The Future of Minimal-Access Myoma Surgery with In-Bag Contained Morcellation
Abstract
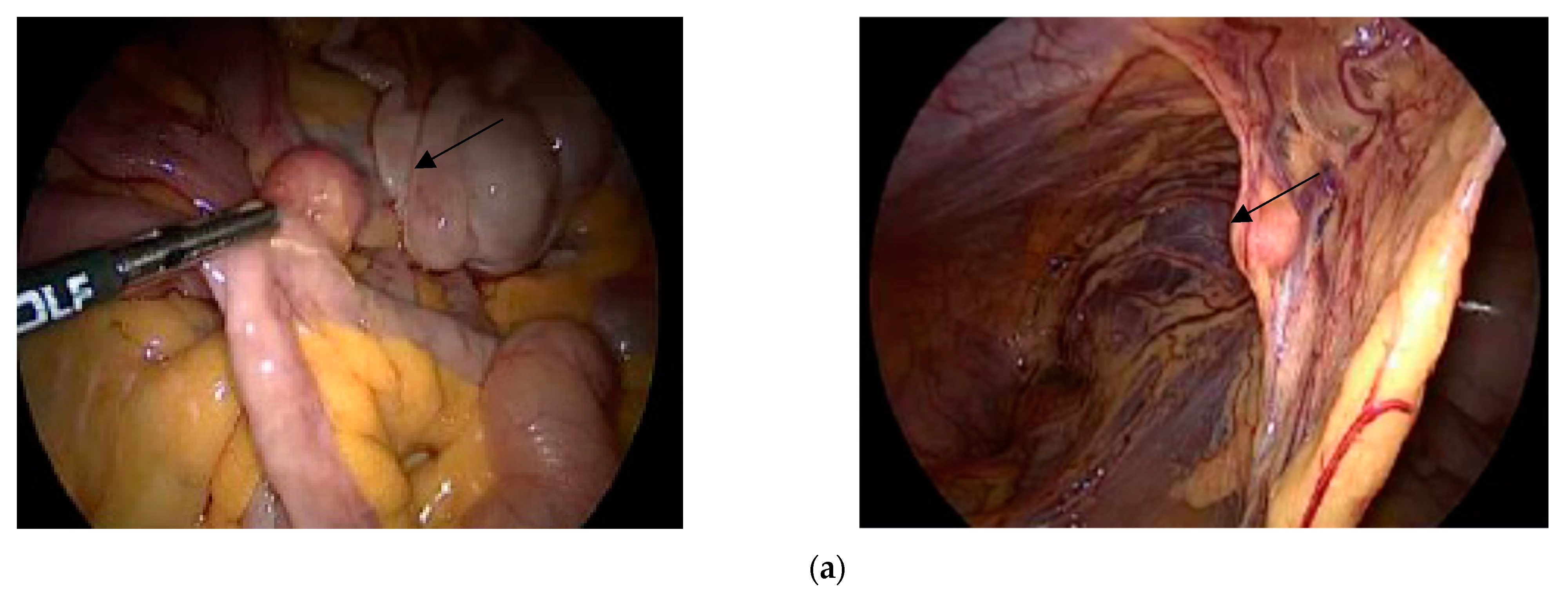
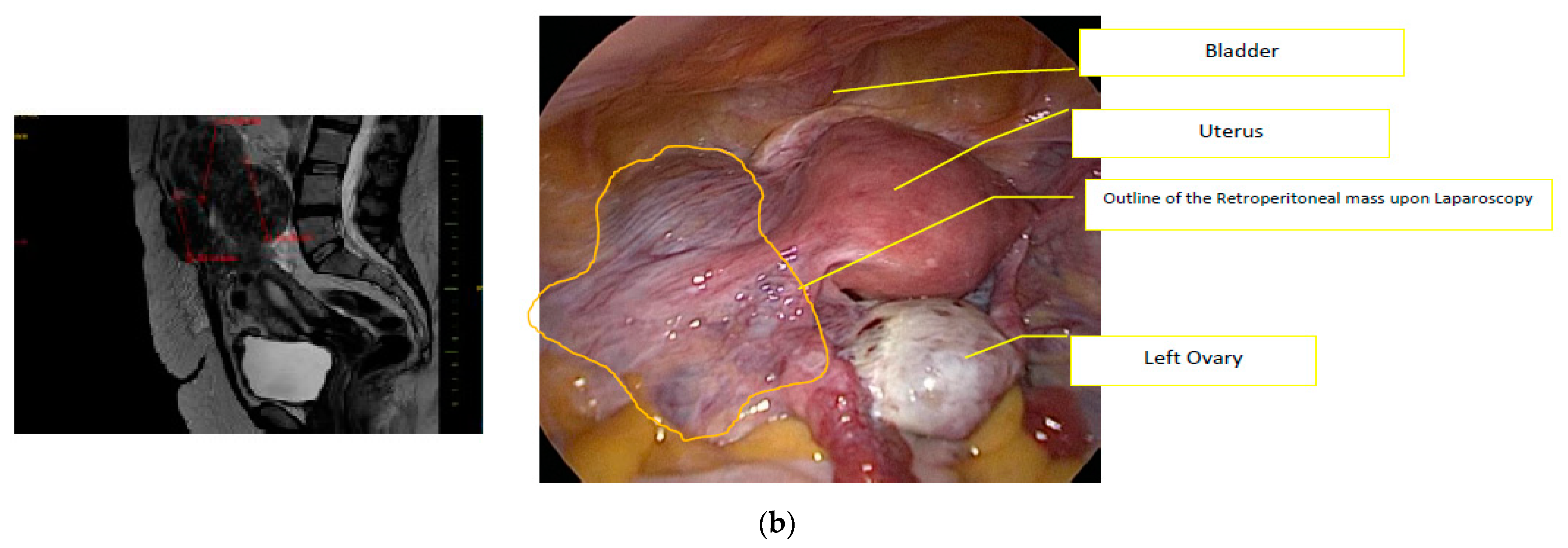
1. Introduction
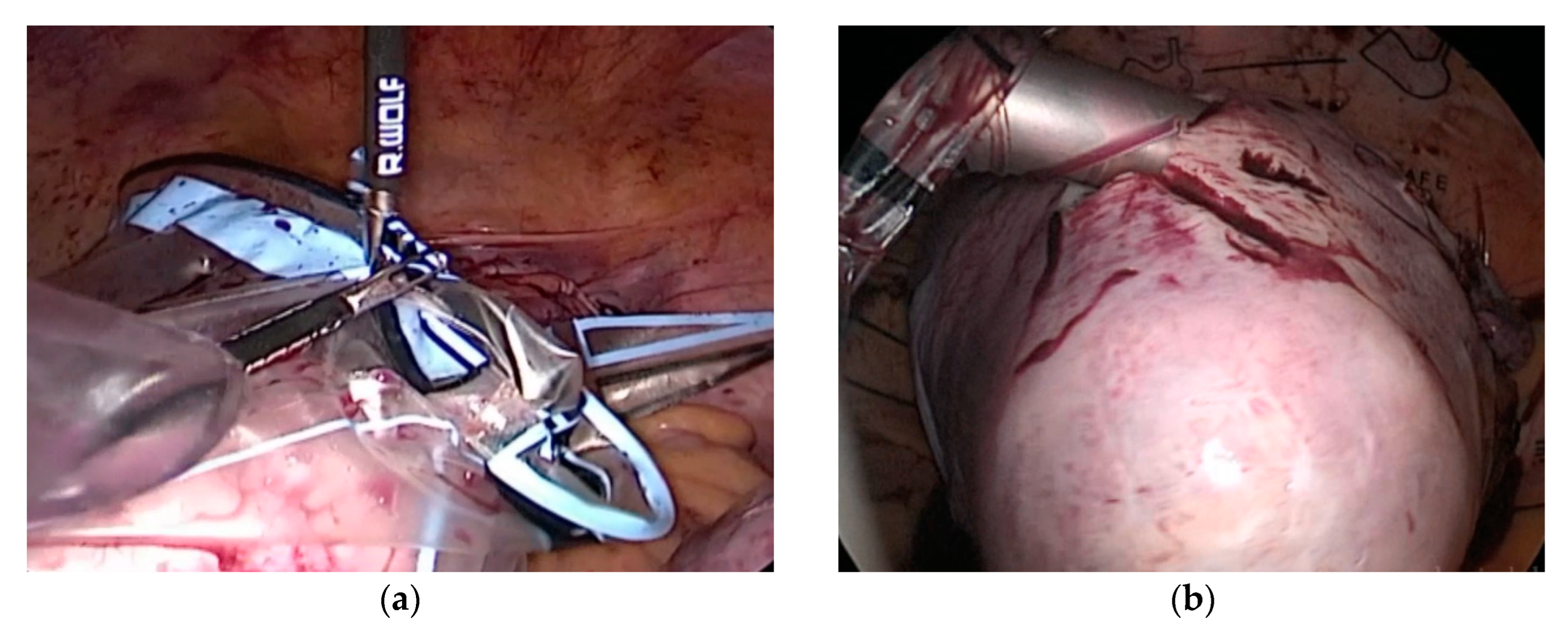
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Devassy, R.; Cezar, C.; Krentel, H.; Verhoeven, H.C.; Devassy, R.; de Wilde, M.S.; Torres-de la Roche, L.A.; Wilde, R.L. Feasibility of myomatous tissue extraction in laparoscopic surgery by contained in—Bag morcellation: A retrospective single arm study. Int. J. Surg. 2019, 62, 22–27. [Google Scholar] [CrossRef]
- Cezar, C.; Korell, M.; Tchartchian, G.; Ziegler, N.; Senshu, K.; Herrmann, A.; Larbig, A.; De Wilde, R.L. How to avoid risks for patients in minimal-access trials: Avoiding complications in clinical first-in-human studies by example of the ADBEE study. Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 35, 84–96. [Google Scholar] [CrossRef][Green Version]
- Brown, J. AAGL Advancing Minimally Invasive Gynecology Worldwide: Statement to the FDA on Power Morcellation. J. Minim. Invasive Gynecol. 2014, 21, 970–971. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, A.; De Wilde, R.L. Laparoscopic myomectomy—The gold standard. Gynecol. Minim. Invasive Ther. 2014, 3, 31–38. [Google Scholar] [CrossRef][Green Version]
- AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL Position Statement: Route of Hysterectomy to Treat Benign Uterine Disease. J. Minim. Invasive Gynecol. 2011, 18, 1–3. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. Laparoscopic Uterine Power Morcellation in Hysterectomy and Myomectomy: FDA Safety Communication. Available online: http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm (accessed on 8 February 2022).
- US Food and Drug Administration Safety Communication. Perform Only Contained Morcellation When Laparoscopic Power Morcellation Is Appropriate. 29 December 2020 Update. Available online: https://www.fda.gov/medical-devices/safety-communications/update-fda-recommends-performing-contained-morcellation-women-when-laparoscopic-power-morcellation (accessed on 8 February 2022).
- Cohen, S.L.; Einarsson, J.I.; Wang, K.C.; Brown, D.; Boruta, D.; Scheib, S.A.; Fader, A.N.; Shibley, T. Contained Power Morcellation within an Insufflated Isolation Bag. Obstet. Gynecol. 2014, 124, 491–497. [Google Scholar] [CrossRef][Green Version]
- Srouji, S.S.; Kaser, D.J.; Gargiulo, A.R. Techniques for contained morcellation in gynecologic surgery. Fertil. Steril. 2015, 103, e34. [Google Scholar] [CrossRef]
- Semm, K. Morzellieren und Nähen per pelviskopiam—Kein Problem mehr. Geburtshilfe Frauenheilkd. 1991, 51, 843–846. [Google Scholar] [CrossRef]
- Leren, V.; Langebrekke, A.; Qvigstad, E. Parasitic leiomyomas after laparoscopic surgery with morcellation. Acta Obstet. Gynecol. Scand. 2012, 91, 1233–1236. [Google Scholar] [CrossRef]
- Bortoletto, P.; Friedman, J.; Milad, M.P. Economics of gynecologic morcellation. Curr. Opin. Obstet. Gynecol. 2018, 30, 89–95. [Google Scholar] [CrossRef]
- Cusidó, M.; Fargas, F.; Baulies, S.; Plana, A.; Rodríguez, I.; Tresserra, F.; Pascual, M.; Fábregas, R. Impact of Surgery on the Evolution of Uterine Sarcomas. J. Minim. Invasive Gynecol. 2015, 22, 1068–1074. [Google Scholar] [CrossRef] [PubMed]
- Kho, K.A.; Anderson, T.L.; Nezhat, C.H. Intracorporeal Electromechanical Tissue Morcellation: A critical review and recommen-dations for clinical practice. Obstet. Gynecol. 2014, 124, 787–793. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bojahr, B.; De Wilde, R.L.; Tchartchian, G. Malignancy rate of 10,731 uteri morcellated during laparoscopic supracervical hysterectomy (LASH). Arch. Gynecol. Obstet. 2015, 292, 665–672. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brölmann, H.; Tanos, V.; Grimbizis, G.; Ind, T.; Philips, K.; Bosch, T.V.D.; Sawalhe, S.; Haak, L.V.D.; Jansen, F.-W.; Pijnenborg, J.; et al. Options on fibroid morcellation: A literature review. Gynecol. Surg. 2015, 12, 3–15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Milad, M.P.; Milad, E.A. Laparoscopic Morcellator-Related Complications. J. Minim. Invasive Gynecol. 2014, 21, 486–491. [Google Scholar] [CrossRef]
- Cholkeri-Singh, A.; Miller, C.E. Power Morcellation in a Specimen Bag. J. Minim. Invasive Gynecol. 2015, 22, 160. [Google Scholar] [CrossRef]
- Zaami, S.; Zupi, E.; Lazzeri, L.; Stark, M.; Malvasi, A.; Signore, F.; Marinelli, E. Medicolegal Issues in Power Morcellation: Cautionary Rules for Gynecologists to Avoid Unfavorable Outcomes. J. Minim. Invasive Gynecol. 2020, 27, 583–592. [Google Scholar] [CrossRef]
- Xu, X.; Desai, V.B.; Wright, J.D.; Lin, H.; Schwartz, P.E.; Gross, C.P. Hospital variation in responses to safety warnings about power morcellation in hysterectomy. Am. J. Obstet. Gynecol. 2020, 224, 589.e1–589.e13. [Google Scholar] [CrossRef]
- Torres-de la Roche, L.A.; Devassy, R.; Makhlouf, G.; Juan, J.S.; Eidswick, J.; De Wilde, R.L. Retroperitoneal angioleiomyomatosis. J. Obstet. Gynecol. India 2020, 71, 337–341. [Google Scholar] [CrossRef]
- Torres-de la Roche, L.A.; Becker, S.; Cezar, C.; Hermann, A.; Larbig, A.; Leicher, L.; Sardo, A.D.S.; Tanos, V.; Wallwiener, M.; Verhoeven, H.; et al. Pathobiology of myomatosis uteri: The underlying knowledge to support our clinical practice. Arch. Gynecol. Obstet. 2017, 296, 701–707. [Google Scholar] [CrossRef]
- Shifrin, G.; Engelhardt, M.; Gee, P.; Pschadka, G. Transcervical fibroid ablation with the Sonata™ system for treatment of submucous and large uterine fibroids. Int. J. Gynecol. Obstet. 2021, 155, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Gothwal, M.; Devkare, V. Successful laparoscopic myomectomy in giant myoma. Int. J. Appl. Basic Med. Res. 2021, 11, 108–110. [Google Scholar] [CrossRef] [PubMed]
- AAGL Advancing Minimally Invasive Gynecology Worldwide. AAGL Practice Report: Morcellation during Uterine Tissue Extraction. J. Minim. Invasive Gynecol. 2014, 21, 517–530. [Google Scholar] [CrossRef]
- Koivisto-Korander, R.; Martinsen, J.; Weiderpass, E.; Leminen, A.; Pukkala, E. Incidence of uterine leiomyosarcoma and endometrial stromal sarcoma in Nordic countries: Results from NORDCAN and NOCCA databases. Maturitas 2012, 72, 56–60. [Google Scholar] [CrossRef]
- Parker, W.H.; Fu, Y.S.; Berek, J.S. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leio-myoma. Obstet. Gynecol. 1994, 83, 414–418. [Google Scholar]
- Denschlag, D.; Ackermann, S.; Battista, M.J.; Cremer, W.; Egerer, G.; Follmann, M.; Haas, H.; Harter, P.; Hettmer, S.; Horn, L.-C.; et al. Sarcoma of the Uterus. Guideline of the DGGG and OEGGG (S2k Level, AWMF Register Number 015/074, February 2019). Geburtshilfe Frauenheilkd. 2019, 79, 1043–1060. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Theben, J.U.; Schellong, A.R.M.; Altgassen, C.; Kelling, K.; Schneider, S.; Große-Drieling, D. Unexpected malignancies after laparoscopic-assisted supracervical hysterectomies (LASH): An analysis of 1584 LASH cases. Arch. Gynecol. Obstet. 2012, 287, 455–462. [Google Scholar] [CrossRef]
- Seidman, M.A.; Oduyebo, T.; Muto, M.G.; Crum, C.P.; Nucci, M.R.; Quade, B.J. Peritoneal Dissemination Complicating Morcellation of Uterine Mesenchymal Neoplasms. PLoS ONE 2012, 7, e50058. [Google Scholar] [CrossRef][Green Version]
- Grady, D. Uterine Surgical Technique Is Linked to Abnormal Growths and Cancer Spread. The New York Times. 2016. Available online: http://www.nytimes.com/2014/02/07/health/uterine-surgical-technique-is-linked-totoabnormal (accessed on 2 February 2022).
- Park, J.-Y.; Park, S.-K.; Kim, D.-Y.; Kim, J.-H.; Kim, Y.-M.; Kim, Y.-T.; Nam, J.-H. The impact of tumor morcellation during surgery on the prognosis of patients with apparently early uterine leiomyosarcoma. Gynecol. Oncol. 2011, 122, 255–259. [Google Scholar] [CrossRef]
- De Wilde, R.L. Regarding “Incidence of Occult Uterine Malignancy Following Vaginal Hysterectomy with Morcellation”. J. Minim. Invasive Gynecol. 2017, 25, 186. [Google Scholar] [CrossRef][Green Version]
- Graebe, K.; Garcia-Soto, A.; Aziz, M.; Valarezo, V.; Heller, P.B.; Tchabo, N.; Tobias, D.H.; Salamon, C.; Ramieri, J.; Dise, C.; et al. Incidental power morcellation of malignancy: A retrospective cohort study. Gynecol. Oncol. 2015, 136, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.D.; Tergas, A.I.; Cui, R.; Burke, W.M.; Hou, J.Y.; Ananth, C.V.; Chen, L.; Richards, C.; Neugut, A.I.; Hershman, D.L. Use of Electric Power Morcellation and Prevalence of Underlying Cancer in Women Who Undergo Myomectomy. JAMA Oncol. 2015, 1, 69–77. [Google Scholar] [CrossRef][Green Version]
- Turner, T.; Secord, A.A.; Lowery, W.J.; Sfakianos, G.; Lee, P.S. Metastatic adenocarcinoma after laparoscopic supracervical hysterectomy with morcellation: A case report. Gynecol. Oncol. Case Rep. 2013, 5, 19–21. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huss, A.; Klar, M.; Hasanov, M.F.; Juhasz-Böss, I.; Bossart, M. Prognostic factors and survival of patients with uterine sarcoma: A German unicenter analysis. Arch. Gynecol. Obstet. 2022. epub ahead of print. [Google Scholar] [CrossRef]
- Tulandi, T.; Leung, A.; Jan, N. Nonmalignant Sequelae of Unconfined Morcellation at Laparoscopic Hysterectomy or Myomectomy. J. Minim. Invasive Gynecol. 2016, 23, 331–337. [Google Scholar] [CrossRef]
- Van Der Meulen, J.F.; Pijnenborg, J.; Boomsma, C.M.; Verberg, M.F.G.; Geomini, P.M.A.J.; Bongers, M.Y. Parasitic myoma after laparoscopic morcellation: A systematic review of the literature. BJOG Int. J. Obstet. Gynaecol. 2015, 123, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Mowers, E.L.; Skinner, B.; McLean, K.; Reynolds, R.K. Effects of Morcellation of Uterine Smooth Muscle Tumor of Uncertain Malignant Potential and Endometrial Stromal Sarcoma: Case Series and Recommendations for Clinical Practice. J. Minim. Invasive Gynecol. 2015, 22, 601–606. [Google Scholar] [CrossRef]
- Cucinella, G.; Granese, R.; Calagna, G.; Somigliana, E.; Perino, A. Parasitic myomas after laparoscopic surgery: An emerging complication in the use of morcellator? Description of four cases. Fertil. Steril. 2011, 96, e90–e96. [Google Scholar] [CrossRef][Green Version]
- Panesar, H.; Dhaliwal, H.S. Iatrogenic parasitic leiomyomas: A late and uncommon complication after laparoscopic morcellation. Cureus 2022, 14, e24718. [Google Scholar] [CrossRef]
- Wong, M.; De Wilde, R.L.; Isaacson, K. Reducing the spread of occult uterine sarcoma at the time of minimally invasive gynecologic surgery. Arch. Gynecol. Obstet. 2017, 297, 285–293. [Google Scholar] [CrossRef]
- Krentel, H.; De Wilde, R.L. Laparoscopic Supracervical Hysterectomy with In-Bag Morcellation in Very Large Uterus. Case Rep. Med. 2017, 2017, 9410571. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rimbach, S.; Holzknecht, A.; Schmedler, C.; Nemes, C.; Offner, F. First clinical experiences using a new in-bag morcellation system during laparoscopic hysterectomy. Arch. Gynecol. Obstet. 2015, 294, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Rimbach, S.; Schempershofe, M. In-Bag Morcellation as a Routine for Laparoscopic Hysterectomy. BioMed Res. Int. 2017, 2017, 6701916. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rimbach, S.; Holzknecht, A.; Nemes, C.; Offner, F.; Craina, M. A new in-bag system to reduce the risk of tissue morcellation: Development and experimental evaluation during laparoscopic hysterectomy. Arch. Gynecol. Obstet. 2015, 292, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Kanade, T.T.; McKenna, J.B.; Choi, S.; Tsai, B.P.; Rosen, D.M.; Cario, G.M.; Chou, D. Sydney Contained in Bag Morcellation for Laparoscopic Myomectomy. J. Minim. Invasive Gynecol. 2014, 21, 981. [Google Scholar] [CrossRef]
- Serur, E.; Zambrano, N.; Brown, K.; Clemetson, E.; Lakhi, N. Extracorporeal Manual Morcellation of Very Large Uteri within an Enclosed Endoscopic Bag: Our 5-Year Experience. J. Minim. Invasive Gynecol. 2016, 23, 903–908. [Google Scholar] [CrossRef]
- Vargas, M.V.; Cohen, S.L.; Fuchs-Weizman, N.; Wang, K.C.; Manoucheri, E.; Vitonis, A.F.; Einarsson, J.I. Open Power Morcellation Versus Contained Power Morcellation Within an Insufflated Isolation Bag: Comparison of Perioperative Outcomes. J. Minim. Invasive Gynecol. 2015, 22, 433–438. [Google Scholar] [CrossRef]
- Gil-Gimeno, A.; Laberge, P.Y.; Lemyre, M.; Gorak, E.; Maheux-Lacroix, S. Morcellation During Total Laparoscopic Hysterectomies: Implications of the Use of a Contained Bag System. J. Obstet. Gynaecol. Can. 2020, 42, 839–845. [Google Scholar] [CrossRef]
- Jin, C.; Hu, Y.; Chen, X.-C.; Zheng, F.-Y.; Lin, F.; Zhou, K.; Chen, F.-D.; Gu, H.-Z. Laparoscopic versus open myomectomy—A meta-analysis of randomized controlled trials. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 145, 14–21. [Google Scholar] [CrossRef]
- Donnez, O.; Jadoul, P.; Squifflet, J.; Donnez, J. A series of 3190 laparoscopic hysterectomies for benign disease from 1990 to 2006: Evaluation of complications compared with vaginal and abdominal procedures. BJOG Int. J. Obstet. Gynaecol. 2008, 116, 492–500. [Google Scholar] [CrossRef]
- Wright, J.D.; Herzog, T.J.; Tsui, J.; Ananth, C.V.; Lewin, S.N.; Lu, Y.-S.; Neugut, A.I.; Hershman, D.L. Nationwide Trends in the Performance of Inpatient Hysterectomy in the United States. Obstet. Gynecol. 2013, 122 Pt 1, 233–241. [Google Scholar] [CrossRef]
- Cezar, C.; Becker, S.; di Spiezio Sardo, A.; Herrmann, A.; Larbig, A.; Tanos, V.; Torres-de la Roche, L.A.; Verhoeven, H.C.; Wallwiener, M.; De Wilde, R.L. Laparoscopy or laparotomy as the way of entrance in myoma enucleation. Arch. Gynecol. Obstet. 2017, 296, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Nieboer, T.; Johnson, N.; Lethaby, A.; Tavender, E.; Curr, E.; Garry, R.; Van Voorst, S.; Mol, B.W.J.; Kluivers, K.B. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst. Rev. 2009, 8, CD003677. [Google Scholar] [CrossRef][Green Version]
- Taylan, E.; Sahin, C.; Zeybek, B.; Akdemir, A. Contained Morcellation: Review of Current Methods and Future Directions. Front. Surg. 2017, 4, 15. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Wilde, R.L.; Herrmann, A.; Torres-de la Roche, L.A.; Krentel, H.; Cezar, C.; de Wilde, M.S.; Devassy, R. Adhesions after laparoscopic myomectomy: Incidence, risk factors, complications, and prevention. Gynecol. Minim. Invasive Ther. 2020, 9, 190–197. [Google Scholar] [CrossRef]


| Bag Size | Bigger Opening Diameter | Telescopic Diameter Required | Volume | Bag Schema |
|---|---|---|---|---|
| Small (S) | 12.5 cm | 6 mm | 1600 | 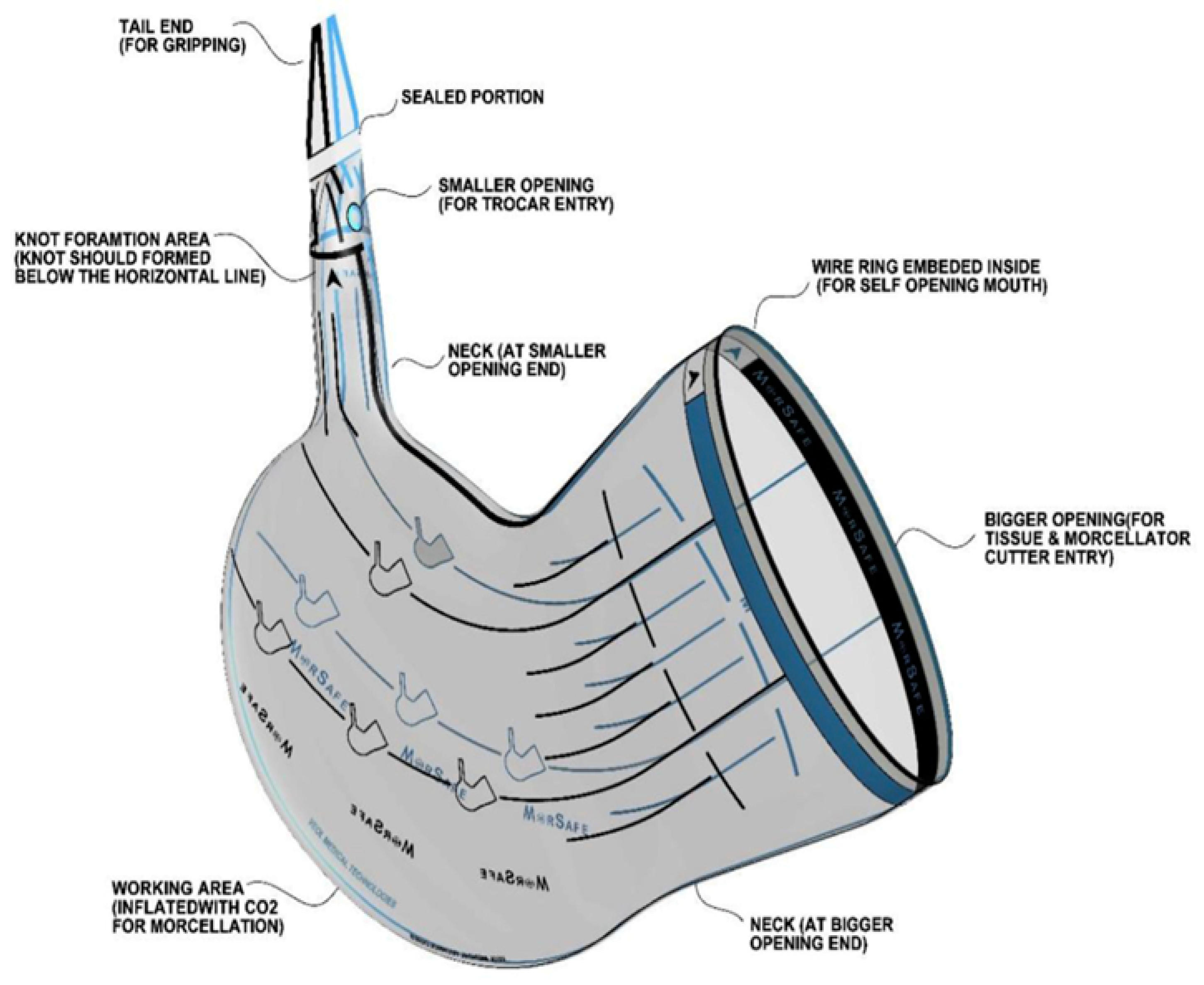 |
| Medium (M) | 13.5 cm | 6 mm | 2100 | |
| Large (L) | 14.5 cm | 6 mm | 2600 | |
| Customized XL variant * | 28.5 cm | 6 mm | 5200/5500 |
| Characteristic | Mean (n = 1120) | Range | |
|---|---|---|---|
| Patient age (years) | 39.3 | 21–71 | |
| Patient weight (kg) | 67.0 | 41–127 | |
| Nulliparous | 562 | NA | |
| Parous | 558 | 1–15 | |
| Type of Surgery | n= 1120 | % | |
| Giant myomectomy | 3 | 0.27 | |
| Large myomectomy | 744 | 66.43 | |
| Small myomectomy | 35 | 3.12 | |
| Parasitic myomectomy | 10 | 0.89 | |
| Adenomyomectomy | 12 | 1.07 | |
| Giant LASH | 1 | 0.09 | |
| Large LASH | 148 | 13.21 | |
| Small LASH | 67 | 5.98 | |
| Large LASH + BSO | 26 | 2.32 | |
| LTH | 73 | 6.52 | |
| Retroperitoneal angioleiomyomatosis | 1 | 0.09 | |
| Specimen weight in grams | Mean | n= 1120 | % |
| <250 | 131.8 ± 0.8 | 239 | 21.34% |
| 250–499 | 349.8 ± 0.8 | 414 | 36.96% |
| 500–999 | 606.4 ± 0.4 | 386 | 34.46% |
| >1000 | 1718.9 ± 0.9 | 81 | 7.23% |
| Type of Tumor | N = 1120 | % |
|---|---|---|
| Adenomyosis | 159 | 14 |
| Adenomyosis with leiomyoma | 14 | 1 |
| Degenerated myoma | 108 | 10 |
| Leiomyoma | 728 | 65 |
| Leiomyoma cellular variant | 28 | 3 |
| Endometrial complex hyperplasia, polyps | 48 | 4 |
| Endometriosis | 26 | 2 |
| Leiomyosarcoma | 2 | 0.18 |
| Endometrial stromal tumor | 1 | 0.09 |
| Angioleiomyomatosis | 1 | 0.09 |
| No obvious histopathology | 5 | 0.45 |
| Cytological Description | N = 1120 | % |
|---|---|---|
| Severe subacute inflammation with activated mesothelial cells and reactive mesothelial cell hyperplasia suggestive of exudative peritoneal effusion | 167 | 15 |
| Mild subacute inflammation with activated mesothelial cells and reactive mesothelial cell hyperplasia | 138 | 12 |
| Cytology of peritoneal fluid with moderate subacute inflammation | 136 | 12 |
| Moderate subacute inflammation consistent with exudative peritoneal effusion | 98 | 9 |
| Severe subacute inflammation with activated hyperplastic mesothelial cells suggestive of exudative peritoneal effusion created by endometriosis | 86 | 8 |
| Mild subacute inflammation suggestive of exudative peritoneal effusion | 82 | 7 |
| Moderate subacute inflammation with circumscribed mesothelial cell hyperplasia suggestive of exudative peritoneal effusion | 77 | 7 |
| Severe subacute inflammation with reactive mesothelial cell hyperplasia suggestive of exudative peritoneal effusion | 73 | 7 |
| Severe acute organizing inflammation with reactive mesothelial cell hyperplasia suggestive of exudative peritoneal effusion | 51 | 5 |
| Severe subacute inflammation with activated mesothelial cells and reactive mesothelial cell hyperplasia suggestive of exudative peritoneal effusion | 41 | 4 |
| Mild to moderate subacute inflammation with reactive mesothelial cell hyperplasia suggestive of exudative peritoneal effusion | 39 | 3 |
| Severe subacute inflammation with mesothelial cell hyperplasia suggestive of exudative peritoneal effusion | 25 | 2 |
| Smear slides of peritoneal fluid showing clear background with scattered mononucleated and mesothelial cells | 7 | 1 |
| Components of the previously diagnosed simple serous papillary cystadenoma of the right ovary | 1 | 0.09 |
| Severe subacute inflammation suggestive of exudative peritoneal effusion. Activated mesothelial cells and abundant mesothelial cell hyperplasia associated with papillary structures. Clinical correlation ruled out intra-abdominal serous papillary neoplasm | 1 | 0.09 |
| Mean Specimen Weight (g) | Bag Manipulation Average Time H:M:S | Morcellation Average Time H:M:S | |
|---|---|---|---|
| Time | 701.7 | 0:17:38 | 0:31:25 |
| Range | 8–4780 | 0:0:30–0:48:32 | 0:1:16–1:31:50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devassy, R.; Devassy, R.R.; de Wilde, M.S.; Krentel, H.; Adlan, A.; Torres-de la Roche, L.A.; De Wilde, R.L. The Future of Minimal-Access Myoma Surgery with In-Bag Contained Morcellation. J. Clin. Med. 2023, 12, 3628. https://doi.org/10.3390/jcm12113628
Devassy R, Devassy RR, de Wilde MS, Krentel H, Adlan A, Torres-de la Roche LA, De Wilde RL. The Future of Minimal-Access Myoma Surgery with In-Bag Contained Morcellation. Journal of Clinical Medicine. 2023; 12(11):3628. https://doi.org/10.3390/jcm12113628
Chicago/Turabian StyleDevassy, Rajesh, Rohan Rajesh Devassy, Maya Sophie de Wilde, Harald Krentel, Aizura Adlan, Luz Angela Torres-de la Roche, and Rudy Leon De Wilde. 2023. "The Future of Minimal-Access Myoma Surgery with In-Bag Contained Morcellation" Journal of Clinical Medicine 12, no. 11: 3628. https://doi.org/10.3390/jcm12113628
APA StyleDevassy, R., Devassy, R. R., de Wilde, M. S., Krentel, H., Adlan, A., Torres-de la Roche, L. A., & De Wilde, R. L. (2023). The Future of Minimal-Access Myoma Surgery with In-Bag Contained Morcellation. Journal of Clinical Medicine, 12(11), 3628. https://doi.org/10.3390/jcm12113628









