A Retrospective Multicenter Study of the Clinicopathological Characteristics and Prognosis of Young Adult Patients with Colorectal Cancer: Effects of Chemotherapy on Prognosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Follow-Up
2.4. Outcome Measures
2.5. Statistical Analysis
3. Results
3.1. Patients’ Charateristics
3.2. Perioperative Outcomes
3.3. Pathologic Outcomes
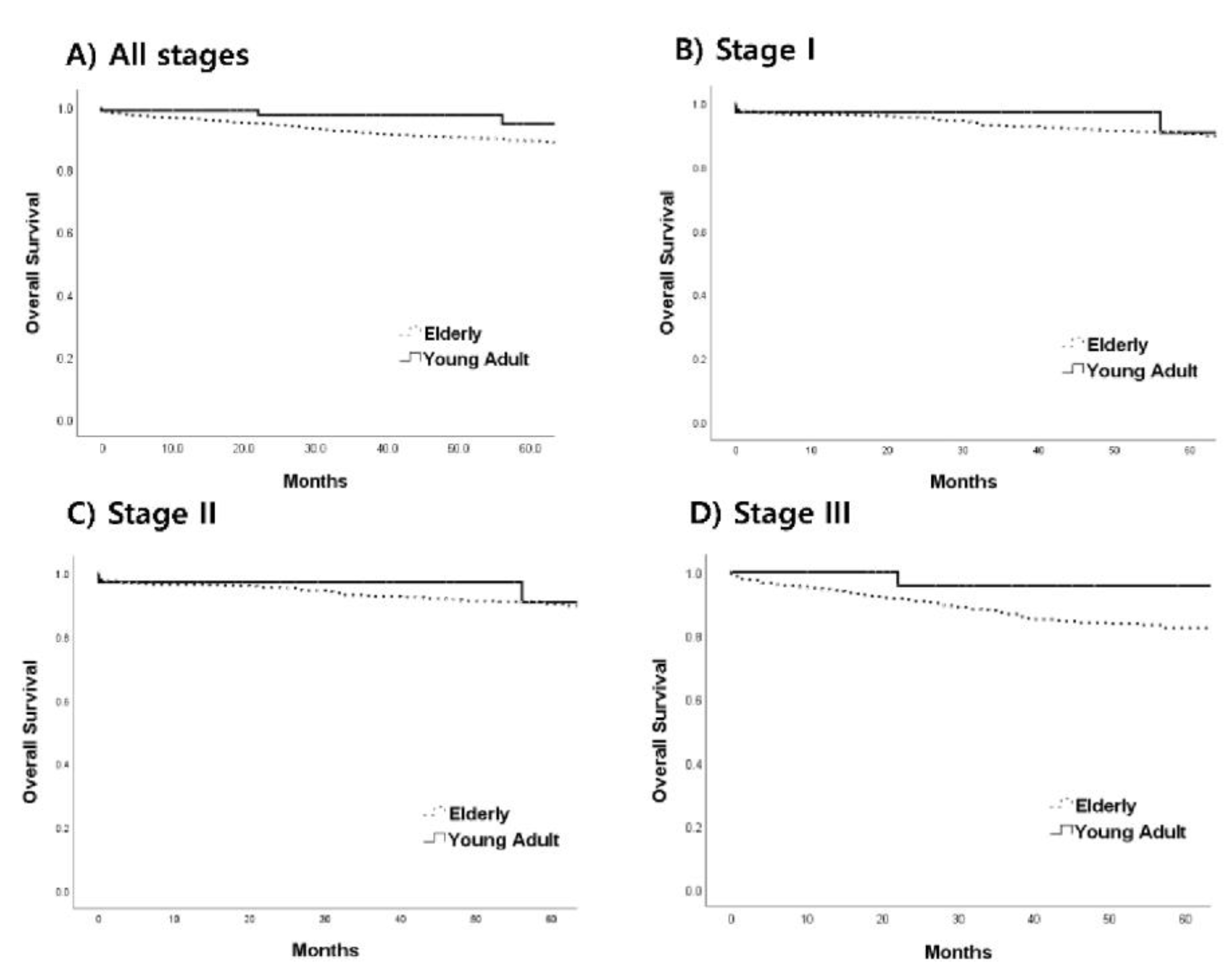
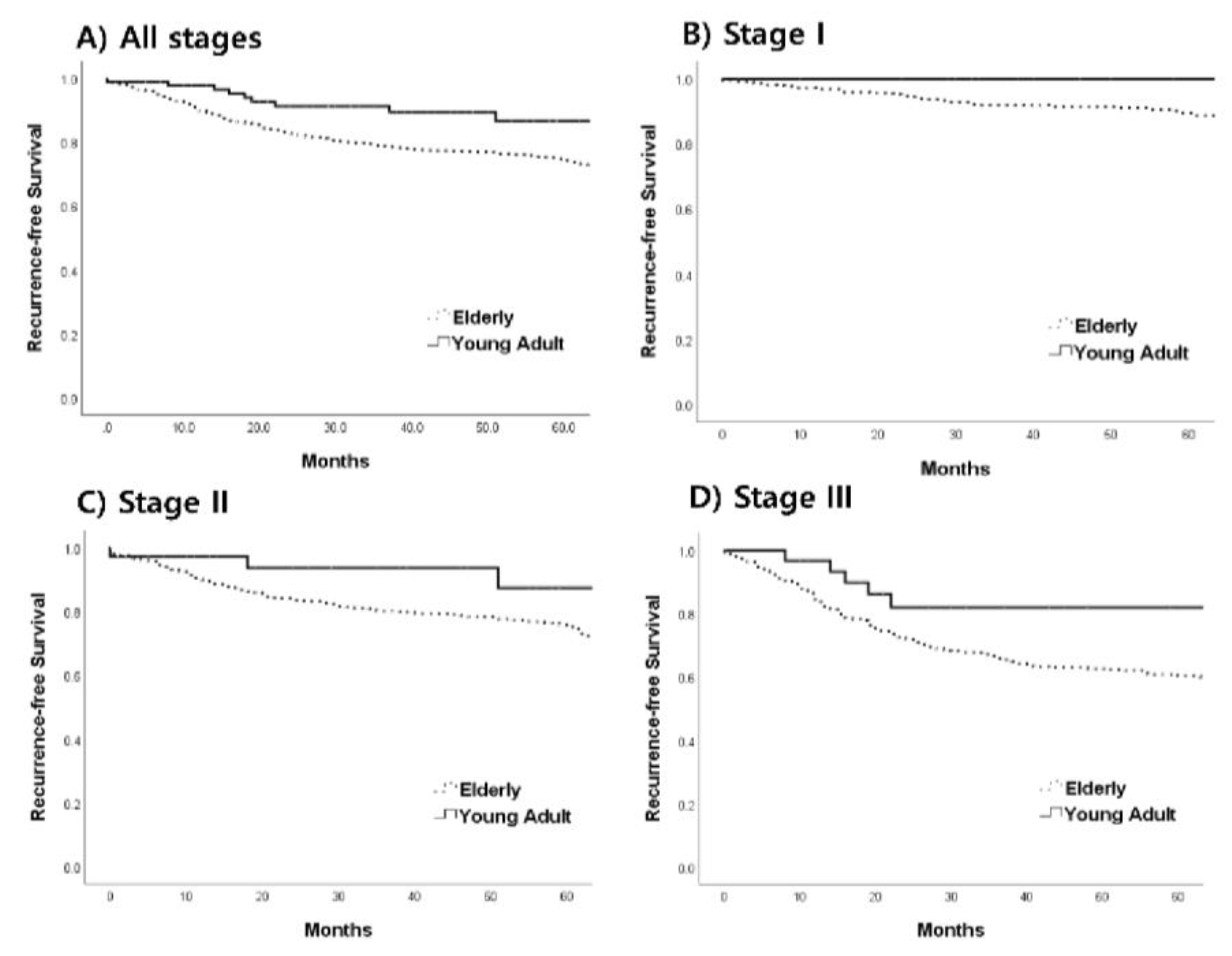
3.4. Prognosis
3.5. Factors Affecting Prognosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Caner J. Clin. 2020, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, M.J.; Ping, J. Clinicopathological Features and Survival Outcomes of Colorectal Cancer in Young Versus Elderly: A Population-Based Cohort Study of SEER 9 Registries Data (1988–2011). Medicine 2015, 94, e1402. [Google Scholar] [CrossRef] [PubMed]
- US Preventive Services Task Force. Screening for colorectal cancer: Recommendation and rationale. Ann. Intern. Med. 2022, 137, 129–131. [Google Scholar]
- Siegel, R.L.; Torre, L.A.; Soerjomataram, I.; Hayes, R.B.; Bray, F.; Weber, T.K.; Jemal, A. Global patterns and trends in colorectal cancer incidence in young adults. Gut 2019, 68, 2179–2185. [Google Scholar] [CrossRef] [PubMed]
- Ahnen, D.J.; Wade, S.W.; Jones, W.F.; Sifri, R.; Mendoza Silveiras, J.; Greenamyer, J.; Guiffre, S.; Axilbund, J.; Spiegel, A.; You, Y.N. The increasing incidence of young-onset colorectal cancer: A call to action. Mayo. Clin. Proc. 2014, 89, 216–224. [Google Scholar] [CrossRef]
- Young, J.P.; Win, A.K.; Rosty, C.; Flight, I.; Roder, D.; Young, G.P.; Frank, O.; Suthers, G.K.; Hewett, P.J.; Ruszkiewicz, A.; et al. Rising incidence of early-onset colorectal cancer in Australia over two decades: Report and review. J. Gastroenterol. Hepatol. 2015, 30, 6–13. [Google Scholar] [CrossRef]
- Davis, D.M.; Marcet, J.E.; Frattini, J.C.; Prather, A.D.; Mateka, J.J.; Nfonsam, V.N. Is it time to lower the recommended screening age for colorectal cancer? J. Am. Coll. Surg. 2011, 213, 352–361. [Google Scholar] [CrossRef]
- Schellerer, V.S.; Hohenberger, W.; Croner, R.S. Is it time to lower the recommended screening age for colorectal cancer? J. Am. Coll. Surg. 2012, 214, 377–378, author reply 378–379. [Google Scholar] [CrossRef]
- Peterse, E.F.P.; Meester, R.G.S.; Siegel, R.L.; Chen, J.C.; Dwyer, A.; Ahnen, D.J.; Smith, R.A.; Zauber, A.G.; Lansdorp-Vogelaar, I. The impact of the rising colorectal cancer incidence in young adults on the optimal age to start screening: Microsimulation analysis I to inform the American Cancer Society colorectal cancer screening guideline. Cancer 2018, 124, 2964–2973. [Google Scholar] [CrossRef]
- Rodriguez, L.; Brennan, K.; Karim, S.; Nanji, S.; Patel, S.V.; Booth, C.M. Disease Characteristics, Clinical Management, and Outcomes of Young Patients with Colon Cancer: A Population-based Study. Clin. Color. Cancer 2018, 17, e651–e661. [Google Scholar] [CrossRef]
- Rho, Y.S.; Gilabert, M.; Polom, K.; Aladashvili, A.; Kopeckova, K.; Megdanova, V.; Coleman, N.; Greally, M.; Marrelli, D.; Roviello, F.; et al. Comparing Clinical Characteristics and Outcomes of Young-onset and Late-onset Colorectal Cancer: An International Collaborative Study. Clin. Colorectal. Cancer 2017, 16, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Bao, F.; Yan, J.; Liu, H.; Li, T.; Chen, H.; Li, G. Poor prognosis of young patients with colorectal cancer: A retrospective study. Int. J. Color. Dis. 2017, 32, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Goldvaser, H.; Purim, O.; Kundel, Y.; Shepshelovich, D.; Shochat, T.; Shemesh-Bar, L.; Sulkes, A.; Brenner, B. Colorectal cancer in young patients: Is it a distinct clinical entity? Int. J. Clin. Oncol. 2016, 21, 684–695. [Google Scholar] [CrossRef]
- Kneuertz, P.J.; Chang, G.J.; Hu, C.Y.; Rodriguez-Bigas, M.A.; Eng, C.; Vilar, E.; Skibber, J.M.; Feig, B.W.; Cormier, J.N.; You, Y.N. Overtreatment of young adults with colon cancer: More intense treatments with unmatched survival gains. JAMA Surg. 2015, 150, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Steele, S.R.; Park, G.E.; Johnson, E.K.; Martin, M.J.; Stojadinovic, A.; Maykel, J.A.; Causey, M.W. The impact of age on colorectal cancer incidence, treatment, and outcomes in an equal-access health care system. Dis. Colon Rectum 2014, 57, 303–310. [Google Scholar] [CrossRef]
- Li, Q.; Cai, G.; Li, D.; Wang, Y.; Zhuo, C.; Cai, S. Better long-term survival in young patients with non-metastatic colorectal cancer after surgery, an analysis of 69,835 patients in SEER database. PLoS ONE 2014, 9, e93756. [Google Scholar] [CrossRef]
- Fu, J.; Yang, J.; Tan, Y.; Jiang, M.; Wen, F.; Huang, Y.; Chen, H.; Yi, C.; Zheng, S.; Yuan, Y. Young patients (≤35 years old) with colorectal cancer have worse outcomes due to more advanced disease: A 30-year retrospective review. Medicine 2014, 93, e135. [Google Scholar] [CrossRef]
- O’Connell, J.B.; Maggard, M.A.; Liu, J.H.; Etzioni, D.A.; Ko, C.Y. Are survival rates different for young and older patients with rectal cancer? Dis. Colon Rectum 2004, 47, 2064–2069. [Google Scholar] [CrossRef]
- Chan, K.K.; Dassanayake, B.; Deen, R.; Wickramarachchi, R.E.; Kumarage, S.K.; Samita, S.; Deen, K.I. Young patients with colorectal cancer have poor survival in the first twenty months after operation and predictable survival in the medium and long-term: Analysis of survival and prognostic markers. World J. Surg. Oncol. 2010, 8, 82. [Google Scholar] [CrossRef]
- Kim, T.J.; Kim, E.R.; Hong, S.N.; Chang, D.K.; Kim, Y.H. Long-Term Outcome and Prognostic Factors of Sporadic Colorectal Cancer in Young Patients: A Large Institutional-Based Retrospective Study. Medicine 2016, 95, e3641. [Google Scholar] [CrossRef]
- Benson, A.B., 3rd; Venook, A.P.; Cederquist, L.; Chan, E.; Chen, Y.J.; Cooper, H.S.; Deming, D.; Engstrom, P.F.; Enzinger, P.C.; Fichera, A.; et al. Colon Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2017, 15, 370–398. [Google Scholar] [CrossRef] [PubMed]
- National Bowel Cancer Audit Annual Report 2019. 2019. Available online: https://www.nboca.org.uk/content/uploads/2020/01/NBOCA-2019-V2.0.pdf (accessed on 9 January 2020).
- Forbes, S.S.; Sutradhar, R.; Paszat, L.F.; Rabeneck, L.; Urbach, D.R.; Baxter, N.N. Long-term survival in young adults with colorectal cancer: A population-based study. Dis. Colon Rectum 2010, 53, 973–978. [Google Scholar] [CrossRef]
- Murata, A.; Akiyoshi, T.; Ueno, M.; Fukunaga, Y.; Nagayama, S.; Fujimoto, Y.; Konishi, T.; Nagasaki, T.; Nagata, J.; Ohno, R. Clinicopathological characteristics of young patients with sporadic colorectal cancer. Surg. Today 2016, 46, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Shemesh-Bar, L.; Kundel, Y.; Idelevich, E.; Sulkes, J.; Sulkes, A.; Brenner, B. Colorectal cancer in young patients in Israel: A distinct clinicopathological entity? World J. Surg. 2010, 34, 2701–2709. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.A.; Chew, M.H.; Koh, P.K.; Tang, C.L. Young colorectal carcinoma patients do not have a poorer prognosis: A comparative review of 2426 cases. Tech. Cloproctol. 2013, 17, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Quah, H.M.; Joseph, R.; Schrag, D.; Shia, J.; Guillem, J.G.; Paty, P.B.; Temple, L.K.; Wong, W.D.; Weiser, M.R. Young age influences treatment but not outcome of colon cancer. Ann. Surg. Oncol. 2007, 14, 2759–2765. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.M.; Park, J.H.; Kim, B.C.; Son, I.T.; Kim, J.Y.; Kim, J.W. Self-expandable metallic stents as a bridge to surgery in obstructive right- and left-sided colorectal cancer: A multicenter cohort study. Sci. Rep. 2023, 13, 438. [Google Scholar] [CrossRef]
- Weiser, M.R. AJCC 8th Edition: Colorectal Cancer. Ann. Surg. Oncol. 2018, 25, 1454–1455. [Google Scholar] [CrossRef]
- Vatandoust, S.; Price, T.J.; Ullah, S.; Roy, A.C.; Beeke, C.; Young, J.P.; Townsend, A.; Padbury, R.; Roder, D.; Karapetis, C.S. Metastatic Colorectal Cancer in Young Adults: A Study from the South Australian Population-Based Registry. Clin. Color. Cancer 2016, 15, 32–36. [Google Scholar] [CrossRef]
- Schellerer, V.S.; Merkel, S.; Schumann, S.C.; Schlabrakowski, A.; Förtsch, T.; Schildberg, C.; Hohenberger, W.; Croner, R.S. Despite aggressive histopathology survival is not impaired in young patients with colorectal cancer: CRC in patients under 50 years of age. Int. J. Color. Dis. 2012, 27, 71–79. [Google Scholar] [CrossRef]
- Chiang, J.M.; Yeh, C.Y.; Changchien, C.R.; Chen, J.S.; Tang, R.; Chen, J.R. Mucinous adenocarcinoma showing different clinicopathological and molecular characteristics in relation to different colorectal cancer subgroups. Int. J. Color. Dis. 2010, 25, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.T.; Huang, K.C.; Cheng, A.L.; Jeng, Y.M.; Wu, M.S.; Wang, S.M. Clinicopathological and molecular biological features of colorectal cancer in patients less than 40 years of age. Br. J. Surg. 2003, 90, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Taggarshe, D.; Rehil, N.; Sharma, S.; Flynn, J.C.; Damadi, A. Colorectal cancer: Are the “young” being overlooked? Am. J. Surg. 2013, 205, 312–316; discussion 316. [Google Scholar] [CrossRef]
- Goodwin, R.A.; Asmis, T.R. Overview of systemic therapy for colorectal cancer. Clin. Colon Rectal Surg. 2009, 22, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Chew, M.H.; Koh, P.K.; Ng, K.H.; Eu, K.W. Improved survival in an Asian cohort of young colorectal cancer patients: An analysis of 523 patients from a single institution. Int. J. Color. Dis. 2009, 24, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
| Young Adult Group (n = 93) | Older Group (n = 1899) | p | |
|---|---|---|---|
| Age | 38.8 (±6.1) | 67.7 (±10.6) | <0.001 |
| Gender | 0.192 | ||
| Men | 48 (51.6) | 1110 (58.5) | |
| Women | 45 (48.4) | 789 (41.5) | |
| ASA | <0.001 | ||
| I | 43 (46.2) | 196 (10.3) | <0.001 |
| II | 43 (46.2) | 1102 (58.0) | |
| III/IV | 7 (7.5) | 601 (31.6) | |
| BMI (kg/m2) | 23.2 (±3.2) | 23.5 (±3.4) | 0.391 |
| CEA (ng/mL) | 8.3(±12.4) | 11.0 (±43.7) | 0.443 |
| Location | 0.444 | ||
| Right colon | 30 (32.3) | 576 (30.4) | |
| Left colon | 36 (38.7) | 683 (36.0) | |
| Rectum | 27 (29.0) | 638 (33.6) | |
| Symptom | 64 (68.8) | 1062 (55.9) | 0.014 |
| Abdominal pain | 41 (44.1) | 420 (22.1) | <0.001 |
| Hematochezia/melena | 13 (14.0) | 392 (20.6) | 0.119 |
| Bowel habit change | 12 (12.9) | 225 (11.8) | 0.759 |
| Body weight change | 7 (7.5) | 57 (3.0) | 0.016 |
| Dyspepsia | 3 (3.2) | 51 (2.7) | 0.738 |
| Tenesmus | 2 (2.2) | 16 (0.8) | 0.204 |
| obstruction | 23 (24.7) | 270 (14.2) | 0.010 |
| Perforation | 5 (5.4) | 32 (1.7) | 0.027 |
| Regular screening | 30 (32.3) | 784 (41.3) | 0.084 |
| Family history of cancer | 15 (16.1) | 238 (12.6) | 0.314 |
| Family history of CRC | 3 (3.2) | 4.2 (2.2) | 0.523 |
| Young Adult Group (n = 93) | Older Group (n = 1899) | p | |
|---|---|---|---|
| Operation time (min) | 223.9 (±77.8) | 232.6 (±90.7) | 0.368 |
| Emergent operation | 15 (16.1) | 161 (8.5) | 0.011 |
| MIS | 73 (78.5) | 1481 (78.2) | 0.945 |
| Diversion | 16 (17.2) | 349 (18.4) | 0.775 |
| Duration of POD (days) | 12.1 (±7.9) | 13.4 (±11.9) | 0.289 |
| Complications | 8 (8.6) | 197 (10.4) | 0.583 |
| Complications ≥ 2 | 2 (2.2) | 24 (1.3) | 0.345 |
| Mortality within 30 days | 1 (1.1) | 2.6 (1.4) | 1.000 |
| Use of CTx | 58 (62.4) | 860 (45.3) | 0.002 |
| 5FU-LV/XELODA/UFT | 16 (17.2) | 356 (18.9) | |
| FOLFOX/XELOX | 40 (43.0) | 464 (24.6) | |
| FOLFIRI | 1 (1.1) | 20 (1.1) | |
| others | 1 (1.1) | 20 (1.1) | |
| Discontinuation of CTx | 5 (8.8) | 172 (20.0) | 0.037 |
| Multidrug regimen | 42 (45.2) | 512 (27.0) | <0.001 |
| Young Adult Group (n = 93) | Older Group (n = 1899) | p | |
|---|---|---|---|
| Histologic type | 0.010 | ||
| Well/moderate | 82 (88.2) | 1794 (94.6) | |
| Poorly/undifferentiated | 11 (11.8) | 103 (5.4) | |
| LVI | 42 (45.2) | 737 (38.8) | 0.220 |
| PNI | 25 (26.9) | 354 (18.7) | 0.047 |
| n of harvested LN | 21.9 (±11.9) | 23.2 (±15.1) | 0.400 |
| T | 0.233 | ||
| T0 | 4 (4.3) | 82 (4.3) | |
| T1 | 10 (10.8) | 311 (16.4) | |
| T2 | 12 (12.9) | 236 (12.5) | |
| T3 | 55 (59.1) | 1060 (55.9) | |
| T4 | 12 (12.9) | 206 (10.9) | |
| N | 0.670 | ||
| N0 | 59 (63.4) | 1218 (64.2) | |
| N1 | 20 (21.5) | 441 (23.2) | |
| N2/N3 | 14 (15.1) | 237 (12.5) | |
| TNM stage 1 | 0.785 | ||
| 0 | 5 (5.4) | 83 (4.4) | |
| I | 19 (20.4) | 476 (25.1) | |
| II | 36 (38.7) | 665 (35.0) | |
| III | 33 (35.5) | 675 (35.5) |
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age < 45 | 0.339 (0.106–1.083) | 0.058 | 0.440 (0.139–1.392) | 0.162 |
| Men | 1.042 (0.760–1.428) | 0.800 | 0.954 (0.702–1.296) | 0.763 |
| ASA ≥ 3 | 1.799 (1.309–2.471) | <0.001 | 1.749 (1.272–2.404) | 0.001 |
| Obstruction | 2.003 (1.380–2.906) | <0.001 | 1.818 (1.260–2.623) | 0.001 |
| Rectal cancer | 1.221 (0.884–1.686) | 0.225 | 1.435 (1.045–1.972) | 0.026 |
| T4 | 1.993 (1.319–3.010) | 0.001 | 1.737 (1.140–2.646) | 0.010 |
| Presence of LN (+) | 2.275 (1.660–3.117) | <0.001 | 2.727 (1.870–3.979) | <0.001 |
| PNI | 1.322 (0.911–1.920) | 0.141 | 0.927 (0.626–1.371) | 0.702 |
| LVI | 2.814 (1.593–2.993) | <0.001 | 1.717 (1.204–2.451) | 0.003 |
| Poorly/undifferentiation | 1.932 (1.125–3.318) | 0.015 | 1.561 (0.927–2.626) | 0.094 |
| Chemotherapy | 0.778 (0.567–1.068) | 0.120 | 0.344 (0.241–0.490) | <0.001 |
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| Age < 45 | 0.392 (0.195–0.785) | 0.006 | 0.438 (0.225–0.853) | 0.015 |
| Men | 1.083 (0.871–1.347) | 0.474 | 1.046 (0.860–1.273) | 0.650 |
| ASA ≥ 3 | 1.695 (1.354–2.123) | <0.001 | 1.757 (1.434–2.153) | <0.001 |
| Obstruction | 2.133 (1.625–2.799) | <0.001 | 1.689 (1.340–2.131) | <0.001 |
| Rectal cancer | 1.558 (1.248–1.945) | <0.001 | 1.695 (1.387–2.071) | <0.001 |
| T4 | 2.906 (2.161–3.908) | <0.001 | 1.920 (1.485–2.482) | <0.001 |
| Presence of LN (+) | 2.723 (2.184–3.396) | <0.001 | 2.111 (1.661–2.683) | <0.001 |
| PNI | 2.092 (1.629–2.687) | <0.001 | 1.297 (1.024–1.641) | 0.031 |
| LVI | 2.386 (1.916–2.972) | <0.001 | 1.510 (1.204–1.894) | <0.001 |
| Poorly/undifferentiation | 1.892 (1.258–2.845) | 0.002 | 1.320 (0.933–1.867) | 0.117 |
| Chemotherapy | 1.567 (1.260–1.947) | <0.001 | 0.696 (0.555–0.873) | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, I.T.; Kang, J.H.; Kim, B.C.; Park, J.H.; Kim, J.W. A Retrospective Multicenter Study of the Clinicopathological Characteristics and Prognosis of Young Adult Patients with Colorectal Cancer: Effects of Chemotherapy on Prognosis. J. Clin. Med. 2023, 12, 3634. https://doi.org/10.3390/jcm12113634
Son IT, Kang JH, Kim BC, Park JH, Kim JW. A Retrospective Multicenter Study of the Clinicopathological Characteristics and Prognosis of Young Adult Patients with Colorectal Cancer: Effects of Chemotherapy on Prognosis. Journal of Clinical Medicine. 2023; 12(11):3634. https://doi.org/10.3390/jcm12113634
Chicago/Turabian StyleSon, Il Tae, Jae Hyun Kang, Byung Chun Kim, Jun Ho Park, and Jong Wan Kim. 2023. "A Retrospective Multicenter Study of the Clinicopathological Characteristics and Prognosis of Young Adult Patients with Colorectal Cancer: Effects of Chemotherapy on Prognosis" Journal of Clinical Medicine 12, no. 11: 3634. https://doi.org/10.3390/jcm12113634
APA StyleSon, I. T., Kang, J. H., Kim, B. C., Park, J. H., & Kim, J. W. (2023). A Retrospective Multicenter Study of the Clinicopathological Characteristics and Prognosis of Young Adult Patients with Colorectal Cancer: Effects of Chemotherapy on Prognosis. Journal of Clinical Medicine, 12(11), 3634. https://doi.org/10.3390/jcm12113634





