Rosenberg, J. Preferred Presentation of the Visual Analog Scale for Measurement of Postoperative Pain. Pain Pract. 2016, 16, 980–984. [Google Scholar] [CrossRef]
- Wang, W. Tolerability of Hypertonic Injectables. Int. J. Pharm. 2015, 490, 308–315. [Google Scholar] [CrossRef]
- Hogan, M.-E.; Perampaladas, K.; Machado, M.; Einarson, T.R.; Taddio, A. Systematic Review and Meta-Analysis of the Effect of Warming Local Anesthetics on Injection Pain. Ann. Emerg. Med. 2011, 58, 86–98. [Google Scholar] [CrossRef]
| Case No. | Age/Sex | Tooth No | Hemophilia | Factor Deficient | Blood Group | Severity of Hemophilia | Factor Replacement Per Appointment [IU/dL] [Eloctate] | Frequency |
|---|---|---|---|---|---|---|---|---|
| 1 | 20/M | 46 | A | VIII | A+ | Severe | 1000 units | Once in every 2 days |
| 2 | 17/M | 36 | A | VIII | B+ | mild | 250 units | 3 days/week |
| 3 | 15/M | 36 | A | VIII | AB+ | mild | 250 units | 2 days/week |
| 4 | 19/F | 46 | A | VIII | AB+ | moderate | 1000 units | 2 days/week |
| 5 | 25/M | 37 | A | VIII | AB+ | moderate | 1000 units | 3 days/week |
| 6 | 30/M | 37 | A | VIII | A+ | Severe | 1500 units | 3 days/week |
| 7 | 22/M | 47 | A | VIII | B+ | Severe | 1500 units | Once in every 2 days |
| 8 | 14/M | 46 | A | VIII | A+ | Severe | 500 units | Once in every 2 days |
| 9 | 16/F | 36 | A | VIII | AB+ | moderate | 500 units | 2 days/week |
| 10 | 28/M | 37 | A | VIII | B+ | moderate | 1000 units | 3 days/week |
| 11 | 27/M | 37 | A | VIII | B+ | mild | 500 units | 2 days/week |
| 12 | 30/M | 47 | A | VIII | AB+ | mild | 500 units | 2 days/week |
| 13 | 24/M | 46 | A | VIII | AB+ | mild | 500 units | 2 days/week |
| 14 | 15/M | 37 | A | VIII | A+ | mild | 500 units | 2 days/week |
| 15 | 24/M | 36 | A | VIII | B+ | mild | 500 units | 2 days/week |
| 16 | 15/M | 46 | A | VIII | AB+ | mild | 500 units | 2 days/week |
| 17 | 43/M | 38 | A | VIII | AB+ | mild | 500 units | 3 days/week |
| 18 | 29/M | 46 | A | VIII | B+ | moderate | 1000 units | 3 days/week |
| 19 | 28/M | 36 | A | VIII | AB+ | Severe | 1500 units | 3 days/week |
| 20 | 26/M | 47 | A | VIII | A+ | Severe | 1000 units | Once in every 2 days |
| 21 | 33/M | 46 | A | VIII | A+ | Severe | 1000 units | Once in every 2 days |
| 22 | 35/M | 46 | A | VIII | A+ | mild | 500 units | 2 days/week |
| 23 | 14/M | 36 | A | VIII | A+ | mild | 500 units | 2 days/week |
| 24 | 18/M | 46 | A | VIII | AB+ | mild | 500 units | 2 days/week |
| 25 | 27/M | 36 | A | VIII | AB+ | mild | 1000 units | 2 days/week |
| 26 | 14/M | 36 | A | VIII | B+ | mild | 500 units | 2 days/week |
| 27 | 20/M | 47 | A | VIII | B+ | Severe | 1000 units | 3 days/week |
| 28 | 22/M | 46 | A | VIII | B+ | mild | 500 units | 2 days/week |
| 29 | 18/M | 36 | A | VIII | AB+ | moderate | 1000 units | 2 days/week |
| 30 | 20/M | 46 | A | VIII | AB+ | mild | 1000 units | 2 days/week |
| S No. | Age/Sex | Tooth No. | Type of Thalassemia | Blood Transfusion | Patient with Iron-Chelation Therapy | Patient without Iron-Chelation Therapy | Splenectomy | Disease Onset |
|---|---|---|---|---|---|---|---|---|
| 1 | 48/M | 47 | B-TI | Once a year | ✓ | 4 years old | ||
| 2 | 50/F | 46 | B-TI | Once in 6 months | ✓ | 6 years old | ||
| 3 | 38/M | 36 | B-TMi | No | ✓ | 13 years old | ||
| 4 | 44/F | 37 | B-TMi | No | ✓ | 16 years old | ||
| 5 | 29/F | 46 | B-TM | Every 2 weeks | ✓ | 2 years old | ||
| 6 | 20/M | 37 | B-TM | Every 2 weeks | ✓ | At birth | ||
| 7 | 35/F | 46 | B-TM | Every 2 weeks | ✓ | ✓ | 2 monthsold | |
| 8 | 18/M | 48 | B-TM | Every 3 weeks | ✓ | At birth | ||
| 9 | 16/M | 47 | B-TM | Every 3 weeks | ✓ | 6 months old | ||
| 10 | 30/M | 47 | B-TM | Every 2 weeks | ✓ | ✓ | 5 months old | |
| 11 | 15/M | 36 | B-TM | Every 3 weeks | ✓ | 6 months old | ||
| 12 | 19/M | 36 | B-TM | Every 3 weeks | ✓ | 2 months old | ||
| 13 | 27/M | 36 | B-TM | Every 2 weeks | ✓ | ✓ | At birth | |
| 14 | 26/M | 48 | B-TM | Every 2 weeks | ✓ | ✓ | At birth | |
| 15 | 15/M | 46 | B-TM | Every 3 weeks | ✓ | 2 months old | ||
| 16 | 58/F | 37 | B-TMi | No | ✓ | 18 years old | ||
| 17 | 14/F | 36 | B-TM | Every 3 weeks | ✓ | |||
| 18 | 17/M | 36 | B-TM | Every 3 weeks | ✓ | 4 months old | ||
| 19 | 20/M | 47 | B-TM | Every 3 weeks | ✓ | 5 months old | ||
| 20 | 32/F | 46 | B-TI | Once in 6 months | ✓ | 10 months | ||
| 21 | 33/F | 36 | B-TI | Once in 6 months | ✓ | 5 years old | ||
| 22 | 38/M | 37 | B-TI | Once a year | ✓ | 7 years old | ||
| 23 | 41/F | 46 | B-TI | Once a year | ✓ | 4 years old | ||
| 24 | 33/F | 38 | B-TI | Once in 6 months | ✓ | 4yrs old | ||
| 25 | 30/M | 46 | B-TM | Every 2 weeks | ✓ | ✓ | At birth | |
| 26 | 26/F | 36 | B-TM | Every 2 weeks | ✓ | 2 months old | ||
| 27 | 32/F | 36 | B-TM | Every 2 weeks | ✓ | ✓ | 5 months old | |
| 28 | 31/M | 46 | B-TM | Every 2 weeks | ✓ | 8 months old | ||
| 29 | 34/M | 46 | B-TMi | No | ✓ | 10 years old | ||
| 30 | 22/F | 37 | B-TM | Every 2 weeks | ✓ | 2 months old |
| Age Group Years | Hemophilic | Thalassemic | Control | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| 14–20 | 14 | 46.7 | 9 | 30 | 6 | 20 |
| 21–29 | 11 | 36.7 | 5 | 16.7 | 7 | 23.3 |
| 30–39 | 4 | 13.3 | 11 | 36.7 | 10 | 33.3 |
| ≥40 | 1 | 3.3 | 5 | 16.7 | 7 | 23.3 |
| Total | 30 | 100 | 30 | 100 | 30 | 100 |
| Mean ± SD (Years) | 22.93 ± 7.17 | 29.7 ± 11.24 | 30.33 ± 10.4 | |||
| Group | Male | Female | Total | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Hemophilic | 28 | 93.3 | 2 | 6.7 | 30 | 100 |
| Thalassemic | 17 | 56.7 | 13 | 43.3 | 30 | 100 |
| Control | 13 | 43.3 | 17 | 56.7 | 30 | 100 |
| Total | 58 | 64.4 | 32 | 35.6 | 90 | 100 |
| Groups | N | Mean | Std. Deviation | Std. Error | ANOVA (p Value) | |
|---|---|---|---|---|---|---|
| Onset time of LA | Hemophilia | 30 | 45.967 | 34.3215 | 6.2662 | 0.56 |
| Thalassemia | 30 | 42.400 | 23.3631 | 4.2655 | ||
| Control | 30 | 38.967 | 12.8532 | 2.3467 | ||
| Total | 90 | 42.444 | 24.9755 | 2.6327 |
| Group | Yes | No | Total | |||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Hemophilic | 6 | 20 | 24 | 80 | 30 | 100 |
| Thalassemic | 4 | 13.3 | 26 | 86.7 | 30 | 100 |
| Control | 4 | 13.3 | 26 | 86.7 | 30 | 100 |
| Total | 14 | 15.6 | 76 | 84.4 | 90 | 100 |
| N | Mean | Std. Deviation | Std. Error | ANOVA (p Value) | ||
|---|---|---|---|---|---|---|
| Pre-op VAS | Hemophilia | 30 | 7.467 | 1.6554 | 0.3022 | 0.17 |
| Thalassemia | 30 | 8.000 | 1.3646 | 0.2491 | ||
| Control | 30 | 8.133 | 1.2521 | 0.2286 | ||
| Total | 90 | 7.867 | 1.4472 | 0.1525 | ||
| LA-VAS | Hemophilia | 30 | 2.733 | 2.5722 | 0.4696 | 0.16 |
| Thalassemia | 30 | 3.300 | 2.1838 | 0.3987 | ||
| Control | 30 | 2.133 | 2.2854 | 0.4173 | ||
| Total | 90 | 2.722 | 2.3751 | 0.2504 | ||
| PE-VAS | Hemophilia | 30 | 1.267 | 2.0833 | 0.3804 | 0.82 |
| Thalassemia | 30 | 1.000 | 1.8004 | 0.3287 | ||
| Control | 30 | 1.000 | 1.7019 | 0.3107 | ||
| Total | 90 | 1.089 | 1.8521 | 0.1952 | ||
| CI-VAS | Hemophilia | 30 | 0.100 | 0.3051 | 0.0557 | 0.55 |
| Thalassemia | 30 | 0.100 | 0.3051 | 0.0557 | ||
| Control | 30 | 0.033 | 0.1826 | 0.0333 | ||
| Total | 90 | 0.078 | 0.2693 | 0.0284 | ||
| N | Mean | Std. Deviation | Std. Error | ANOVA (p Value) | ||
|---|---|---|---|---|---|---|
| Pre-op VAS | Mild | 30 | 1.000 | 0.0000 | 0.0000 | |
| Moderate | 30 | 1.000 | 0.0000 | 0.0000 | ||
| Severe | 30 | 1.000 | 0.0000 | 0.0000 | ||
| Total | 90 | 1.000 | 0.0000 | 0.0000 | ||
| LA-VAS | Mild | 30 | 0.600 | 0.4983 | 0.0910 | 0.048 † |
| Moderate | 30 | 0.700 | 0.4661 | 0.0851 | ||
| Severe | 30 | 0.833 | 0.3790 | 0.0692 | ||
| Total | 90 | 0.711 | 0.4558 | 0.0480 | ||
| PE-VAS | Mild | 30 | 0.567 | 0.7279 | 0.1329 | 0.926 |
| Moderate | 30 | 0.567 | 0.8172 | 0.1492 | ||
| Severe | 30 | 0.500 | 0.7311 | 0.1335 | ||
| Total | 90 | 0.544 | 0.7519 | 0.0793 | ||
| CI-VAS | Mild | 30 | 0.033 | 0.1826 | 0.0333 | 0.547 |
| Moderate | 30 | 0.100 | 0.3051 | 0.0557 | ||
| Severe | 30 | 0.100 | 0.3051 | 0.0557 | ||
| Total | 90 | 0.078 | 0.2693 | 0.0284 | ||
| Model | Coefficients | ||||
|---|---|---|---|---|---|
| Unstandardized Coefficients | Standardized Coefficients | t | Sig. | ||
| B | Std. Error | Beta | |||
| Pre-op VAS | 7.300 | 0.296 | 0.230 | 24.696 | 0.000 |
| LA-VAS | 1.368 | 0.470 | 0.335 | 3.341 | 0.001 |
| PE-VAS | −0.253 | 0.352 | 0.426 | −0.720 | 0.473 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, S.; Govind, S.; Jena, D.; Dash, S.; Jena, S.P.; Yadav, D.; Karan, S.; Kancherla, J.; Jena, A.; Mishra, L.; et al. Local Anesthesia Onset and Pain Perception in Hemophilic and Thalassemic Conditions. J. Clin. Med. 2023, 12, 3646. https://doi.org/10.3390/jcm12113646
Das S, Govind S, Jena D, Dash S, Jena SP, Yadav D, Karan S, Kancherla J, Jena A, Mishra L, et al. Local Anesthesia Onset and Pain Perception in Hemophilic and Thalassemic Conditions. Journal of Clinical Medicine. 2023; 12(11):3646. https://doi.org/10.3390/jcm12113646
Chicago/Turabian StyleDas, Supriya, Shashirekha Govind, Debkant Jena, Sumit Dash, Siba Prasad Jena, Deepika Yadav, Smita Karan, Jyothsna Kancherla, Amit Jena, Lora Mishra, and et al. 2023. "Local Anesthesia Onset and Pain Perception in Hemophilic and Thalassemic Conditions" Journal of Clinical Medicine 12, no. 11: 3646. https://doi.org/10.3390/jcm12113646
APA StyleDas, S., Govind, S., Jena, D., Dash, S., Jena, S. P., Yadav, D., Karan, S., Kancherla, J., Jena, A., Mishra, L., Bal, S. C. B., & Pattanaik, S. (2023). Local Anesthesia Onset and Pain Perception in Hemophilic and Thalassemic Conditions. Journal of Clinical Medicine, 12(11), 3646. https://doi.org/10.3390/jcm12113646








