Triticum vulgare Extract and Polyhexanide (Fitostimoline® Hydrogel/Fitostimoline® Plus Gauze) versus Saline Gauze Dressing in Patients with Diabetic Foot Ulcers: Results of a Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
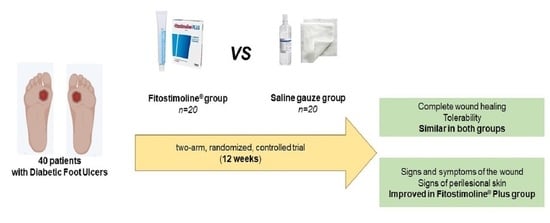
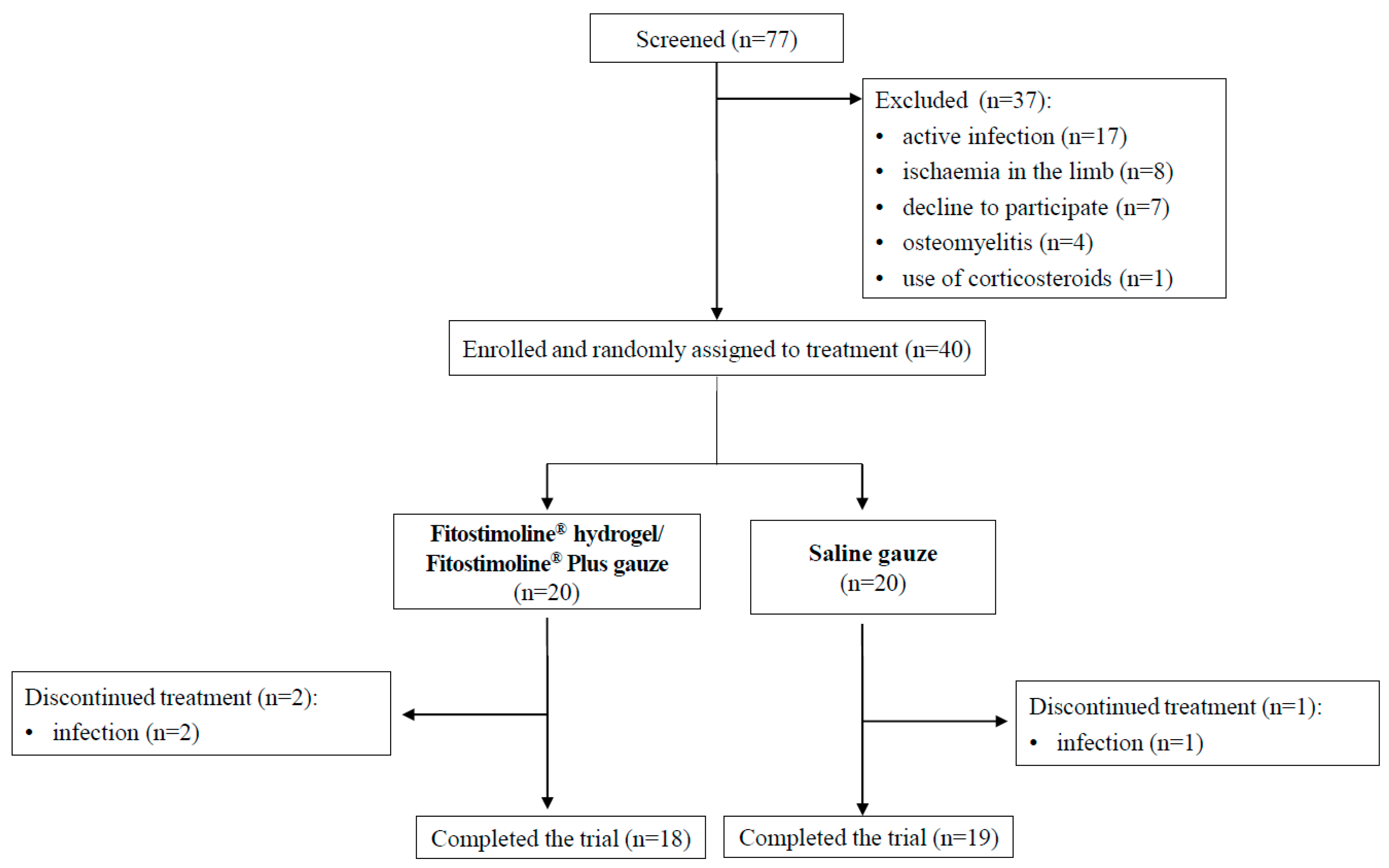
2.1. Study Design and Participants
2.2. Other Outcomes
2.3. Sample Size
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Participants
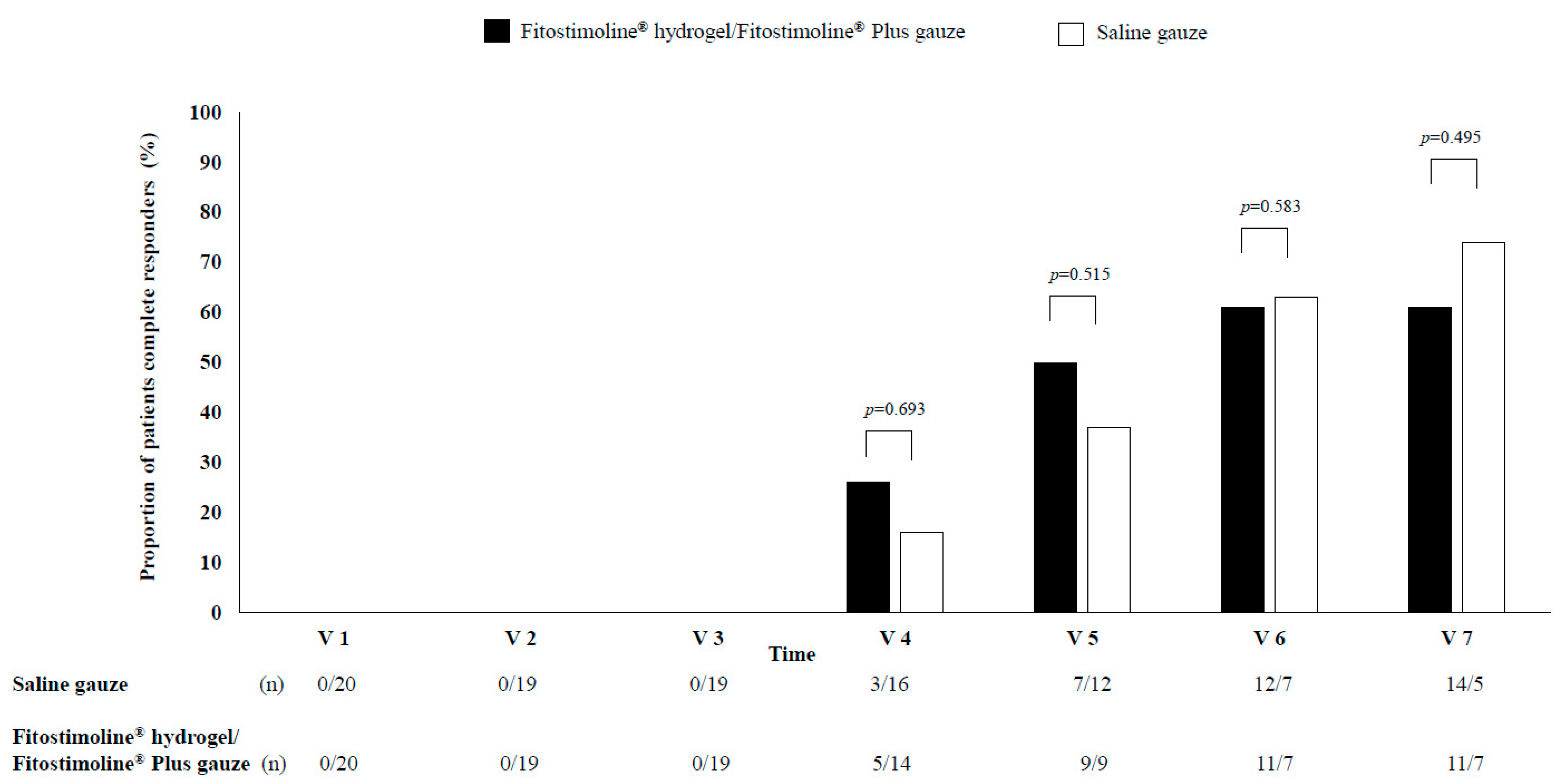
3.2. Effects of the Two Treatments on the Wound Sizes
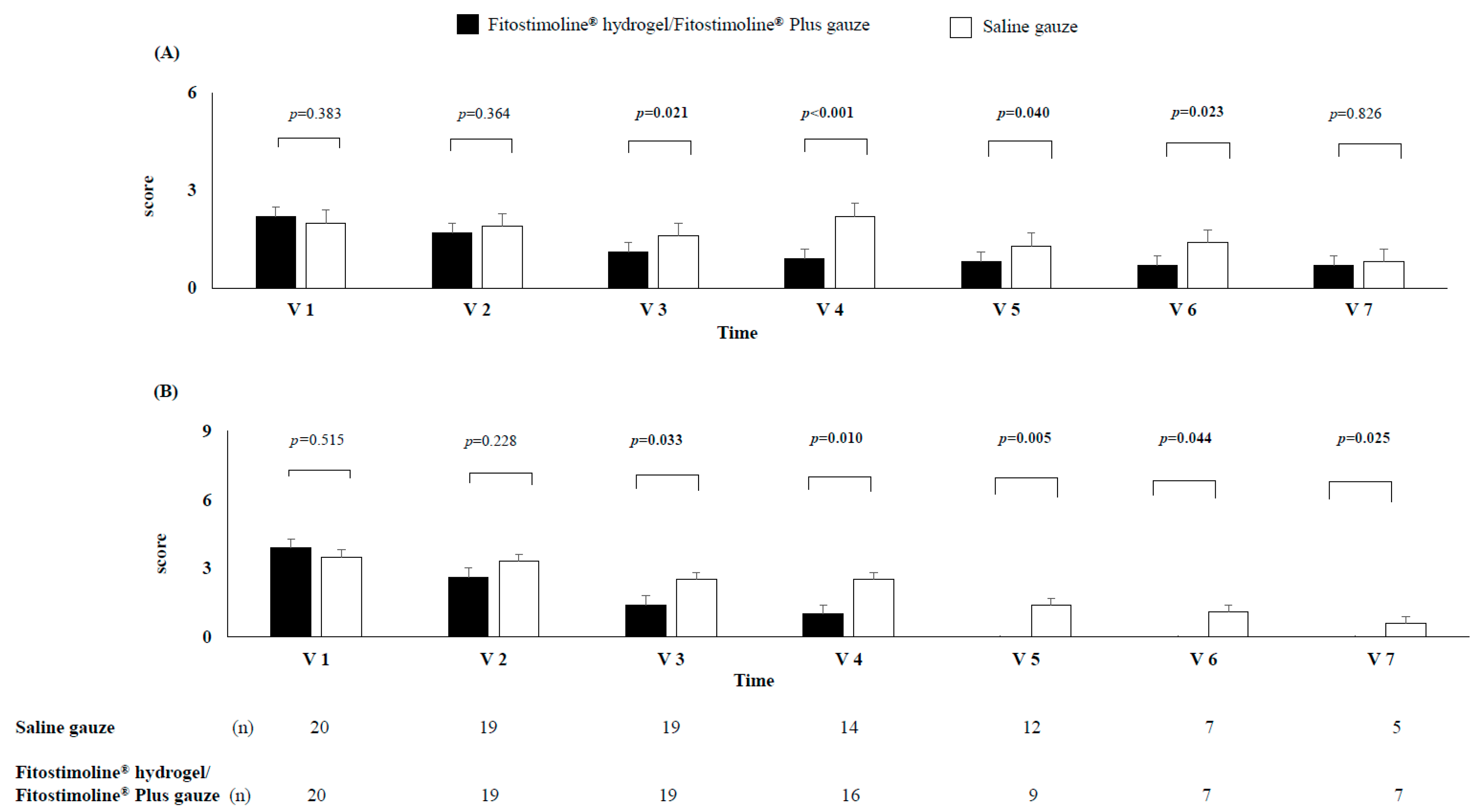
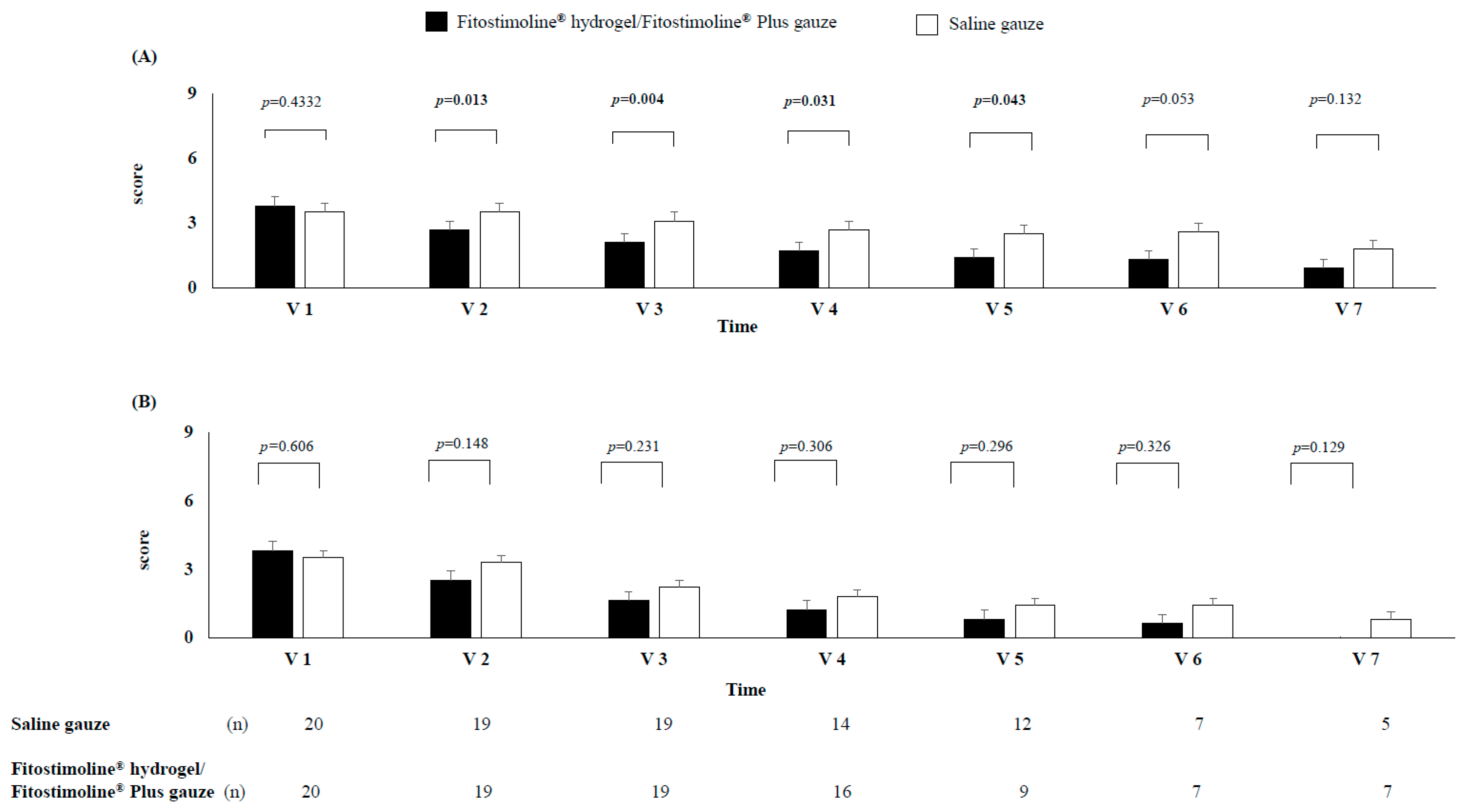
3.3. Effects of the Two Treatments on Signs and Symptoms of the Wound and Perilesional Skin
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Lazzarini, P.A.; Pacella, R.E.; Armstrong, D.G.; van Netten, J.J. Diabetes-related lower-extremity complications are a leading cause of the global burden of disability. Diabetes Med. 2018, 35, 1297–1299. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- McDermott, K.; Fang, M.; Boulton, A.J.M.; Selvin, E.; Hicks, C.V. Etiology, Epidemiology, and Disparities in the Burden of Diabetic Foot Ulcers. Diabetes Care 2023, 46, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Senneville, É.; Abbas, Z.G.; Aragón-Sánchez, J.; Diggle, M.; Embil, J.M.; Kono, S.; Lavery, L.A.; Malone, M.; van Asten, S.A.; et al. Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab. Res. Rev. 2020, 36 (Suppl. S1), e3280. [Google Scholar] [CrossRef] [PubMed]
- Rayman, G.; Vas, P.; Dhatariya, K.; Driver, V.; Hartemann, A.; Londahl, M.; Piaggesi, A.; Apelqvist, J.; Attinger, C.; Game, F.; et al. Guidelines on use of interventions to enhance healing of chronic foot ulcers in diabetes (IWGDF 2019 update). Diabetes Metab. Res. Rev. 2020, 36 (Suppl. S1), e3283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yin, H.; Lei, X.; Lau, J.N.Y.; Yuan, M.; Wang, X.; Zhang, F.; Zhou, F.; Qi, S.; Shu, B.; et al. A Systematic Review and Meta-Analysis of Clinical Effectiveness and Safety of Hydrogel Dressings in the Management of Skin Wounds. Front. Bioeng. Biotechnol. 2019, 7, 342. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chao, N.; Yin, D. A Net Meta-Analysis of the Effectiveness of Different Types of Dressings in the Treatment of Diabetic Foot. Comput. Math. Methods Med. 2022, 18, 4915402. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Wang, C.; Liu, H.; Li, Q.; Li, R.; Zhang, Y.; Liu, Y.; Shao, Y.; Wang, J. Selection of Appropriate Wound Dressing for Various Wounds. Front. Bioeng. Biotechnol. 2020, 8, 182. [Google Scholar] [CrossRef] [PubMed]
- Sanguigno, L.; Casamassa, A.; Funel, N.; Minale, M.; Riccio, R.; Riccio, S.; Boscia, F.; Brancaccio, P.; Pollina, L.E.; Anzilotti, S.; et al. Triticum vulgare extract exerts an anti-inflammatory action in two in vitro models of inflammation in microglial cells. PLoS ONE 2018, 13, e0197493. [Google Scholar] [CrossRef] [PubMed]
- Falciglia, M.D.; Palladino, R.; Maglione, B.; Schiavo, G. In Vitro Antimicrobial Activity Evaluation of a Novel Fitostimoline® Plus Spray Formulation. Int. J. Microbiol. 2021, 2, 1114853. [Google Scholar] [CrossRef] [PubMed]
- Martini, P.; Mazzatenta, C.; Saponati, G. Efficacy and tolerability of fitostimoline in two different forms (soaked gauzes and cream) and citrizan gel in the topical treatment of second-degree superficial cutaneous burns. Dermatol. Res. Pract. 2011, 2011, 978291. [Google Scholar] [CrossRef] [PubMed]
- Firlar, I.; Altunbek, M.; McCarthy, C.; Ramalingam, M.; Camci-Unal, G. Functional Hydrogels for Treatment of Chronic Wounds. Gels 2022, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Lavery, L.A.; Armstrong, D.G.; Harkless, L.B. Classification of diabetic foot wounds. J. Foot. Ankle Surg. 1996, 35, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Products—Damor Farmaceutici (damorpharma.it). Available online: https://www.damorpharma.it/en/products/ (accessed on 30 April 2023).
- Koburger, T.; Hubner, N.O.; Braun, M.; Siebert, J.; Kramer, A. Standardized comparison of antiseptic efficacy of triclosan, PVPiodine, octenidine dihydrochloride, polyhexanide and chlorhexidine digluconate. J. Antimicrob. Chemother. 2010, 65, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- Koehler, J.; Verheyen, L.; Hedtrich, S.; Brandl, F.P.; Goepferich, A.M. Alkaline poly(ethylene glycol)-based hydrogels for a potential use as bioactive wound dressings. J. Biomed. Mater. Res. A 2017, 105, 3360–3368. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Wang, X.; Nune, K.C.; Misra, R. Biodegradable hydrogel-based biomaterials with high absorbent properties for non-adherent wound dressing. Int. Wound J. 2017, 14, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound Dressings and Comparative Effectiveness Data. Adv. Wound Care 2014, 3, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.K.; Saliba, L.; Smith, M.J.; McTavish, J.; Raine, C.; Curtin, P. Normal saline wound dressing—Is it really normal? Br. J. Plast. Surg. 2000, 53, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Tito, A.; Minale, M.; Riccio, S.; Grieco, F.; Colucci, M.G.; Apone, F. A Triticum vulgare Extract Exhibits Regenerating Activity During the Wound Healing Process. Clin. Cosmet. Investig. Dermatol. 2020, 13, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; McAnulty, J.F.; Schurr, M.J.; Murphy, C.J.; Abbott, N.L. Polymeric materials for chronic wound and burn dressings. In Advanced Wound Repair Therapies; Farrar, D., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 186–208. [Google Scholar]
| Fitostimoline® Hydrogel/ Fitostimoline® Plus Gauze (n = 20) | Saline Gauze (n = 20) | |
|---|---|---|
| Sex (female/male) | 4/16 | 7/13 |
| Age (years) | 65 ± 10 | 63 ± 9 |
| BMI (kg/m2) | 31 ± 5 | 30 ± 5 |
| HbA1c (%) | 7.7 ± 1.3 | 7.4 ± 0.7 |
| Total Cholesterol (mg/dL) | 157 ± 37 | 143 ± 29 |
| HDL Cholesterol (mg/dL) | 43 ± 11 | 45 ± 9 |
| Triglycerides (mg/dL) | 131 ± 61 | 124 ± 53 |
| LDL Cholesterol (mg/dL) | 89 ± 37 | 74 ± 34 |
| Wound duration (months) | ||
| 6 ± 6 | 7 ± 6 | |
| Wound etiology | ||
| Neuropathic | 8 | 9 |
| Ischemic | 7 | 6 |
| Neuroischemic | 5 | 5 |
| Wound size | ||
| Wound largest diameter (cm) | 1.7 ± 0.9 | 1.6 ± 1 |
| Wound smallest diameter (cm) | 1.1 ± 0.5 | 1.1 ± 0.8 |
| Wound depth (cm) | 0.4 ± 0.2 | 0.4 ± 0.2 |
| Wound area (cm2) | 2.1 ± 1.8 | 2.3 ± 2.7 |
| Fitostimoline® Hydrogel/ Fitostimoline® Plus Gauze (n = 7) | Saline Gauze (n = 5) | p † | |||||
|---|---|---|---|---|---|---|---|
| Wound Size | Baseline | 12 Weeks | Δ | Baseline | 12 Weeks | Δ | |
| Wound largest diameter (cm) | 2.2 ± 1 | 0.8 ± 0.6 * | −1.4 ± 0.6 | 2.8 ± 1.1 | 1.4 ± 0.9 * | −1.5 ± 0.5 | 0.831 |
| Wound smallest diameter (cm) | 1.4 ± 0.6 | 0.4 ± 0.2 * | −1.0 ± 0.5 | 1.9 ± 0.8 | 0.8 ± 0.9 * | −1.0 ± 0.5 | 0.992 |
| Wound depth (cm) | 0.5 ± 0.2 | 0.3 ± 0.2 * | −0.3 ± 0.2 | 0.7 ± 0.3 | 0.5 ± 0.4 * | −0.3 ± 0.2 | 0.697 |
| Wound area (cm2) | 3.2 ± 1.9 | 0.3 ± 0.2 * | −2.7 ± 1.7 | 5.5 ± 3.0 | 1.6 ± 2.0 * | −3.9 ± 1.9 | 0.300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Della Pepa, G.; Lombardi, G.; Gianfrancesco, S.; Piccolo, R.; Chirico, G.; Pellegrino, M.; Santella, L.; Tecce, N.; Volpicelli, A.; Sollo, E.; et al. Triticum vulgare Extract and Polyhexanide (Fitostimoline® Hydrogel/Fitostimoline® Plus Gauze) versus Saline Gauze Dressing in Patients with Diabetic Foot Ulcers: Results of a Randomized Controlled Trial. J. Clin. Med. 2023, 12, 3596. https://doi.org/10.3390/jcm12103596
Della Pepa G, Lombardi G, Gianfrancesco S, Piccolo R, Chirico G, Pellegrino M, Santella L, Tecce N, Volpicelli A, Sollo E, et al. Triticum vulgare Extract and Polyhexanide (Fitostimoline® Hydrogel/Fitostimoline® Plus Gauze) versus Saline Gauze Dressing in Patients with Diabetic Foot Ulcers: Results of a Randomized Controlled Trial. Journal of Clinical Medicine. 2023; 12(10):3596. https://doi.org/10.3390/jcm12103596
Chicago/Turabian StyleDella Pepa, Giuseppe, Gianluca Lombardi, Salvatore Gianfrancesco, Roberto Piccolo, Giovanni Chirico, Micaela Pellegrino, Luigi Santella, Nicola Tecce, Anastasia Volpicelli, Elena Sollo, and et al. 2023. "Triticum vulgare Extract and Polyhexanide (Fitostimoline® Hydrogel/Fitostimoline® Plus Gauze) versus Saline Gauze Dressing in Patients with Diabetic Foot Ulcers: Results of a Randomized Controlled Trial" Journal of Clinical Medicine 12, no. 10: 3596. https://doi.org/10.3390/jcm12103596
APA StyleDella Pepa, G., Lombardi, G., Gianfrancesco, S., Piccolo, R., Chirico, G., Pellegrino, M., Santella, L., Tecce, N., Volpicelli, A., Sollo, E., Bozzetto, L., Masulli, M., Riccardi, G., Rivellese, A. A., & Saldalamacchia, G. (2023). Triticum vulgare Extract and Polyhexanide (Fitostimoline® Hydrogel/Fitostimoline® Plus Gauze) versus Saline Gauze Dressing in Patients with Diabetic Foot Ulcers: Results of a Randomized Controlled Trial. Journal of Clinical Medicine, 12(10), 3596. https://doi.org/10.3390/jcm12103596