Abstract
This scoping review summarizes what is known about kidney metabolism during hypothermic perfusion preservation. Papers studying kidney metabolism during hypothermic (<12 °C) perfusion were identified (PubMed, Embase, Web of Science, Cochrane). Out of 14,335 initially identified records, 52 were included [dog (26/52), rabbit (2/52), pig (20/52), human (7/52)]. These were published between 1970–2023, partially explaining study heterogeneity. There is a considerable risk of bias in the reported studies. Studies used different perfusates, oxygenation levels, kidney injury levels, and devices and reported on perfusate and tissue metabolites. In 11 papers, (non)radioactively labeled metabolites (tracers) were used to study metabolic pathways. Together these studies show that kidneys are metabolically active during hypothermic perfusion, regardless of the perfusion setting. Although tracers give us more insight into active metabolic pathways, kidney metabolism during hypothermic perfusion is incompletely understood. Metabolism is influenced by perfusate composition, oxygenation levels, and likely also by pre-existing ischemic injury. In the modern era, with increasing donations after circulatory death and the emergence of hypothermic oxygenated perfusion, the focus should be on understanding metabolic perturbations caused by pre-existing injury levels and the effect of perfusate oxygen levels. The use of tracers is indispensable to understanding the kidney’s metabolism during perfusion, given the complexity of interactions between different metabolites.
1. Introduction
Hypothermic perfusion preservation, also called hypothermic machine perfusion, reduces the risk of delayed graft function [1]. Studies also suggest improved graft survival in kidneys donated after brain death (DBD), though this is not the case for kidneys donated after circulatory death (DCD) [2,3,4]. A recent randomized controlled trial suggests that actively oxygenating the perfusate during hypothermic perfusion of older DCD kidneys improves kidney function and survival [5]. The underlying mechanisms by which hypothermic perfusion exerts its effect are still incompletely understood.
To understand the mechanisms that drive the effect of hypothermic perfusion preservation and how this technique might be further improved, it is essential to understand “on-pump” kidney behavior. A key concept of hypothermic preservation is that metabolism, and therefore cellular metabolic requirements, are minimized. However, although the metabolic rate below 4 °C is reported to be about 5–10% of that at body temperature [6,7], there is still active metabolism in the cold. Furthermore, the preservation temperature during hypothermic perfusion often does not reach below 4 °C. The metabolic activity in this ex situ, hypothermic environment and how it is influenced by oxygenation is poorly understood [5,8,9].
This review assesses, appraises, and summarizes our current understanding of the kidney’s metabolism during hypothermic perfusion preservation.
2. Materials and Methods
2.1. Search Strategy
This review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) guidelines. The protocol was prospectively registered [10]. With the help of an experienced biomedical information specialist, a search strategy was built and PubMed, Embase, Cochrane Library, and Web of Science Core Collection were searched. The following concepts: “metabolism”, “kidney”, and “perfusion” were developed. The complete search strategy can be found in Table S1.
2.2. Study Selection and Eligibility Criteria
Two authors independently assessed the eligibility of the articles based on the title and abstract, conducted full-text analysis, and extracted data. In case of disagreements, a third experienced researcher was consulted. Studies were included from database inception with final searches carried out on 2 February 2023.
Studies were eligible for inclusion if they reported on any of the pre-specified outcomes. Only studies in mammals providing data on perfusate or tissue metabolites of kidneys undergoing hypothermic perfusion were included. Table S2 lists the inclusion and exclusion criteria. Articles written in a language other than English, French, or Dutch; articles with no full text available; review articles, letters, editorials, and conference abstracts were also excluded. Reference lists of included studies were also searched using the same inclusion and exclusion criteria (‘snowballing’).
2.3. Data Extraction and Processing
The results of the search were imported into Endnote (Version 20, Clearview Analytics, Philadelphia, PA, USA). Duplicates were removed using the “Find duplicates” tool in Endnote. The remaining articles were imported into Rayyan [11] and screened according to prespecified inclusion and exclusion criteria (Table S2). A data extraction table was designed and tested before extraction of data began. The full data extraction table is publicly accessible and contains information on title, authors, year of publication, study type, experimental set-up, group characteristics, perfusion characteristics, analyses, perfusate results, urine results, tissue results, and post-transplant results [12]. If details of the experimental design were not reported, we attempted to derive information from referenced studies.
Kidneys were categorized as “minimally injured” when they endured less than 5 min of warm ischemia and <30 min of cold ischemia and as “injured” in all other cases. Some articles report on multiple experiments that include different species, oxygenation methods, and perfusates. Therefore, percentages do not always add up to 100%.
2.4. Quality Assessment and Data Analysis
Concerning experimental animal studies, the ‘systematic review center for laboratory animal experimentation (SYRCLE) risk of bias tool’ was used to assess the quality of the animal experiments and the article. This tool is based on the Cochrane risk-of-bias tool and adapted for aspects of bias that play a specific role in animal intervention studies [13]. Signal questions were formulated by Hooijmans et al. to facilitate judgment and reported to increase transparency and applicability of results [13]. For studies with human organs, methodological quality was assessed using the National Institutes of Health (NIH) scoring tools. These tools include items for evaluating potential flaws in study methods or implementation, including sources of bias (e.g., patient selection, performance, attrition, and detection), confounding, study power, the strength of causality in the association between interventions and outcomes, and other factors [14].
3. Results
A systematic search of online databases, performed on 2 February 2023, resulted in the identification of 14,335 records. After duplicate removal, 9794 records remained, of which 9596 were excluded based upon predefined inclusion and exclusion criteria at the time of initial screening and 152 at the time of full-text screening, leaving 46 included articles. From the reference lists, another 1507 potential papers were identified leading to six additional inclusions. In total, 52 papers were included in this scoping review. Figure S1 shows the flowchart. The full data extraction table can be accessed online [12]. Included articles were published between 1970 and 2023 (Table S3).
3.1. Quality and Risk of Bias Assessment
For animal studies, the risk of bias was most often “unclear” or “high” because essential information was often not reported (Figure S2, Table S4). For human studies, the overall quality was better, most often “good” or “fair” (Figure S2, Table S5).
3.2. Hypothermic Perfusion Set-up
The majority of articles reported on animal experiments (48/52) in dogs (26/52), rabbits (2/52), and pigs (20/52) (Figure 1). Seven studies reported on the perfusion of human kidneys (7/52), of which three were transplant studies [8,15,16]. Some papers report on both animal and human kidney perfusions (3/51) (Table S3). Kidneys were exposed to variable ischemic injury, induced by introducing warm ischemia (5 to 240 min; clamping of the renal artery (and vein) before procurement or procurement after death) or exposure to cold storage before perfusion (up to 20 h) (Table S3). After hypothermic perfusion, kidneys were transplanted in 22 studies [8,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35] or re-perfused with a blood-based perfusate in one study [36]. Kidneys were flushed with different solutions (detailed in the extraction table [12]) before mounting on the perfusion device.
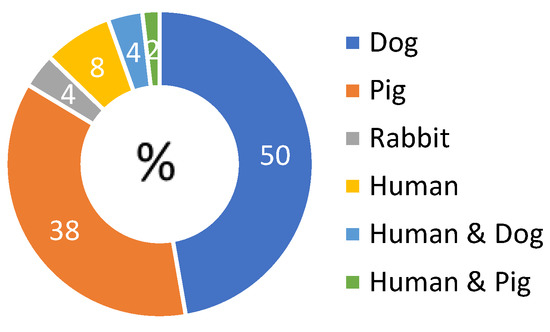
Figure 1.
Diagram of distribution of included papers according to the species studied. Some articles report on multiple experiments in different species, therefore percentages do not add up to 100%.
Several perfusion devices were used, from homemade to commercially available devices (Table S3). The latter were sometimes adjusted to fit the aim of the research. Common features of these perfusion circuits were a reservoir and tubing, and a pump to circulate the perfusate. Cooling (2–12 °C) was accomplished by a heat exchanger, ice surrounding the reservoir, or a combination of both. The majority of the circuits used pulsatile perfusion (roller, centrifugal, or peristaltic pump); continuous non-pulsatile perfusion was used in two studies [37,38]. In most studies, perfusion was pressure controlled (25 to 60 mmHg) with a maximal pressure of 30 mmHg in recent studies.
Acellular perfusates were used without the use of an oxygen carrier. Between 1970 and 1986, these were plasma- or albumin-based and often a vasodilator, heparin, antibiotics, corticosteroids, allopurinol, insulin, and buffers to maintain pH were added (Table S6). As of 1980, synthetic perfusates were introduced. Albumin was replaced by synthetic oncotic products (mannitol, hydroxyethyl starch, etc.) and the addition of less permeable anions (like gluconate and lactobionate) prevented further hypothermic-induced cell swelling in contrast with other anions (like chloride) [39]. Adaptations were made to include adenine nucleotide derivates and electrolytes were added in extracellular or intracellular concentrations. After 1986, all studies were performed with synthetic solutions, mostly formulations of Belzer’s Machine Perfusion Solution (MPS) [39]. In some studies, carbohydrates, amino acids, or fatty acids were added to the perfusate (Table S6).
In the majority of studies, the perfusate was oxygenated (40/52; 77%), most commonly by using a membrane oxygenator (26/40; 65%) (Table S2). In these studies, a mixture of O2 and CO2 [17,19,20,21,22,25,34,40,41,42,43,44,45] or a mixture of O2, CO2, and nitrogen [26,27,38,46,47,48,49] was given. Surface oxygenation with room air or O2 (13/40; 33%) [20,32,37,38,41,42,43,50,51,52,53,54,55] was also used. In one article (3%), bubble and surface oxygenation were combined [20]. Other methods were film oxygenation [43], run-off and tube oxygenation [33,56], hyperbaric oxygenation in a pressure chamber [45], and non-specified oxygenation [28,57]. Some studies investigated different oxygenation types. In the remaining studies, the perfusate was not actively oxygenated (10/52; 19%) [8,15,16,18,30,35,58,59,60,61] or it was not clear if the perfusate was oxygenated (2/52; 4%) [31,62].
3.3. Metabolism during Hypothermic Perfusion with Plasma-Based Perfusates
Studies were categorized as either studying carbohydrate (7/9), amino acid (1/9), fatty acid (2/9) metabolism, or the metabolism of high-energy molecules (3/9). All studies used oxygen during perfusion and one study [40] compared oxygenated and non-oxygenated perfusion. Details are listed in Table 1 and Table S7.

Table 1.
Summary of studies reporting on kidney metabolism with plasma-based perfusate.
3.3.1. Carbohydrate Metabolism
In six studies, minimally injured kidneys were hypothermically perfused with oxygen and all perfusates contained glucose/dextrose at the start [17,23,29,40,44,57]. (Table S6). Studies show increasing [29], decreasing [44], and similar [40] perfusate glucose concentrations over time. The perfusate lactate/pyruvate ratio, said to be an indicator of the redox potential, increased over time [17,23,29,44,57].
When ischemically injured kidneys were perfused with an oxygenated glucose-containing perfusate, glucose concentrations decreased with increasing levels of lactate and pyruvate [25].
3.3.2. Amino Acid Metabolism
Many amino acids (alanine, glutamate, serine, glycine, valine, threonine, (iso)leucine, methionine, aspartate, phenylalanine, lysine, (hydroxy)proline, tyrosine, and arginine) were released during the oxygenated perfusion of minimally injured kidneys [44].
3.3.3. Fatty Acid Metabolism
Perfusate-free fatty acids [29] and lipids [63] decreased during the oxygenated perfusion of minimally injured kidneys. The decrease in perfusate triglycerides and tissue phospholipids was less pronounced when oleate (a long-chain fatty acid) was administered during perfusion, with a more pronounced decrease in perfusate neutral lipids but high retention of tissue triglycerides and neutral lipids [63].
3.3.4. Energy Metabolism
In minimally injured kidneys, ATP levels increased during oxygenated perfusion while in injured kidneys, tissue ATP levels decreased rapidly during warm ischemia, with restoration during oxygenated perfusion [56]. Nevertheless, Pegg et al. did not observe adenine nucleotide restoration [25] and Kahng et al. described a variable range for each adenine nucleotide and a suboptimal energy charge during perfusion [57].
3.4. Metabolism during Hypothermic Perfusion with Albumin-Based Perfusates
Studies were categorized as either studying carbohydrate (10/16; 63%), amino acid (2/16; 13%), fatty acid (10/16; 63%) metabolism, or the metabolism of high energy molecules (3/16; 19%). All studies used oxygenated perfusion. Details are listed in Table 2 and Table S8.

Table 2.
Summary of studies reporting on kidney metabolism with an albumin-based perfusate.
In nine studies, radioactively labeled metabolites—also called tracers—were added to the perfusion circuit to investigate active metabolic pathways: 14C-glucose [27,42,46,47,48], 14C-mevalonate [53], 14C-acetate [47], 14C-lactate [47], 14C-labeled amino acids (14C-cycloleucine [42], 14C-leucine [41], 14C-threonine [41]), 14C-labeled fatty acids (14C-linoleate [46,48,49], 14C-palmitate [26,46,48,49], 14C-caprylate [42,46,48], 14C-myristic acid [48]).
3.4.1. Carbohydrate Metabolism
A decrease in perfusate glucose and an increase in perfusate lactate concentration was seen during oxygenated perfusion [27,42,46,47,48]. Only one study showed no change in perfusate glucose level with a low lactate and non-measurable pyruvate during oxygenated perfusion with bovine serum albumin [34]. This glucose drop was more pronounced when glucose was a component of the perfusate [27] and less pronounced when amino acids [42] or fatty acids [46,47] were added. Interestingly, Slaattelid et al. also found a different perfusate glucose uptake and release pattern when glucose was administered intravenously before kidney procurement [27]. Three studies found a decrease in tissue glucose regardless of whether the perfusion medium was glucose-rich or -poor [27,37,38]. Tracer studies showed active glucose metabolism, with the detection of 14C-CO2 and 14C-lactate [27,46,47,48]. An increase in the lactate/pyruvate ratio was seen in the studies by Grundmann et al. [23] and Pettersson et al. [48]. Tissue lactate showed no change [38] and was the highest metabolite measured [57], even increasing after 72 h when glucose was added [37].
3.4.2. Amino Acid Metabolism
Many amino acids were released during the oxygenated perfusion of minimally injured kidneys, with an increase in urea and ammonia [42]. The increase was most pronounced for alanine and taurine [41]. No increase was seen for glutamine, proline, aspartate, and cystine [42].
In contrast, when amino acids were added to the perfusate, a decrease in many amino acids (glutamine, proline, glycine, aspartate, arginine, (iso)leucine, methionine, valine, and serine) was seen with an increase only in threonine, tyrosine, ornithine, taurine, and alanine levels [41,42]. Labeled (cyclo)leucine and threonine were incorporated into proteins; more so for leucine which is also reflected in the higher label recovery in CO2 from leucine than threonine [41].
3.4.3. Fatty Acid Metabolism
In minimally injured kidneys, a decrease in fatty acids during oxygenated perfusion with a fatty acid-rich perfusate was seen [26,27,38,42,46,47,48,49]. When a defatted perfusate was used, perfusate-free fatty acids increased [24]. Perfusate short-chain fatty acids (caprylate and acetate) decreased rapidly [38,42,46,47,48,49], while a decrease in medium-chain fatty acids (laureate and myristic acid) was seen after a few days when short-chain fatty acids were depleted [46,48]. Labeled short-chain fatty acids were mainly incorporated into CO2 [42,46,47,48], glucose [47,48], and lactate [47,48], indicative of fatty acid oxidation and gluconeogenesis. Long-chain fatty acids (palmitate, oleate, linoleate, stearate) decreased more slowly than short- and medium-chain fatty acids [26,38,46,48]. Labeled long-chain fatty acids were incorporated into phospholipids [48,49] and triglycerides [48,49], and to a very low extent into CO2 [46,48,49], indicative of utilization as a membrane stabilizer rather than as a source for oxidation.
In tissue, phospholipids decreased with fatty acid-free perfusate [47]. When a fatty acid-rich perfusate was used, phospholipid levels did not change [49] or decreased [48]. Tissue cholesterol levels remained unchanged in three studies in which kidneys were pumped for 2 to 6 days [47,49,53] and decreased in one study after 6 days of perfusion [48].
3.4.4. Metabolism of High-Energy Molecules
In minimally injured kidneys, ATP levels and total adenine nucleotide levels decreased during oxygenated perfusion but the energy charge potential was optimal [37]. While in ischemically injured kidneys, ATP levels increased to near normal after 24 h of oxygenated perfusion but total adenine nucleotide levels remained the same [28] or had a variable range with suboptimal energy charge [57].
3.5. Metabolism during Hypothermic Perfusion with Synthetic Perfusates
Studies were categorized as either studying carbohydrate (20/31 65%), amino acid (14/31; 45%), fatty acid (9/31; 29%) metabolism, metabolism of high-energy molecules (23/31; 74%), or TCA cycle metabolism (8/31; 26%). In 19 studies, oxygen was added to the perfusate, in ten studies no oxygen was given, and in two studies it was not clear if oxygen was given.
In two studies, non-radioactively labeled 13C-glucose [60,64] was used to further clarify the metabolism. Details are listed in Table 3 and Table S9.

Table 3.
Summary of studies reporting on kidney metabolism with a synthetic perfusate.
3.5.1. Carbohydrate Metabolism
Perfusion with oxygenated glucose-free perfusate led to low and decreasing levels of tissue glucose and very low and stable lactate levels in minimally injured kidneys [37].
In ischemically injured kidneys, perfused without active oxygenation, glucose changes seem dependent on the glucose concentrations in the perfusate. Indeed, no changes in glucose levels were seen during long-term perfusion with MPS (72 h perfusion with perfusate changes every 24 h) [59]. In a similar 72 h experiment with 24 h renewals of UHK solution, containing less glucose and mannitol compared to MPS, perfusate glucose levels dropped [59]. Similarly, other studies investigating short- and long-term non-oxygenated perfusions of pig kidneys with MPS found no statistical change in perfusate glucose levels [8,16,58] or an increase in glucose [15] with increases in perfusate and tissue lactate [8,15,16]. A similar study with human kidneys showed a glucose and lactate increase in the perfusate [15]. Only one group studied glucose changes during the oxygenated perfusion of injured kidneys and found increased perfusate glucose and lactate levels with similar or increased tissue lactate [19,20,21,22]. Perfusate lactate levels were lower when high oxygen concentrations were given [20,21,22,64].
Tracer studies show active glucose metabolism during (non-)oxygenated perfusion of ischemically injured kidneys. When 13C-glucose was infused, 13C-lactate appeared in the perfusate and tissue [60,64]. Less labeled lactate was recovered in tissue after highly oxygenated perfusion [64].
Mannitol did not change with non-oxygenated MPS [21] and increased during oxygenated perfusion [21]. Ribose levels remained stable in two studies [8,15] and decreased in one study [16].
3.5.2. Amino Acid Metabolism
Amino acid release in the perfusate was shown for minimally and ischemically injured kidneys, regardless of oxygenation status and a correlation between an increase in amino acids and perfusion duration has been suggested [16]. Glutamate [8,15,16,18,21,58,59,61], alanine [8,15,16,18,21,35,60,61,64], valine [8,15,16,18,35], glycine [8,15,16,18,21], and (iso)leucine [16] were most often studied. A glutamate increase was also seen during hypothermic perfusion with UHK-solution [59]. Glutamate levels were lower in the tissue of highly oxygenated kidneys compared to non- or low-oxygenated kidneys [20,21,22,64].
There is some evidence of metabolization of glucose to amino acids as the infusion of 13C-glucose during (non-)oxygenated perfusion resulted in the detection of labeled alanine [60,64] in the perfusate and tissue and labeled glutamate in the tissue [60].
Glutathione (GSH), a tripeptide (cysteine, glycine, glutamate) and powerful antioxidant, is a component of MPS. All studies investigating perfusate GSH showed a decrease during the non-oxygenated and oxygenated perfusion of injured kidneys [8,15,21,35,51,61,64].
Oxidized glutathione in the perfusate also decreased or was undetectable [15,16]. There is some suggestion that higher oxygen concentrations increase the levels of tissue glutathione [64].
3.5.3. Fatty Acid Metabolism
Acetate, a short-chain fatty acid, did not change [8,15,60,64] or increased [35] during non-oxygenated perfusion. Acetate remained unchanged or decreased with oxygenated perfusion [21,64]. The ketone body 3-hydroxybutyrate seems to behave differently in humans (increases) [8,15] and pigs (no change) [8] during non-oxygenated perfusion. All kidneys suffered ischemic injury.
During oxygenated perfusion of minimally injured kidneys with gluconate-based perfusates, tissue phospholipids were studied. An initial decrease was seen in the first 24 h followed by an increase in phospholipids [54].
3.5.4. Energy Metabolism
Belzer’s MPS contains adenine and ribose after studies showed higher ATP concentrations with these additives compared to adenosine during oxygenated perfusion of minimally injured kidneys [32,52,55]. Furthermore, with MPS, ATP content increases with higher oxygen concentrations [50].
In ischemically injured kidneys, ATP content decreased during warm ischemia [30,31,36,65,66] and restoration occurred during perfusion [20,21,22,30,66]. ATP increased more when the perfusate was actively oxygenated [20,21,36,64,65,66]. A similar ATP increase during highly oxygenated perfusion with Celsior [45] and a Haemaccel-based perfusate [37,43] has been shown. An increase in tissue ADP was also observed [21,22]; AMP decreased [21] or remained unchanged [22]. Similar studies in injured kidneys showed an increase in perfusate (hypo)xanthine [8,15,16,21], inosine [8,15,16], and adenosine [16], while no changes [8,15,21] or a decrease [16] in adenine concentration was seen.
3.5.5. TCA Cycle Metabolism
Only a few studies examined TCA cycle intermediates [8,15,16,20,21,22,61,64]. Administration of 13C-glucose resulted in the formation of 13C-citrate, 13C-malate, and 13C-succinate in the cortex and medulla, indicative of TCA cycle activity [64]. Tissue 13C-succinate was higher if the perfusate was highly oxygenated [64].
4. Discussion
Commendable work contributing to our understanding of kidney metabolic behavior during cold perfusion has been performed over the past 50 years. Nevertheless, it remains incompletely understood.
From compiling the findings of this scoping review, and in particular those of “tracer studies” during which a (non)radioactively labeled metabolite is added to the perfusion circuit, it is clear that kidneys are metabolically active during cold perfusion. However, key pieces of the puzzle are missing. The fact that metabolism is intrinsically a complex network of interacting biochemical reactions that can be influenced by numerous factors complicates the interpretation of findings. Indeed, perfusate composition, oxygenation, pre-existing kidney injury, and perhaps even pre-donation nutrient availability seem to influence metabolism during hypothermic perfusion preservation. Furthermore, key enzymes are likely to be influenced by the low temperatures and this in turn will influence metabolism.
Tracer studies have shown the oxidation of glucose, amino acids, and fatty acids to CO2 [27,41,42,46,47,48,49] but it is unclear if and how oxygenation levels and pre-existing injury affect this. A few older studies suggest gluconeogenesis from fatty acids [47,48] and the incorporation of amino acids into proteins when these are provided [41]. Indeed, kidney metabolism seems dependent on the perfusate composition. Administration of carbohydrates, amino acids, and lipids seems to change the uptake and release patterns of these metabolites. In almost all studies, glucose was added to the perfusate as an energy source. Interestingly, glucose and lactate metabolism changed when other substrates such as amino acids or fatty acids were added to the perfusate, suggesting these metabolites could have competing interests as energy sources. In vivo, fatty acids and amino acids can feed into the TCA cycle either as acetyl-coenzyme A or another TCA-cycle intermediate and serve as important energy sources [67]. In physiological, in vivo conditions, the kidneys play a role in the synthesis and inter-organ exchange of amino acids [68]. It is unclear how the absence of inter-organ exchange influences amino acid metabolism during cold perfusion. Furthermore, the in vivo renal metabolism of alanine, (iso)leucine, and valine changes during fasting and feeding states [68,69] and this might influence metabolism during cold perfusion as well.
The oxygenation level of the perfusate also seems to affect kidney metabolism during cold perfusion. Indeed, a reasonable number of studies showed differences in kidney metabolism when comparing different oxygen levels. Concerning lactate metabolism, there seems to be a reduced increase in lactate when the perfusate was actively oxygenated compared to non-oxygenated perfusates [21,22,40,43,64]. ATP levels and the supported energy charge are higher when kidneys are oxygenated during perfusion [20,21,36,43,45,50,64,65,66].
Injury levels are likely to play a role as well, but this is less clear as only a few studies directly compared kidneys with different injury levels. Pre-existing warm ischemia depletes ATP levels and studies suggest variable reconstitution of ATP during oxygenated perfusion [28,30,36,43,56,65,66]. Observations of other metabolites in minimally injured kidneys are less clear.
The potential implications of these observations in a clinical setting are important. Indeed, supporting the metabolically active kidney during hypothermic perfusion seems the logical next step. Actively oxygenating the perfusate during the hypothermic perfusion of older DCD kidneys improved graft outcomes compared to standard non-oxygenated hypothermic perfusion in a recent randomized controlled trial [5]. On the other hand, there was no evidence that short (2 h) reconditioning of expanded-criteria donor kidneys, following cold storage, improved graft survival compared to cold storage alone [70]. Perhaps 2 h is too short to change metabolic behavior after a long period of cold storage or perhaps DBD organs respond differently to additional oxygen compared to DCD.
These findings need to be interpreted with a degree of caution. The majority of studies have a considerable risk of bias and studies were conducted over the course of 50 years with numerous changes in perfusate and perfusion conditions. Many studies report on experiments with dog kidneys. It is important to realize that dog kidneys have a higher tolerance to ischemia reperfusion injury, while pig kidneys are more similar to human kidneys [71,72]. Even though pig kidneys are physiologically and anatomically comparable to human kidneys, there is only limited evidence that pig kidney metabolism is the same as that of human kidneys, however, it is reassuring that Nath et al. found comparable metabolites in pig and human kidney perfusates [8].
As with all scoping reviews, it is possible that some relevant articles were not identified or that relevant studies were published after the search. We limited the chance of missing relevant articles by setting up a broad search strategy in collaboration with experienced biomedical reference librarians. Furthermore, the references of included articles were searched to identify any articles that might have been missed in the search.
5. Conclusions
In conclusion, kidneys are metabolically active during cold perfusion preservation. This metabolic activity is incompletely understood and is influenced by a multitude of factors including perfusate composition, oxygenation level, and likely pre-existing injury. It is clear that a greater number of well-designed (pre-)clinical studies are necessary to understand this behavior. In the modern era, with increasing DCD donations and the emergence of hypothermic oxygenated perfusion, the focus should be on understanding metabolic perturbations caused by pre-existing injury levels and on the effect of perfusate oxygen levels. The use of tracers in such studies is indispensable to understanding the metabolism, given the complexity of interactions between different metabolites.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12113613/s1, Figure S1 Flow chart of the systematic search identifying published papers reporting on kidney metabolism during hypothermic perfusion; Figure S2: Risk of bias assessment in 30 studies identified, using SYRCLEs tool [13]; Table S1: Search string in databases Pubmed, Embase, Cochrane Library and Web of Science Core Collection; Table S2: Inclusion and exclusion criteria; Table S3: Overview of study set-up and perfusion characteristics; Table S4: Detailed Risk of Bias Assessment using SYRCLE’s tool for articles reporting on animal studies; Table S5: Quality assessment of studies including human kidneys according to the NIH quality assessment score; Table S6: Composition of different perfusion solutions studied in this review; Table S7: Extended summary of studies reporting on kidney metabolism with plasma-based perfusates; Table S8: Extended summary of studies reporting on kidney metabolism with albumin-based perfusates; Table S9: Extended summary of studies reporting on kidney metabolism with synthetic perfusates. Refs. [73,74] are cited in the Supplemental File.
Author Contributions
Conceptualization I.J. and L.V.; methodology L.V. and R.D.a.; validation L.V., R.D.a. and I.J.; formal analysis; L.V., R.D.a. and I.J.; investigation L.V. and R.D.a.; resources L.V. and R.D.a.; data curation L.V., R.D.a. and I.J.; writing—original draft preparation L.V. and R.D.a.; writing—review and editing I.J. and B.G.; visualization L.V., R.D.a. and I.J.; supervision I.J.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received was supported by an FTBO grant from KU Leuven/UZ Leuven and a KOOR grant from UZ Leuven.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The dataset has been deposited in RDR, KU Leuven’s data repository, and is publicly available via https://doi.org/10.48804/AMSYVO [12].
Acknowledgments
We thank Veerle Heedfeld for her help with cleaning the extraction table. We also thank Thomas Vandendriessche, Chayenne Van Meel and Krizia Tuand, biomedical reference librarians of the KU Leuven Libraries—2Bergen—Learning Centre Désiré Collen (Leuven, Belgium) for their help in conducting the systematic literature search.
Conflicts of Interest
B.G. and I.J. are listed as co-inventor of a patent application on methods and applications of analyzing the perfusate of an ex situ perfused kidney (EP 22155190.6). I.J. received speaker fees from XVIVO Perfusion paid to her institution.
References
- Tingle, S.J.; Figueiredo, R.S.; Moir, J.A.; Goodfellow, M.; Talbot, D.; Wilson, C.H. Machine perfusion preservation versus static cold storage for deceased donor kidney transplantation. Cochrane Database Syst. Rev. 2019, 3, CD011671. [Google Scholar] [CrossRef]
- Moers, C.; Smits, J.M.; Maathuis, M.H.; Treckmann, J.; van Gelder, F.; Napieralski, B.P.; van Kasterop-Kutz, M.; van der Heide, J.J.; Squifflet, J.P.; van Heurn, E.; et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N. Engl. J. Med. 2009, 360, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Moers, C.; Pirenne, J.; Paul, A.; Ploeg, R.J.; Na, N.A. Machine perfusion or cold storage in deceased-donor kidney transplantation. N. Engl. J. Med. 2012, 366, 770–771. [Google Scholar] [CrossRef]
- Jochmans, I.; Moers, C.; Smits, J.M.; Leuvenink, H.G.D.; Treckmann, J.; Paul, A.; Rahmel, A.; Squifflet, J.P.; Van Heurn, E.; Monbaliu, D.; et al. Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: A multicenter, randomized, controlled trial. Ann. Surg. 2010, 252, 756–762. [Google Scholar] [CrossRef]
- Jochmans, I.; Brat, A.; Davies, L.; Hofker, H.S.; van de Leemkolk, F.E.M.; Leuvenink, H.G.D.; Knight, S.R.; Pirenne, J.; Ploeg, R.J.; COMPARE Trial Collaboration and Consortium for Organ Preservation in Europe (COPE). Oxygenated versus standard cold perfusion preservation in kidney transplantation (COMPARE): A randomised, double-blind, paired, phase 3 trial. Lancet 2020, 396, 1653–1662. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.A.; Nicholson, H.F.; Nicholson, M.L. Oxygenated kidney preservation techniques. Transplantation 2012, 93, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Guibert, E.E.; Petrenko, A.Y.; Balaban, C.L.; Somov, A.Y.; Rodriguez, J.V.; Fuller, B.J. Organ preservation: Current concepts and new strategies for the next decade. Transfus. Med. Hemotherapy 2011, 38, 125–142. [Google Scholar] [CrossRef]
- Nath, J.; Guy, A.; Smith, T.B.; Cobbold, M.; Inston, N.G.; Hodson, J.; Tennant, D.A.; Ludwig, C.; Ready, A.R. Metabolomic perfusate analysis during kidney machine perfusion: The pig provides an appropriate model for human studies. PLoS ONE 2014, 9, e114818. [Google Scholar] [CrossRef]
- Jochmans, I. Improving Organ Preservation: The Trick Is to Keep (Cells) Breathing. Transplantation 2022, 106, 904–907. [Google Scholar] [CrossRef]
- Verstraeten, L.; Denabt, R.; Jochmans, I. Current insights into the metabolome during hypothermic isolated kidney perfusion—A scoping review. Open. Sci. Framew. 2022. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Verstraeten, L.; Denabt, R.; Jochmans, I. Replication Data for: Current Insights into the Metabolome during Hypothermic Kidney Perfusion—A Systematic Review. 2023. Available online: https://doi.org/10.48804/AMSYVO (accessed on 7 April 2023).
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Study Quality Assessment Tools, NIH. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 24 February 2023).
- Guy, A.J.; Nath, J.; Cobbold, M.; Ludwig, C.; Tennant, D.A.; Inston, N.G.; Ready, A.R. Metabolomic analysis of perfusate during hypothermic machine perfusion of human cadaveric kidneys. Transplantation 2015, 99, 754–759. [Google Scholar] [CrossRef]
- Faucher, Q.; Alarcan, H.; Sauvage, F.L.; Forestier, L.; Miquelestorena-Standley, E.; Nadal-Desbarats, L.; Arnion, H.; Venhard, J.C.; Brichart, N.; Bruyère, F.; et al. Perfusate Metabolomics Content and Expression of Tubular Transporters During Human Kidney Graft Preservation by Hypothermic Machine Perfusion. Transplantation 2022, 106, 1831–1843. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.L.; Dmochowski, J.R.; Murray, J.E.; Couch, N.P. Successful 24 hour renal perfusion-preservation with monitoring by surface electrometry during the storage interval. Surgery 1970, 67, 944–950. [Google Scholar] [PubMed]
- Bon, D.; Billault, C.; Thuillier, R.; Hebrard, W.; Boildieu, N.; Celhay, O.; Irani, J.; Seguin, F.; Hauet, T. Analysis of perfusates during hypothermic machine perfusion by NMR spectroscopy: A potential tool for predicting kidney graft outcome. Transplantation 2014, 97, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Darius, T.; Gianello, P.; Vergauwen, M.; Mourad, N.I.; Buemi, A.; De Meyer, M.; Mourad, M. The effect on early renal function of various dynamic preservation strategies in a preclinical pig ischemia–reperfusion autotransplant model. Am. J. Transpl. 2018, 19, 752–762. [Google Scholar] [CrossRef]
- Darius, T.; Vergauwen, M.; Mueller, M.; Aydin, S.; Dutkowski, P.; Gianello, P.; Mourad, M. Brief Bubble and Intermittent Surface Oxygenation Is a Simple and Effective Alternative for Membrane Oxygenation During Hypothermic Machine Perfusion in Kidneys. Transpl. Direct 2020, 6, e571. [Google Scholar] [CrossRef]
- Darius, T.; Vergauwen, M.; Smith, T.; Gerin, I.; Joris, V.; Mueller, M.; Aydin, S.; Muller, X.; Schlegel, A.; Nath, J.; et al. Brief O2 uploading during continuous hypothermic machine perfusion is simple yet effective oxygenation method to improve initial kidney function in a porcine autotransplant model. Am. J. Transplant. 2020, 20, 2030–2043. [Google Scholar] [CrossRef]
- Darius, T.; Vergauwen, M.; Smith, T.B.; Patel, K.; Craps, J.; Joris, V.; Aydin, S.; Ury, B.; Buemi, A.; De Meyer, M.; et al. Influence of Different Partial Pressures of Oxygen During Continuous Hypothermic Machine Perfusion in a Pig Kidney Ischemia-reperfusion Autotransplant Model. Transplantation 2020, 104, 731–743. [Google Scholar] [CrossRef]
- Grundmann, R.; Berr, F.; Pitschi, H. Ninety six hour preservation of canine kidneys. Transplantation 1974, 17, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Halasz, N.A.; Collins, G.M. Fatty acid utilization during perfusion. Transplantation 1975, 20, 354–356. [Google Scholar] [PubMed]
- Pegg, D.E.; Foreman, J.; Rolles, K. Metabolism during preservation and viability of ischemically injured canine kidneys. Transplantation 1984, 38, 78–81. [Google Scholar] [PubMed]
- Slaattelid, O.; Flatmark, A.; Skrede, S. The importance of perfusate content of free fatty acids for dog kidney preservation. Scand. J. Clin. Lab. Investig. 1976, 36, 239–245. [Google Scholar] [CrossRef]
- Slaattelid, O.; Gronnerod, O.; Horn, A. Preservation of dog kidneys by hypothermic perfusion with glucose-free and glucose-rich perfusates. Eur. Surg. Res. 1976, 8, 515–527. [Google Scholar] [CrossRef]
- Collins, G.M.; Taft, P.; Green, R.D.; Ruprecht, R.; Halasz, N.A. Adenine nucleotide levels in preserved and ischemically injured canine kidneys. World J. Surg. 1977, 2, 237–243. [Google Scholar] [CrossRef]
- Grundmann, R.; Landes, T.; Pichlmaier, H. Hypothermic pulsatile perfusion of dog kidneys. Factors limiting successful preservation time. Transplantation 1972, 14, 742–747. [Google Scholar] [CrossRef]
- Kaminski, J.; Delpech, P.O.; Kaaki-Hosni, S.; Promeyrat, X.; Hauet, T.; Hannaert, P. Oxygen Consumption by Warm Ischemia-Injured Porcine Kidneys in Hypothermic Static and Machine Preservation. J. Surg. Res. 2019, 242, 78–86. [Google Scholar] [CrossRef]
- La Manna, G.; Conte, D.; Cappuccilli, M.L.; Nardo, B.; D’Addio, F.; Puviani, L.; Comai, G.; Bianchi, F.; Bertelli, R.; Lanci, N.; et al. An in vivo autotransplant model of renal preservation: Cold storage versus machine perfusion in the prevention of ischemia/reperfusion injury. Artif. Organs 2009, 33, 565–570. [Google Scholar] [CrossRef]
- McAnulty, J.F.; Southard, J.H.; Belzer, F.O. Comparison of the effects of adenine-ribose with adenosine for maintenance of ATP concentrations in 5-day hypothermically perfused dog kidneys. Cryobiology 1988, 25, 409–416. [Google Scholar] [CrossRef]
- Minor, T.; Sitzia, M.; Dombrowski, F. Kidney transplantation from non-heart-beating donors after oxygenated low-flow machine perfusion preservation with histidine-tryptophan-ketoglutarate solution. Transpl. Int. 2005, 17, 707–712. [Google Scholar] [CrossRef]
- Pegg, D.E.; Green, C.J. Renal preservation by hypothermic perfusion using a defined perfusion fluid. Cryobiology 1972, 9, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Simona, M.-S.; Alessandra, V.; Emanuela, C.; Elena, T.; Michela, M.; Fulvia, G.; Vincenzo, S.; Ilaria, B.; Federica, M.; Eloisa, A.; et al. Evaluation of Oxidative Stress and Metabolic Profile in a Preclinical Kidney Transplantation Model According to Different Preservation Modalities. Int. J. Mol. Sci. 2023, 24, 1029. [Google Scholar] [CrossRef] [PubMed]
- Venema, L.H.; Brat, A.; Moers, C.; Hart, N.A.; Ploeg, R.J.; Hannaert, P.; Minor, T.; Leuvenink, H. Effects of Oxygen During Long-term Hypothermic Machine Perfusion in a Porcine Model of Kidney Donation After Circulatory Death. Transplantation 2019, 103, 2057–2064. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.H.; Armbruster, D.; Grebe, W.; Czerniak, A.; Isselhard, W. Effects of differences in substrate supply on the energy metabolism of hypothermically perfused canine kidneys. Cryobiology 1980, 17, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.H.; Isselhard, W.; Hauer, U.; Menge, M. Free fatty acid and glucose metabolism during hypothermic perfusion of canine kidneys. Eur. Surg. Res. 1979, 11, 107–121. [Google Scholar] [CrossRef]
- Belzer, F.O.; Glass, N.R.; Sollinger, H.W.; Hoffmann, R.M.; Southard, J.H. A new perfusate for kidney preservation. Transplantation 1982, 33, 322–323. [Google Scholar]
- Pedersen, F.B.; Hrynczuk, J.R.; Scheibel, J.H.; Sorensen, B.L. Urine production and metabolism of glucose and lactic acid in the kidney during 36 hours of cooling and perfusion with diluted plasma. Scand. J. Urol. Nephrol. 1973, 7, 68–73. [Google Scholar] [CrossRef]
- Lundstam, S.; Jagenburg, R.; Jonsson, O.; Lundholm, K.; Naucler, J.; Pettersson, S.; Schersten, T. Metabolism in the hypothermically perfused dog kidney. Incorporation rate of leucine and threonine into proteins. Eur. Surg. Res. 1977, 9, 206–216. [Google Scholar] [CrossRef]
- Lundstam, S.; Jagenburg, R.; Jonsson, O.; Lundholm, K.; Naucler, J.; Pettersson, S.; Schersten, T. Metabolism in the hypothermically perfused dog kidney. Utilization and production of amino acids. Eur. Surg. Res. 1977, 9, 191–205. [Google Scholar] [CrossRef]
- Pegg, D.E.; Wusteman, M.C.; Foreman, J. Metabolism of normal and ischemically injured rabbit kidneys during perfusion for 48 hours at 10 C. Transplantation 1981, 32, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Verkh, L.; Freier, D.T.; Celik, C. Changes in concentration of amino acids and other metabolites during hypothermic perfusion of the canine kidney. Cryobiology 1986, 23, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Ravaioli, M.; Baldassare, M.; Vasuri, F.; Pasquinelli, G.; Laggetta, M.; Valente, S.; De Pace, V.; Neri, F.; Siniscalchi, A.; Zanfi, C.; et al. Strategies to Restore Adenosine Triphosphate (ATP) Level After More than 20 Hours of Cold Ischemia Time in Human Marginal Kidney Grafts. Ann. Transplant. 2018, 23, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Lundstam, S.; Claes, G.; Jonsson, O.; Naucler, J.; Pettersson, S.; Schersten, T. metabolic studies on kidney in hypothermic perfusion. J. D. Urol. Et. De. Nephrol. 1975, 81, 716–720. [Google Scholar]
- Lundstam, S.; Claes, G.; Jonsson, O.; Pettersson, S.; Schersten, T. Metabolism in the hypothermically perfused kidney. Production and utilization of lactate and utilization of acetate in the dog kidney. Eur. Surg. Res. 1976, 8, 300–310. [Google Scholar] [CrossRef]
- Pettersson, S.; Claes, G.; Schersten, T. Fatty acid and glucose utilization during continuous hypothermic perfusion of dog kidney. Eur. Surg. Res. 1974, 6, 79–94. [Google Scholar] [CrossRef]
- Skrede, S.; Slaattelid, O. Fatty acid metabolism during hypothermic perfusion of the isolated dog kidney. Scand. J. Clin. Lab. Invest. 1979, 39, 765–771. [Google Scholar] [CrossRef]
- Lazeyras, F.; Buhler, L.; Vallee, J.P.; Hergt, M.; Nastasi, A.; Ruttimann, R.; Morel, P.; Buchs, J.B. Detection of ATP by “in line” 31P magnetic resonance spectroscopy during oxygenated hypothermic pulsatile perfusion of pigs’ kidneys. Magn. Reson. Mater. Phys. Biol. Med. 2012, 25, 391–399. [Google Scholar] [CrossRef]
- Boudjema, K.; Lindell, S.L.; Southard, J.H.; Belzer, F.O. Changes in glutathione concentration in hypothermically perfused dog kidneys. J. Lab. Clin. Med. 1991, 117, 131–137. [Google Scholar]
- Southhard, J.H.; Kuniyoshi, M.; Lutz, M.F.; Ametani, M.; Belzer, F.O. Comparison of the effect of 3- and 5-day hypothermic perfusion of dog kidneys on metabolism of tissue slices. Cryobiology 1984, 21, 285–295. [Google Scholar] [CrossRef]
- Kleist, H.; Jonsson, O.; Lundstam, S.; Naucler, J.; Pettersson, S.; Schersten, T. Metabolism in the hypothermically perfused kidney: Utilization of mevalonate in the human and in the dog kidney. Eur. Surg. Res. 1982, 14, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Southard, J.H.; Ametani, M.S.; Lutz, M.F.; Belzer, F.O. Effects of hypothermic perfusion of kidneys on tissue and mitochondrial phospholipids. Cryobiology 1984, 21, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Southard, J.H.; Lutz, M.F.; Ametani, M.S.; Belzer, F.O. Stimulation of ATP synthesis in hypothermically perfused dog kidneys by adenosine and PO4. Cryobiology 1984, 21, 13–19. [Google Scholar] [CrossRef]
- Collste, H.; Bergström, J.; Hultman, E.; Melin, B. ATP in the cortex of canine kidneys undergoing hypothermic storage. Life Sci. 1971, 10, 1201–1206. [Google Scholar] [CrossRef]
- Kahng, M.W.; Trifillis, A.L.; Hall-Craggs, M.; Regec, A.; Trump, B.F. Biochemical and morphological studies on human kidneys preserved for transplantation. Am. J. Clin. Pathol. 1983, 80, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Baicu, S.C.; Simmons, P.M.; Campbell, L.H.; Taylor, M.J.; Brockbank, K.G. Interstitial fluid analysis for assessment of organ function. Clin. Transplant. 2004, 18 (Suppl. S12), 16–21. [Google Scholar] [CrossRef]
- Baicu, S.C.; Taylor, M.J.; Brockbank, K.G. Modulating biochemical perturbations during 72-hour machine perfusion of kidneys: Role of preservation solution. Cryobiology 2007, 54, 114–120. [Google Scholar] [CrossRef]
- Nath, J.; Smith, T.; Hollis, A.; Ebbs, S.R.; Canbilen, S.W.; Tennant, D.A.; Ready, A.R.; Ludwig, C. 13 C glucose labelling studies using 2D NMR are a useful tool for determining ex vivo whole organ metabolism during hypothermic machine perfusion of kidneys. Transplant. Res. 2016, 5, 7. [Google Scholar] [CrossRef]
- Nath, J.; Smith, T.B.; Patel, K.; Ebbs, S.R.; Hollis, A.; Tennant, D.A.; Ludwig, C.; Ready, A.R. Metabolic differences between cold stored and machine perfused porcine kidneys: A (1)H NMR based study. Cryobiology 2017, 74, 115–120. [Google Scholar] [CrossRef]
- Hamaoui, K.; Gowers, S.; Damji, S.; Rogers, M.; Leong, C.L.; Hanna, G.; Darzi, A.; Boutelle, M.; Papalois, V. Rapid sampling microdialysis as a novel tool for parenchyma assessment during static cold storage and hypothermic machine perfusion in a translational ex vivo porcine kidney model. J. Surg. Res. 2016, 200, 332–345. [Google Scholar] [CrossRef]
- Huang, J.S.; Downes, G.L.; Belzer, F.O. Utilization of fatty acids in perfused hypothermic dog kidney. J. Lipid Res. 1971, 12, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Smith, T.B.; Neil, D.A.H.; Thakker, A.; Tsuchiya, Y.; Higgs, E.B.; Hodges, N.J.; Ready, A.R.; Nath, J.; Ludwig, C. The Effects of Oxygenation on Ex Vivo Kidneys Undergoing Hypothermic Machine Perfusion. Transplantation 2019, 103, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Buchs, J.B.; Lazeyras, F.; Ruttimann, R.; Nastasi, A.; Morel, P. Oxygenated hypothermic pulsatile perfusion versus cold static storage for kidneys from non heart-beating donors tested by in-line ATP resynthesis to establish a strategy of preservation. Perfusion 2011, 26, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Longchamp, A.; Klauser, A.; Songeon, J.; Agius, T.; Nastasi, A.; Ruttiman, R.; Moll, S.; Meier, R.P.H.; Buhler, L.; Corpataux, J.M.; et al. Ex Vivo Analysis of Kidney Graft Viability Using 31P Magnetic Resonance Imaging Spectroscopy. Transplantation 2020, 104, 1825–1831. [Google Scholar] [CrossRef]
- Martinez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef]
- van de Poll, M.C.G.; Soeters, P.B.; Deutz, N.E.P.; Fearon, K.C.H.; Dejong, C.H.C. Renal metabolism of amino acids: Its role in interorgan amino acid exchange. Am. J. Clin. Nutr. 2004, 79, 185–197. [Google Scholar] [CrossRef]
- Pitts, R.F.; Stone, W.J. Renal metabolism of alanine. J. Clin. Invest. 1967, 46, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Husen, P.; Boffa, C.; Jochmans, I.; Krikke, C.; Davies, L.; Mazilescu, L.; Brat, A.; Knight, S.; Wettstein, D.; Cseprekal, O.; et al. Oxygenated End-Hypothermic Machine Perfusion in Expanded Criteria Donor Kidney Transplant: A Randomized Clinical Trial. JAMA Surg. 2021, 156, 517–525. [Google Scholar] [CrossRef]
- Giraud, S.; Favreau, F.; Chatauret, N.; Thuillier, R.; Maiga, S.; Hauet, T. Contribution of large pig for renal ischemia-reperfusion and transplantation studies: The preclinical model. J. Biomed. Biotechnol. 2011, 2011, 532127. [Google Scholar] [CrossRef]
- Lieberthal, W.; Nigam, S.K. Acute renal failure. II. Experimental models of acute renal failure: Imperfect but indispensable. Am. J. Physiol. Renal Physiol. 2000, 278, F1–F12. [Google Scholar] [CrossRef]
- Karangwa, S.A.; Dutkowski, P.; Fontes, P.; Friend, P.J.; Guarrera, J.V.; Markmann, J.F.; Mergental, H.; Minor, T.; Quintini, C.; Selzner, M.; et al. Machine Perfusion of Donor Livers for Transplantation: A Proposal for Standardized Nomenclature and Reporting Guidelines. Am. J. Transplant 2016, 16, 2932–2942. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.F. Removal of Fatty Acids from Serum Albumin by Charcoal Treatment. J. Biol. Chem. 1967, 242, 173–181. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).