Serum Urea-to-Albumin Ratio Is an Independent Predictor of Intra-Hospital Mortality in Neurosurgical Intensive Care Unit Patients with Spontaneous Intracerebral Hemorrhage
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Treatment Regime and Intensive Care Unit Treatment
2.4. Serum Biomarkers
2.5. Radiological Data
2.6. Intra-Hospital Mortality
2.7. Statistical Analysis
3. Results
3.1. Main Characteristics
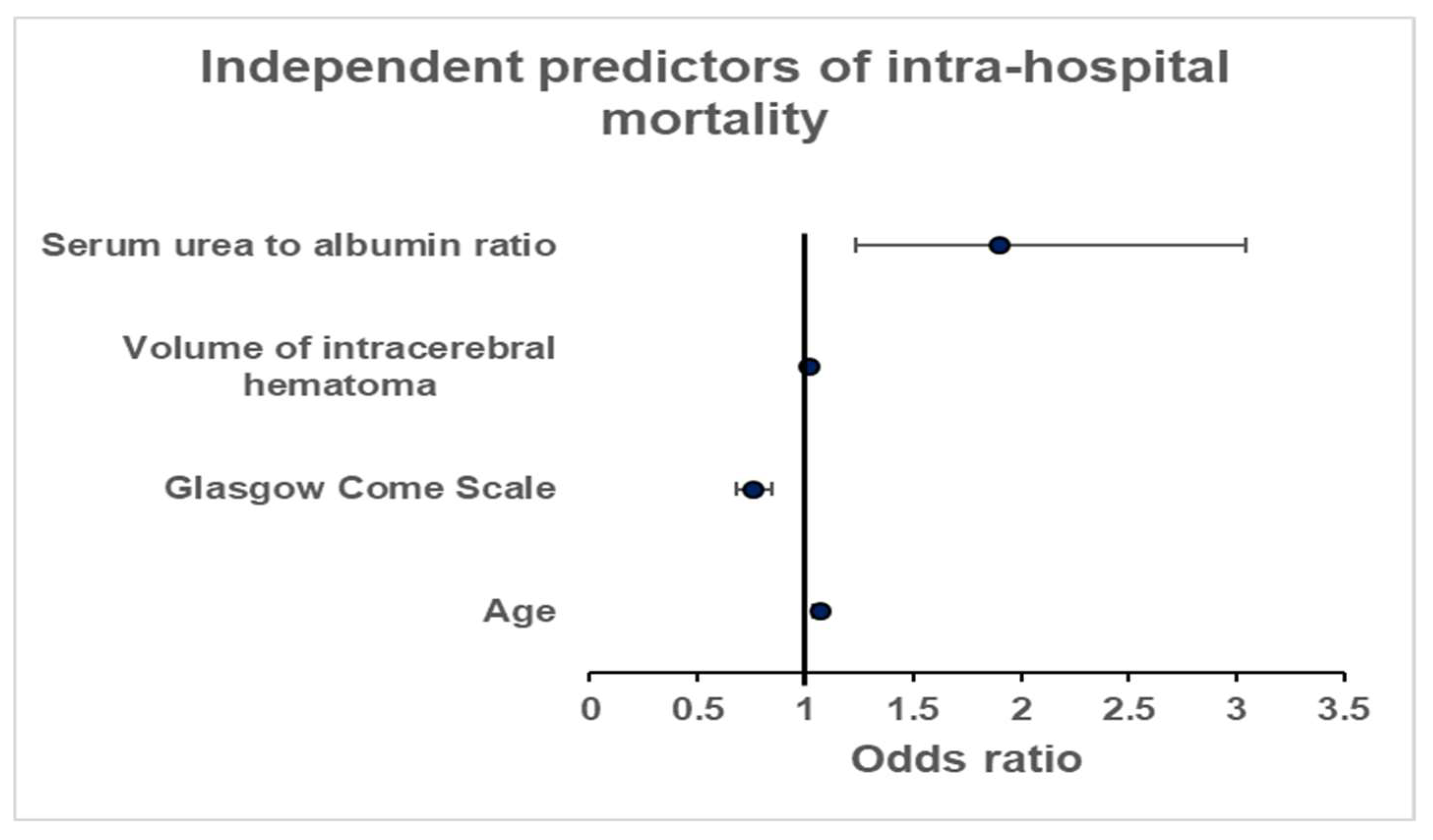
3.2. Intra-Hospital Mortality
4. Discussion
4.1. Summary of Findings
4.2. Intra-Hospital Mortality
4.3. Serum Urea-to-Albumin Ratio
4.4. Limitations and Strengths of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hays, A.; Diringer, M.N. Elevated troponin levels are associated with higher mortality following intracerebral hemorrhage. Neurology 2006, 66, 1330–1334. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.; Luecke, M.; Preuss, M.; Boeker, D.K.; Joedicke, A.; Oertel, M.F. Spontaneous intracerebral hemorrhage with ventricular extension and the grading of obstructive hydrocephalus: The prediction of outcome of a special life-threatening entity. Neurosurgery 2010, 67, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Hjalmarsson, C.; Bergfeldt, L.; Bokemark, L.; Manhem, K.; Andersson, B. Electrocardiographic abnormalities and elevated cTNT at admission for intracerebral hemorrhage: Predictors for survival? Ann. Noninvasive Electrocardiol. 2013, 18, 441–449. [Google Scholar] [CrossRef]
- Ahn, C.S.; Lee, S.K.; Kim, H.S.; Kong, M.H.; Song, K.Y.; Kang, D.S. Surgical outcome of spontaneous intracerebral hemorrhage in less than stuporous mental status. J. Korean Neurosurg. Soc. 2004, 35, 290–296. [Google Scholar]
- Martí-Fàbregas, J.; Belvís, R.; Guardia, E.; Cocho, D.; Muñoz, J.; Marruecos, L.; Martí-Vilalta, J.L. Prognostic value of Pulsatility index in acute intra-cerebral hemorrhage. Neurology 2003, 61, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Garrett, M.C.; Komotar, R.J.; Starke, R.M.; Doshi, D.; Otten, M.L.; Connolly, E.S. Elevated troponin levels are predictive of mortality in surgical intracerebral hemorrhage patients. Neurocrit. Care 2010, 12, 199–203. [Google Scholar] [CrossRef]
- Caplan, L.R. Intracerebral haemorrhage. Lancet 1992, 339, 656–658. [Google Scholar] [CrossRef]
- Tuhrim, S.; Horowitz, D.R.; Sacher, M.; Godbold, J.H. Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Crit. Care Med. 1999, 27, 617–621. [Google Scholar] [CrossRef]
- Juvela, S. Risk factors for impaired outcome after spontaneous intracerebral hemorrhage. Arch. Neurol. 1995, 52, 1193–1200. [Google Scholar] [CrossRef]
- Davis, S.M.; Broderick, J.; Hennerici, M.; Brun, N.C.; Diringer, M.N.; Mayer, S.A.; Begtrup, K.; Steiner, T.; Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006, 66, 1175–1181. [Google Scholar] [CrossRef]
- Bender, M.; Haferkorn, K.; Friedrich, M.; Uhl, E.; Stein, M. Impact of Early C-Reactive Protein/Albumin Ratio on Intra-Hospital Mortality among Patients with Spontaneous Intracerebral Hemorrhage. J. Clin. Med. 2020, 9, 1236. [Google Scholar] [CrossRef]
- Foerch, C.; Curdt, I.; Yan, B.; Dvorak, F.; Hermans, M.; Berkefeld, J.; Raabe, A.; Neumann-Haefelin, T.; Steinmetz, H.; Sitzer, M. Serum glial fibrillary acidic protein as a biomarker for intracerebral haemorrhage in patients with acute stroke. J. Neurol. Neurosurg. Psychiatry 2006, 77, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Gerner, S.T.; Auerbeck, K.; Sprügel, M.I.; Sembill, J.A.; Madžar, D.; Gölitz, P.; Hoelter, P.; Kuramatsu, J.B.; Schwab, S.; Huttner, H.B. Peak Troponin I Levels Are Associated with Functional Outcome in Intracerebral Hemorrhage. Cerebrovasc. Dis. 2018, 46, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Bender, M.; Haferkorn, K.; Tajmiri-Gondai, S.; Uhl, E.; Stein, M. Fibrinogen to Albumin Ratio as Early Serum Biomarker for Prediction of Intra-Hospital Mortality in Neurosurgical Intensive Care Unit Patients with Spontaneous Intracerebral Hemorrhage. J. Clin. Med. 2022, 11, 4214. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ren, W.; Zu, H.; Dong, Q. Evaluate the serum cortisol in patients with intracerebral hemorrhage. Clin. Neurol. Neurosurg. 2014, 123, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, S.; Costa, F.; Seabra, M.; Dias, R.; Soares, A.; Dias, C.; Azevedo, E.; Castro, P. Systemic inflammation status at admission affects the outcome of intracerebral hemorrhage by increasing perihematomal edema but not the hematoma growth. Acta Neurol. Belg. 2021, 121, 649–659. [Google Scholar] [CrossRef]
- Bender, M.; Haferkorn, K.; Nagl, J.; Uhl, E.; Stein, M. Serum Lactate as Serum Biomarker for Cardiopulmonary Parameters within the First 24 Hours after a Spontaneous Intracerebral Hemorrhage. Diagnostics 2022, 12, 2414. [Google Scholar] [CrossRef]
- Diedler, J.; Sykora, M.; Hahn, P.; Rupp, A.; Rocco, A.; Herweh, C.; Steiner, T. C-reactive-protein levels associated with infection predict short- and long-term outcome after supratentorial intracerebral hemorrhage. Cerebrovasc. Dis. 2009, 27, 272–279. [Google Scholar] [CrossRef]
- Agnihotri, S.; Czap, A.; Staff, I.; Fortunato, G.; McCullough, L.D. Peripheral leukocyte counts and outcomes after intracerebral hemorrhage. J. Neuroinflamm. 2011, 8, 160. [Google Scholar] [CrossRef]
- Tibaut, M.; Caprnda, M.; Kubatka, P.; Sinkovič, A.; Valentova, V.; Filipova, S.; Gazdikova, K.; Gaspar, L.; Mozos, I.; Egom, E.E.; et al. Markers of Atherosclerosis: Part 2-Genetic and Imaging Markers. Heart Lung Circ. 2019, 28, 678–689. [Google Scholar] [CrossRef]
- Pereira, A.G.; Costa, N.A.; Gut, A.L.; Azevedo, P.S.; Tanni, S.E.; Mamede Zornoff, L.A.; Rupp de Paiva, S.A.; Polegato, B.F.; Minicucci, M.F. Urea to albumin ratio is a predictor of mortality in patients with septic shock. Clin. Nutr. ESPEN 2021, 42, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Gundpatil, D.B.; Somani, B.L.; Saha, T.K.; Banerjee, M. Serum urea: Albumin ratio as a prognostic marker in critical patients with non-chronic kidney disease. Indian J. Clin. Biochem. 2014, 29, 97–100. [Google Scholar] [CrossRef]
- Ugajin, M.; Yamaki, K.; Iwamura, N.; Yagi, T.; Asano, T. Blood urea nitrogen to serum albumin ratio independently predicts mortality and severity of community-acquired pneumonia. Int. J. Gen. Med. 2012, 5, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Beier, K.; Eppanapally, S.; Bazick, H.S.; Chang, D.; Mahadevappa, K.; Gibbons, F.K.; Christopher, K.B. Elevation of blood urea nitrogen is predictive of long-term mortality in critically ill patients independent of “normal” creatinine. Crit. Care Med. 2011, 39, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Faisst, M.; Wellner, U.F.; Utzolino, S.; Hopt, U.T.; Keck, T. Elevated blood urea nitrogen is an independent risk factor of prolonged intensive care unit stay due to acute necrotizing pancreatitis. J. Crit. Care 2010, 25, 105–111. [Google Scholar] [CrossRef]
- Artero, A.; Zaragoza, R.; Camarena, J.J.; Sancho, S.; González, R.; Nogueira, J.M. Prognostic factors of mortality in patients with community-acquired bloodstream infection with severe sepsis and septic shock. J. Crit. Care 2010, 25, 276–281. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J.; Balk, E.; Kausz, A.T.; Levin, A.; Steffes, M.W.; Hogg, R.J.; Perrone, R.D.; Lau, J.; Eknoyan, G.; et al. National Kidney Foundation practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Ann. Intern. Med. 2003, 139, 137–147. [Google Scholar] [CrossRef]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef]
- Evans, W.A., Jr. An encephalographic ratio for estimating ventricular enlargement and cerebral atrophy. Arch. Neurol. Psychiatry 1942, 47, 931–937. [Google Scholar] [CrossRef]
- Graeb, D.A.; Robertson, W.D.; Lapointe, J.S.; Nugent, R.A.; Harrison, P.B. Computed tomographic diagnosis of intraventricular hemorrhage. Etiology and prognosis. Radiology 1982, 143, 91–96. [Google Scholar] [CrossRef]
- van Swieten, J.C.; Koudstaal, P.J.; Visser, M.C.; Schouten, H.J.; van Gijn, J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988, 19, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, M.; Leira, R.; Tejada, J.; Gil-Peralta, A.; Dávalos, A.; Castillo, J. Stroke Project, Cerebrovascular Diseases Group of the Spanish Neurological Society. Predictors of good outcome in medium to large spontaneous supratentorial intracerebral haemorrhages. J. Neurol. Neurosurg. Psychiatry 2005, 76, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, J.C., 3rd; Greenberg, S.M.; Anderson, C.S.; Becker, K.; Bendok, B.R.; Cushman, M.; Fung, G.L.; Goldstein, J.N.; Macdonald, R.L.; Mitchell, P.H.; et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2015, 46, 2032–2060. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; Zhang, X.C.; Zheng, X.; Rui, M.; Yao, S.C.; Song, Z.J.; Zhao, F.C.; Guo, K.J. Effect of admission blood urea and creatinine levels on mortality in elderly patients with hip fracture. Zhongguo Gu Shang 2017, 30, 901–905. (In Chinese) [Google Scholar] [CrossRef]
- Winter, M.C.; Potter, V.A.; Woll, P.J. Raised serum urea predicts for early death in small cell lung cancer. Clin. Oncol. (R. Coll. Radiol.) 2008, 20, 745–750. [Google Scholar] [CrossRef]
- Zhang, Y.; Churilov, L.; Meretoja, A.; Teo, S.; Davis, S.M.; Yan, B. Elevated urea level is associated with poor clinical outcome and increased mortality post intravenous tissue plasminogen activator in stroke patients. J. Neurol. Sci. 2013, 332, 110–115. [Google Scholar] [CrossRef]
- Idris, I.; Hill, R.; Sharma, J.C. Effects of admission serum urea, glomerular filtration rate, proteinuria and diabetes status on 3-month mortality after acute stroke. Diabetes Vasc. Dis. Res. 2010, 7, 239–240. [Google Scholar] [CrossRef]
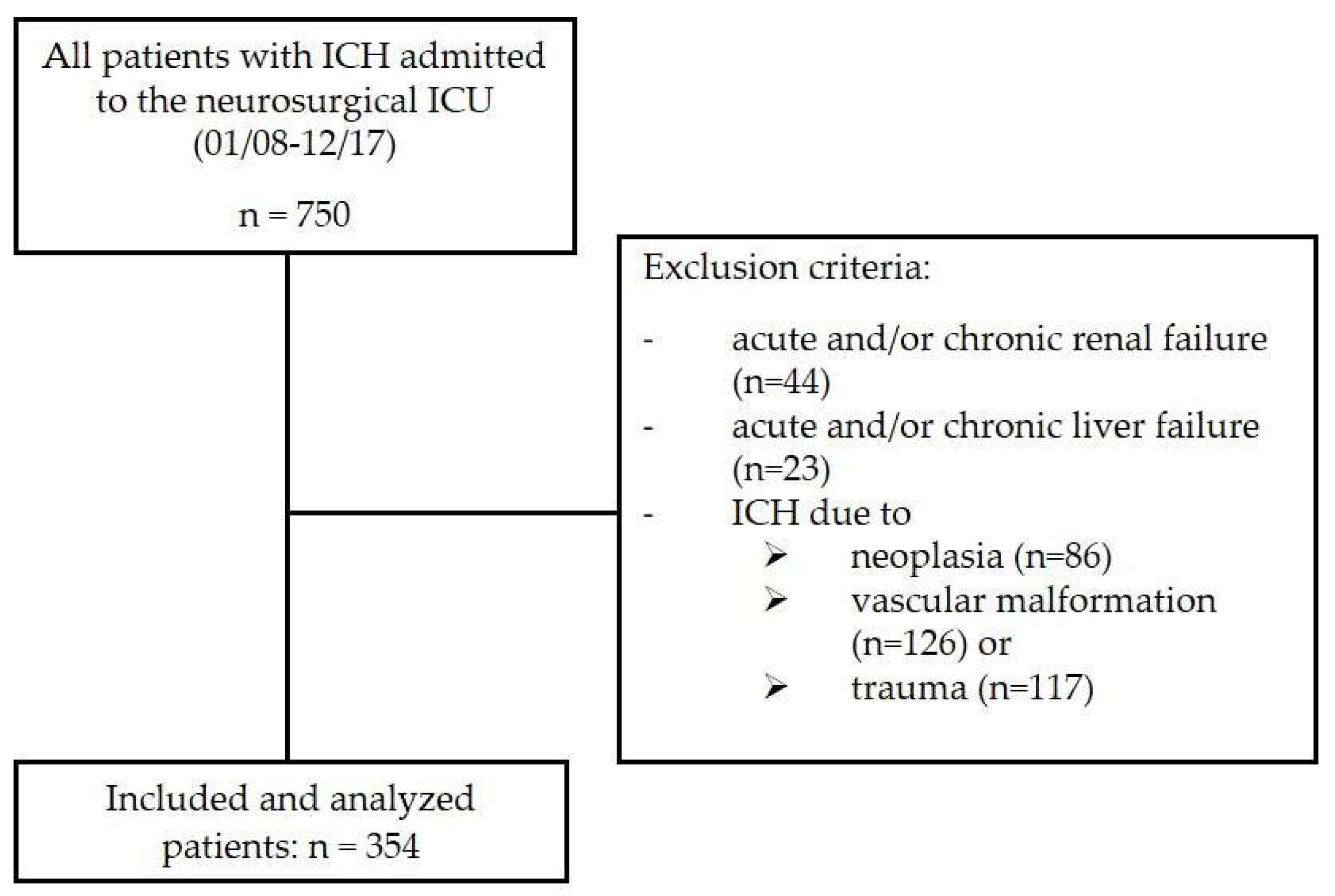
| Parameter | Overall (n = 354) | Survivor (n = 243) | Non-Survivor (n = 111) | p-Value |
|---|---|---|---|---|
| Baseline data | ||||
| Age, years, mean (±SD) * | 68.6 (13.1) | 66.8 (13.3) | 72.8 (11.8) | <0.0001 |
| Women, n (%) * | 161 (45.5) | 119 (49) | 42 (37.8) | 0.05 |
| Men, n (%) * | 193 (54.5) | 124 (51) | 69 (62.2) | |
| Body-Mass-Index, kg/m2, median (IQR) * | 26.1 (24.2–29.3) | 26.6 (24.2–29.4) | 25.7 (23.7–27.8) | 0.16 |
| Glasgow Coma Scale score, median (IQR) * | 8 (3–12) | 10 (6–13) | 4 (3–7) | <0.0001 |
| Intubated patients, n (%) ** | 211 (59.6) | 129 (53.1) | 82 (73.9) | 0.0002 |
| Body temperature, centigrade, median (IQR) * | 36.3 (35.5–36.9) | 36.4 (35.8–37) | 35.9 (35.1–36.6) | <0.0001 |
| Hospital stay, median (IQR) **** | 16.5 (4.8–27) | 22 (13–33) | 3 (1–8) | 0.03 |
| Comorbidities | ||||
| Chronic arterial hypertension, n (%) * | 208 (58.8) | 153 (63) | 55 (49.5) | 0.02 |
| Chronic obstructive pulmonary diseases, n (%) * | 15 (4.2) | 10 (4.1) | 5 (4.5) | 0.87 |
| Cardiac arrhythmia, n (%) * | 70 (19.8) | 45 (18.5) | 25 (22.5) | 0.38 |
| Coronary artery disease, n (%) * | 44 (12.4) | 26 (10.7) | 18 (16.2) | 0.14 |
| Heart failure, n (%) * | 20 (5.6) | 9 (3.7) | 11 (9.9) | 0.02 |
| Diabetes mellitus, n (%) * | 56 (15.8) | 40 (16.5) | 16 (14.4) | 0.62 |
| History of ischemic stroke, n (%) * | 50 (14.1) | 38 (15.6) | 12 (10.8) | 0.23 |
| History of ICH, n (%) * | 17 (4.8) | 9 (3.7) | 8 (7.2) | 0.15 |
| History of cancer without cerebral manifestation, n (%) * | 29 (8.2) | 18 (7.4) | 11 (9.9) | 0.43 |
| Premedication | ||||
| Antihypertensive drugs, n (%) * | 164 (46.3) | 127 (52.3) | 37 (33.3) | 0.0009 |
| Antiobstructive drugs, n (%) * | 5 (1.4) | 2 (0.8) | 3 (2.7) | 0.16 |
| Antidiabetic drugs, n (%) * | 36 (10.2) | 23 (9.5) | 13 (11.7) | 0.52 |
| Antiplatelet agents, n (%) * | 50 (14.1) | 34 (14) | 16 (14.4) | 0.92 |
| New oral anticoagulants, n (%) * | 13 (3.7) | 6 (2.5) | 7 (6.3) | 0.08 |
| Vitamin K antagonist, n (%) * | 73 (20.6) | 50 (20.6) | 23 (20.7) | 0.98 |
| Biomarkers | ||||
| White blood cells, giga/L, mean (± SD) * | 10.9 (4.4) | 10.6 (4) | 11.7 (5.3) | 0.03 |
| Hemoglobin, g/dL, mean (±SD) * | 13.1 (2) | 13.2 (1.9) | 12.9 (2.1) | 0.33 |
| Hematocrit, %, mean (±SD) * | 38.5 (5.5) | 38.6 (5.2) | 38.2 (5.9) | 0.5 |
| Cholinesterase, U/L, mean (±SD) * | 7809.4 (2282.3) | 8115.9 (2226.2) | 7138.5 (2269.5) | <0.0001 |
| Blood glucose, mg/dL, mean (±SD) * | 163.8 (59.3) | 158.1 (55.6) | 176.2 (65.2) | 0.007 |
| Serum lactate, mmol/L, mean (±SD) * | 1.7 (1.5) | 1.6 (1.3) | 2 (1.7) | 0.01 |
| Cortisol, µg/dL, mean (±SD) * | 27.7 (19.4) | 27.2 (19.4) | 29.2 (19.3) | 0.42 |
| C-reactive protein, mg/L, mean (±SD) * | 22 (39.3) | 30.6 (46.2) | 37.1 (17.3) | 0.005 |
| Creatinine, mg/dL, mean (±SD) * | 0.9 (0.6) | 0.9 (0.5) | 1 (0.8) | 0.61 |
| Serum urea, g/L, mean (±SD) * | 0.41 (0.22) | 0.37 (0.17) | 0.50 (0.27) | <0.0001 |
| Albumin, g/L, mean (±SD) * | 38.2 (5.4) | 39 (4.8) | 36.5 (6.3) | <0.0001 |
| Serum urea-to-albumin ratio, mean (±SD) * | 0.01 (0.007) | 0.01 (0.005) | 0.014 (0.009) | <0.0001 |
| Prothrombin time, %, mean (±SD) * | 83.7 (26.2) | 86.1 (25) | 78.4 (28.3) | 0.01 |
| Partial thromboplastin time, seconds, mean (±SD) * | 32.5 (11.4) | 31.3 (8.3) | 35.3 (16.1) | 0.002 |
| Antithrombin III, %/NORM, mean (±SD) * | 88.7 (16) | 89.9 (14.4) | 86.6 (18.3) | 0.16 |
| Treatment | ||||
| Medical treatment, n (%) *** | 151 (42.7) | 98 (40.3) | 53 (47.7) | 0.19 |
| Surgical Treatment, n (%) *** | 203 (57.3) | 145 (59.7) | 58 (52.3) | |
| Insertion EVD, n (%) *** | 77 (37.9) | 53 (36.6) | 24 (41.4) | 0.52 |
| Evacuation ICH, n (%) *** | 62 (30.5) | 46 (31.7) | 16 (27.6) | 0.56 |
| Decompressive craniectomy, n (%) *** | 18 (8.9) | 12 (8.3) | 6 (10.3) | 0.64 |
| Decompressive craniectomy and Evacuation ICH, n (%) *** | 46 (22.7) | 34 (23.4) | 12 (20.7) | 0.67 |
| Radiological data | ||||
| Localization | ||||
| Supratentorial, lobar, n (%) * | 122 (34.5) | 78 (32.1) | 44 (39.6) | 0.17 |
| Supratentorial, deep, n (%) * | 180 (50.8) | 122 (50.2) | 58 (52.3) | 0.72 |
| Infratentorial, n (%) * | 52 (14.7) | 43 (17.7) | 9 (8.1) | 0.02 |
| ICH volume, cm3, mean (±SD) * | 52.3 (42.2) | 42.9 (34.9) | 72.8 (49.1) | <0.0001 |
| IVH, n (%) * | 248 (70.1) | 156 (64.2) | 92 (82.9) | <0.0001 |
| Hydrocephalus, n (%) * | 158 (44.6) | 94 (38.7) | 64 (57.7) | 0.003 |
| mRS score, median (IQR) **** | 5 (4–6) | 4 (4–5) | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bender, M.; Haferkorn, K.; Tajmiri-Gondai, S.; Stein, M.; Uhl, E. Serum Urea-to-Albumin Ratio Is an Independent Predictor of Intra-Hospital Mortality in Neurosurgical Intensive Care Unit Patients with Spontaneous Intracerebral Hemorrhage. J. Clin. Med. 2023, 12, 3538. https://doi.org/10.3390/jcm12103538
Bender M, Haferkorn K, Tajmiri-Gondai S, Stein M, Uhl E. Serum Urea-to-Albumin Ratio Is an Independent Predictor of Intra-Hospital Mortality in Neurosurgical Intensive Care Unit Patients with Spontaneous Intracerebral Hemorrhage. Journal of Clinical Medicine. 2023; 12(10):3538. https://doi.org/10.3390/jcm12103538
Chicago/Turabian StyleBender, Michael, Kristin Haferkorn, Shahin Tajmiri-Gondai, Marco Stein, and Eberhard Uhl. 2023. "Serum Urea-to-Albumin Ratio Is an Independent Predictor of Intra-Hospital Mortality in Neurosurgical Intensive Care Unit Patients with Spontaneous Intracerebral Hemorrhage" Journal of Clinical Medicine 12, no. 10: 3538. https://doi.org/10.3390/jcm12103538
APA StyleBender, M., Haferkorn, K., Tajmiri-Gondai, S., Stein, M., & Uhl, E. (2023). Serum Urea-to-Albumin Ratio Is an Independent Predictor of Intra-Hospital Mortality in Neurosurgical Intensive Care Unit Patients with Spontaneous Intracerebral Hemorrhage. Journal of Clinical Medicine, 12(10), 3538. https://doi.org/10.3390/jcm12103538









