Primary Tumour Treatment in Stage 4 Colorectal Cancer with Unresectable Liver and Lung Metastases and No Peritoneal Carcinomatosis—Current Trends and Attitudes in the Absence of Clear Guidelines
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- International Agency on Research on Cancer. Colorectal Cancer. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/10_8_9-Colorectum-fact-sheet.pdf (accessed on 28 February 2023).
- Ciardiello, F.; Ciardiello, D.; Martini, G.; Napolitano, S.; Tabernero, J.; Cervantes, A. Clinical management of metastatic colorectal cancer in the era of precsion medicine. CA Cancer J. Clin. 2022, 72, 372–401. [Google Scholar] [CrossRef] [PubMed]
- American Joint Committee on Cancer. AJCC Cancer Staging Manual, 8th Edition, Chicago, IL. 5 June 2018. Available online: www.cancerstaging.org (accessed on 28 February 2023).
- Park, E.J.; Baek, J.H.; Choi, G.S.; Park, W.C.; Yu, C.S.; Kang, S.B.; Min, B.S.; Kim, J.H.; Kim, H.R.; Lee, B.H.; et al. The role of primary tumour resection in colorectal cancer patients with asymptomatic, synchronous, unresectable metastasis: A multicenter randomized controlled trial. Cancers 2020, 12, 2306. [Google Scholar] [CrossRef] [PubMed]
- Feo, L.; Polcino, M.; Nash, G.M. Resection of the primary tumour in Stage IV colorectal cancer: When is it necessary? Surg. Clin. N. Am. 2017, 97, 657–669. [Google Scholar] [CrossRef] [PubMed]
- ICMJE. Defining the Role of Authors and Contributors. Available online: https://www.icmje.org/recommendations/browse/roles-and-responsibilities/defining-the-role-of-authors-and-contributors.html (accessed on 28 February 2023).
- National Institute for Health and Care Excellence. Colorectal Cancer. NICE Guideline 151. 29 January 2020. Available online: https://www.nice.org.uk/guidance/ng151/resources/colorectal-cancer-pdf-66141835244485 (accessed on 23 February 2023).
- Gollins, S.; Moran, B.; Adams, R.; Cunningham, C.; Bach, S.; Sun Myint, A.; Renehan, A.; Karandikar, S.; Goh, V.; Prezzi, D.; et al. Association of Coloproctology of Great Britain & Ireland (ACPGBI): Guidelines for the management of cancer of the Colon, Rectum and Anus (2017)—Multidisciplinary management. Color. Dis. 2017, 19 (Suppl. S1), 37–66. [Google Scholar]
- Cervantes, A.; Adam, R.; Rosello, S.; Arnold, D.; Normanno, N.; Taieb, A.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef] [PubMed]
- German Guideline Program in Oncology. Evidence-Based Guideline for Colorectal Cancer. Version 2.1. January 2019. Available online: https://www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downloads/Leitlinien/Kolorektales_Karzinom/Version_2/GGPO_Guideline_Colorectal_Cancer_2.1.pdf (accessed on 24 February 2023).
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines on Oncology. Colon Cancer. Version 3.2022. 2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (accessed on 24 February 2023).
- Vogel, J.D.; Felder, S.I.; Bhama, A.R.; Hawkins, A.T.; Langenfeld, S.J.; Shaffer, V.O.; Thorsen, A.J.; Weiser, M.R.; Chang, G.J.; Lightner, A.L.; et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Colon Cancer. Dis. Colon. Rectum 2022, 65, 148–177. [Google Scholar] [CrossRef] [PubMed]
- Associazione Italiana di Oncologia Medica. Linee Guida Tumori del Colon. Edizione 2020. October 2020. Available online: https://www.aiom.it/wp-content/uploads/2020/10/2020_LG_AIOM_Colon.pdf (accessed on 28 February 2023).
- Kanemitsu, Y.; Shitara, K.; Mizusawa, J.; Hamaguchi, T.; Shida, D.; Komori, K.; Ikeda, S.; Ojima, H.; Ike, H.; Shiomi, A.; et al. Primary Tumor Resection Plus Chemotherapy Versus Chemotherapy Alone for Colorectal Cancer Patients with Asymptomatic, Synchronous Unresectable Metastases (JCOG1007; iPACS): A Randomized Clinical Trial. J. Clin. Oncol. 2021, 39, 1098–1109. [Google Scholar] [CrossRef] [PubMed]
- Dave, E.W.; van der Kruijssen, D.E.W.; Elias, S.G.; Vink, G.R.; van Rooijen, K.L.; Lam-Boer, J.; Mol, L.; Punt, C.J.A.; de Wilt, J.H.W.; Koopman, M.; et al. Sixty-Day Mortality of Patients with Metastatic Colorectal Cancer Randomized to Systemic Treatment vs Primary Tumor Resection Followed by Systemic Treatment. The CAIRO4 Phase 3 Randomized Clinical Trial. JAMA Surg. 2021, 156, 1093–1101. [Google Scholar]
- Ferrand, F.; Malka, D.; Bourredjem, A.; Allonier, C.; Bouche, O.; Louafi, S.; Boige, V.; Mousseau, M.; Raoul, J.L.; Bedenne, L.; et al. Impact of primary tumour resection on survival of patients with colorectal cancer and synchronous metastases treated by chemotherapy. Results from the multicenter randomized trial Federation Francophone de Cancerologie Digestive 9601. Eur. J. Cancer 2013, 49, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Cirocchi, R.; Trastulli, S.; Abraha, I.; Vettoretto, N.; Boselli, C.; Montedori, A.; Parisi, A.; Noya, G.; Plattell, C. Non-resection versus resection for an asymptomatic primary tumour in patients with unresectable stage IV colorectal cancer. Cochrane Database Syst. Rev. 2012, 15, CD008997. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Xu, L.; Yang, W.; Xu, X.; Zheng, S. Asymptomatic primary tumour resection in metastatic colorectal cancer: A systematic review and meta-analysis. Front. Oncol. 2022, 12, 836404. [Google Scholar] [CrossRef] [PubMed]
- Faron, M.; Pignon, J.P.; Malka, D.; Bourredjem, A.; Douillard, J.Y.; Adenis, A.; Elias, D.; Bouché, O.; Ducreux, M. Is primary tumour resection associated with survival improvement in patients with colorectal cancer and unresectable synchronous metastases? A pooled analysis of individual data from four randomised trials. Eur. J. Cancer 2015, 51, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Alawadi, Z.; Phatak, U.R.; Hu, C.Y.; Bailey, C.E.; You, Y.N.; Kao, S.L.; Massarweh, N.N.; Feig, B.W.; Rodriguez-Bigas, M.A.; Skibber, J.M.; et al. Comparative effectiveness of primary tumour resection in patients with stage IV colon cancer. Cancer 2017, 123, 1124–1133. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xia, Z.; Jia, X.; Chen, K.; Li, D.; Dai, Y.; Tao, M.; Mao, Y. Primary tumor resection is associated with improved survival in stage IV colorectal cancer: An instrumental variable analysis. Sci. Rep. 2015, 5, 16516. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. Colorectal Cancer (Update). D1 Surgery for Asymptomatic Primary Tumour. NICE Guideline NG151. January 2020. Available online: https://www.nice.org.uk/guidance/ng151/evidence/d1-surgery-for-asymptomatic-primary-tumour-pdf-253058083671 (accessed on 26 February 2023).
- Rahbari, N.N.; Lordick, F.; Fink, C.; Bork, U.; Stange, A.; Jager, D.; Luntz, S.P.; Englert, S.; Rossion, I.; Koch, M.; et al. Resection of the primary tumour versus no resection prior to systemic therapy in patients with colon cancer and synchronous unresectable metastases (UICC stage IV): SYNCHRONOUS. A randomised controlled multicentre trial. BMC Cancer 2012, 12, 142. [Google Scholar] [CrossRef] [PubMed]
| Factor | Number | % |
|---|---|---|
| Total | 602 | 100 |
| Gender | ||
| Men | 476 | 79.1 |
| Women | 124 | 20.6 |
| Other/Does not respond | 2 | 0.3 |
| Age | ||
| <30 | 21 | 3.5 |
| 30–40 | 239 | 39.7 |
| 40–50 | 137 | 22.8 |
| 50–60 | 125 | 20.8 |
| >60 | 80 | 13.3 |
| Degree of experience | ||
| Trainee | 57 | 9.5 |
| Registrar/Senior Trainee/SAS doctor | 100 | 16.6 |
| Consultant | 368 | 61.1 |
| Other | 75 | 12.5 |
| Missing | 2 | 0.3 |
| Specialty | ||
| General Surgery | 505 | 83.9 |
| Colorectal Surgery | 79 | 13.1 |
| Upper GI Surgery | 5 | 0.8 |
| Medical Oncology | 4 | 0.7 |
| Clinical Oncology/Radiotherapy | 6 | 1.0 |
| Other | 3 | 0.5 |
| Main place of work | ||
| University Hospital | 224 | 37.2 |
| Teaching Hospital | 79 | 13.1 |
| District General Hospital | 190 | 31.6 |
| Community Hospital | 68 | 11.3 |
| Private Hospital | 33 | 5.5 |
| Private Practice/Clinic | 1 | 0.2 |
| Other | 7 | 1.2 |
| Zone | ||
| Northern Europe | 28 | 4.7 |
| Continental Europe | 88 | 14.6 |
| Southern Europe | 449 | 74.6 |
| Eastern Europe | 14 | 2.3 |
| USA/Canada | 2 | 0.3 |
| Central America | 0 | 0.0 |
| South America | 3 | 0.5 |
| North Africa | 3 | 0.5 |
| Central Africa | 2 | 0.3 |
| South Africa | 1 | 0.2 |
| Near East | 0 | 0.0 |
| Middle East | 5 | 0.8 |
| Far East/Asia | 2 | 0.3 |
| Oceania | 0 | 0.0 |
| Other/Does not respond | 5 | 0.8 |
| Is there a regular Colorectal Cancer MDT in your hospital? | ||
| Yes | 542 | 90.0 |
| No | 60 | 10.0 |
| How many colorectal cancers you see/treat in 1 year? | ||
| <20 | 40 | 6.6 |
| 20–40 | 106 | 17.6 |
| 40–60 | 129 | 21.4 |
| 60–80 | 87 | 14.5 |
| 80–100 | 97 | 16.1 |
| >100 | 142 | 23.6 |
| Does not respond | 1 | 0.2 |
| Clinical Cases | Treatment | N. | % |
|---|---|---|---|
| Case 1. Patient with asymptomatic Stage IV left colon cancer with inoperable liver metastases; no other metastases; age 43; ASA 2; WHO Perf. 0; and K-RAS naive. | Resection of the primary tumour + chemotherapy | 284 | 47.2% |
| Chemotherapy | 309 | 51.3% | |
| End-of-life care | 1 | 0.2% | |
| Other | 8 | 1.3% | |
| Total | 602 | 100% | |
| Case 2. Patient with asymptomatic Stage IV sigmoid colon cancer with inoperable liver metastases; no other metastases; age 82; ASA 2; WHO Perf. 0; and K-RAS mutant. | Resection of the primary tumour + chemotherapy | 186 | 30.9% |
| Chemotherapy | 315 | 52.3% | |
| Surveillance | 40 | 6.6% | |
| End-of-life care | 44 | 7.3% | |
| Other | 17 | 2.8% | |
| Total | 602 | 100% | |
| Case 3. Patient with asymptomatic Stage IV right colon cancer with inoperable liver metastases; no other metastases; age 67; ASA 4; WHO Perf. 3; and K-RAS naive. | Resection of the primary tumour + chemotherapy | 117 | 19.4% |
| Chemotherapy | 336 | 55.8% | |
| End-of-life care | 123 | 20.4% | |
| Other | 26 | 4.3% | |
| Total | 602 | 100% | |
| Case 4. Patient with asymptomatic Stage IV rectal cancer with inoperable liver and lung metastases; age 72; ASA 3; WHO Perf. 2; and K-RAS mutant. | Resection of the primary tumour + chemotherapy | 69 | 11.5% |
| Chemotherapy | 371 | 61.6% | |
| Radiotherapy | 89 | 14.8% | |
| Surveillance | 15 | 2.5% | |
| End-of-life care | 25 | 4.2% | |
| Other | 33 | 5.5% | |
| Total | 602 | 100.% | |
| Case 5. Patient with perforated Stage IV sigmoid tumour with inoperable liver and lung metastases; age 65; ASA 1; and WHO Perf. 0. | Emergency resection of the primary tumour + chemotherapy | 455 | 75.6% |
| Emergency resection of the primary tumour + surveillance/end-of-life care | 17 | 2.8% | |
| Emergency drainage + ileostomy/colostomy + elective resection of the primary tumour + chemotherapy | 60 | 10.0% | |
| Emergency drainage + ileostomy/colostomy + chemotherapy | 64 | 10.6% | |
| Other | 6 | 1.0% | |
| Total | 602 | 100% | |
| Case 6. Patient with obstruction due to Stage IV right colon cancer with inoperable liver and lung metastases and ascites; age 54; ASA 2; and WHO Perf. 2. | Emergency resection of the primary tumour + chemotherapy | 265 | 44.0% |
| Emergency ileostomy/caecostomy + elective resection of the primary tumour + chemotherapy | 92 | 15.3% | |
| Emergency ileostomy/caecostomy + chemotherapy | 117 | 19.4% | |
| Stent + chemotherapy | 110 | 18.3% | |
| Stent + surveillance | 12 | 2.0% | |
| End-of-life care | 2 | 0.3% | |
| Other | 4 | 0.7% | |
| Total | 602 | 100% | |
| Case 7. Patient with severe acute anaemia and rectal bleeding; cancer of the caecum with inoperable liver and lung metastases; age 70; ASA 2; and WHO Perf. 1. | Emergency resection of the primary tumour + chemotherapy | 247 | 41.0% |
| Embolization + chemotherapy | 41 | 6.8% | |
| Embolization + elective resection of the primary tumour + chemotherapy | 57 | 9.5% | |
| Transfusions + chemotherapy | 18 | 3.0% | |
| Transfusions + elective resection of the primary tumour + chemotherapy | 229 | 38.0% | |
| Transfusions + surveillance | 6 | 1.0% | |
| Other | 4 | 0.7% | |
| Total | 602 | 100% | |
| Case 8. Patient with asymptomatic Stage IV right colon cancer with inoperable lung metastases and ascites; age 47; ASA 3; and WHO Perf. 3. | Resection of the primary tumour + chemotherapy | 153 | 25.4% |
| Chemotherapy | 392 | 65.1% | |
| Surveillance | 34 | 5.6% | |
| Other | 23 | 3.8% | |
| Total | 602 | 100% | |
| Case 9. Patient with asymptomatic Stage IV distal transverse colon cancer with inoperable liver metastases; age 40; ASA 1; WHO Perf. 0; and K-RAS naive. | Resection of the primary tumour + chemotherapy | 311 | 51.7% |
| Chemotherapy | 283 | 47.% | |
| Surveillance | 2 | 0.3% | |
| Other | 6 | 1.% | |
| Total | 602 | 100% | |
| Case 10. Patient with asymptomatic Stage IV cancer of the proximal transverse colon with inoperable liver metastases; age 55; ASA 1; WHO Perf. 0; and K-RAS mutant. | Resection of the primary tumour + chemotherapy | 313 | 52.% |
| Chemotherapy | 272 | 45.2% | |
| Surveillance | 4 | 0.7% | |
| Other | 13 | 2.2% | |
| Total | 602 | 100% | |
| Case 11. Patient with obstructing Stage IV cancer of the splenic flexure, ascites and inoperable lung and liver metastases; age 65; ASA 4; and WHO Perf. 4. | Emergency resection of the primary tumour + chemotherapy | 109 | 18.1% |
| Emergency ileostomy/colostomy + chemotherapy | 230 | 38.2% | |
| Emergency ileostomy/colostomy + elective resection of the primary + chemotherapy | 54 | 9.% | |
| Emergency ileostomy/colostomy + surveillance | 129 | 21.4% | |
| End-of-life care | 53 | 8.8% | |
| Other | 27 | 4.5% | |
| Total | 602 | 100% | |
| Case 12. Patient with severe anaemia due to bleeding rectal cancer with inoperable liver and lung metastases; age 67; ASA 1; and WHO Perf. 1. | Emergency resection of the primary tumour + chemotherapy | 98 | 16.3% |
| Embolization/endoscopic haemostasis + elective resection of the primary + chemotherapy | 116 | 19.3% | |
| Embolization/endoscopic haemostasis + chemotherapy | 187 | 31.1% | |
| Transfusions + elective resection of the primary + chemotherapy | 132 | 21.9% | |
| Transfusions + chemotherapy | 34 | 5.6% | |
| End-of-life care | 2 | 0.3% | |
| Other | 33 | 5.5% | |
| Total | 602 | 100% |
| What factors do you consider as priority in the decision-making process in a case of a Stage IV colorectal cancer with inoperable liver and lung metastases? (multiple choice) | Age | 444 | 73.8 |
| Emergency presentation | 442 | 73.4 | |
| Symptoms | 423 | 70.3 | |
| ASA class | 421 | 69.9 | |
| Presence of ascites/carcinomatosis | 396 | 65.8 | |
| WHO Performance status | 365 | 60.6 | |
| Guidelines | 305 | 50.7 | |
| K-RAS status | 202 | 33.6 | |
| Number of metastatic sites | 177 | 29.4 | |
| Preference of the patient | 144 | 23.9 | |
| Other | 63 | 10.5 | |
| Availability of a skilled colorectal surgeon | 52 | 8.6 | |
| Local availability of chemotherapy facilities | 52 | 8.6 | |
| Local availability of biologics/third-line chemotherapy | 37 | 6.1 | |
| Cost/Financial implications | 9 | 1.5 | |
| Do you regularly offer/consider resection of the primary tumour in Stage IV colorectal cancer patients with inoperable liver and lung metastases? | Always | 19 | 3.2% |
| Often | 155 | 25.7% | |
| Sometimes | 262 | 43.5% | |
| Rarely | 142 | 23.6% | |
| No | 23 | 3.8% | |
| Missed/Does not answer | 1 | 0.2% |
| Variables | n. | Elective RS | Emergency RS | p | |
|---|---|---|---|---|---|
| Total | 602 | 34.0 ± 30.6 | 45.8 ± 17.1 | 0.000 | |
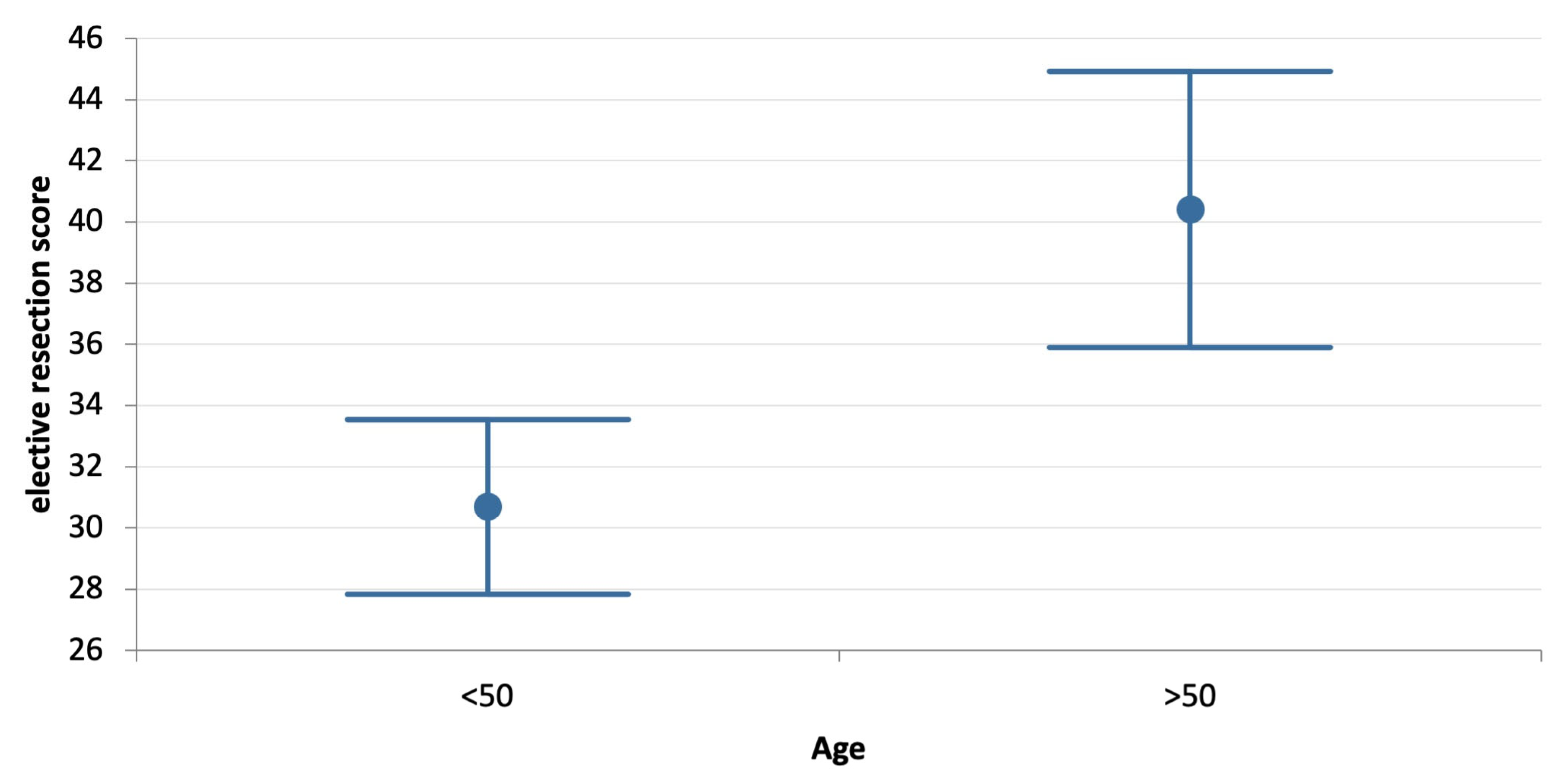
| Age | <50 yo | 397 | 30.7 ± 28.9 | 45.6 ± 16.8 | 0.000 |
| >50 yo | 205 | 40.4 ± 32.8 | 46.3 ± 17.6 | 0.024 | |
| p | 0.000 | 0.645 | |||
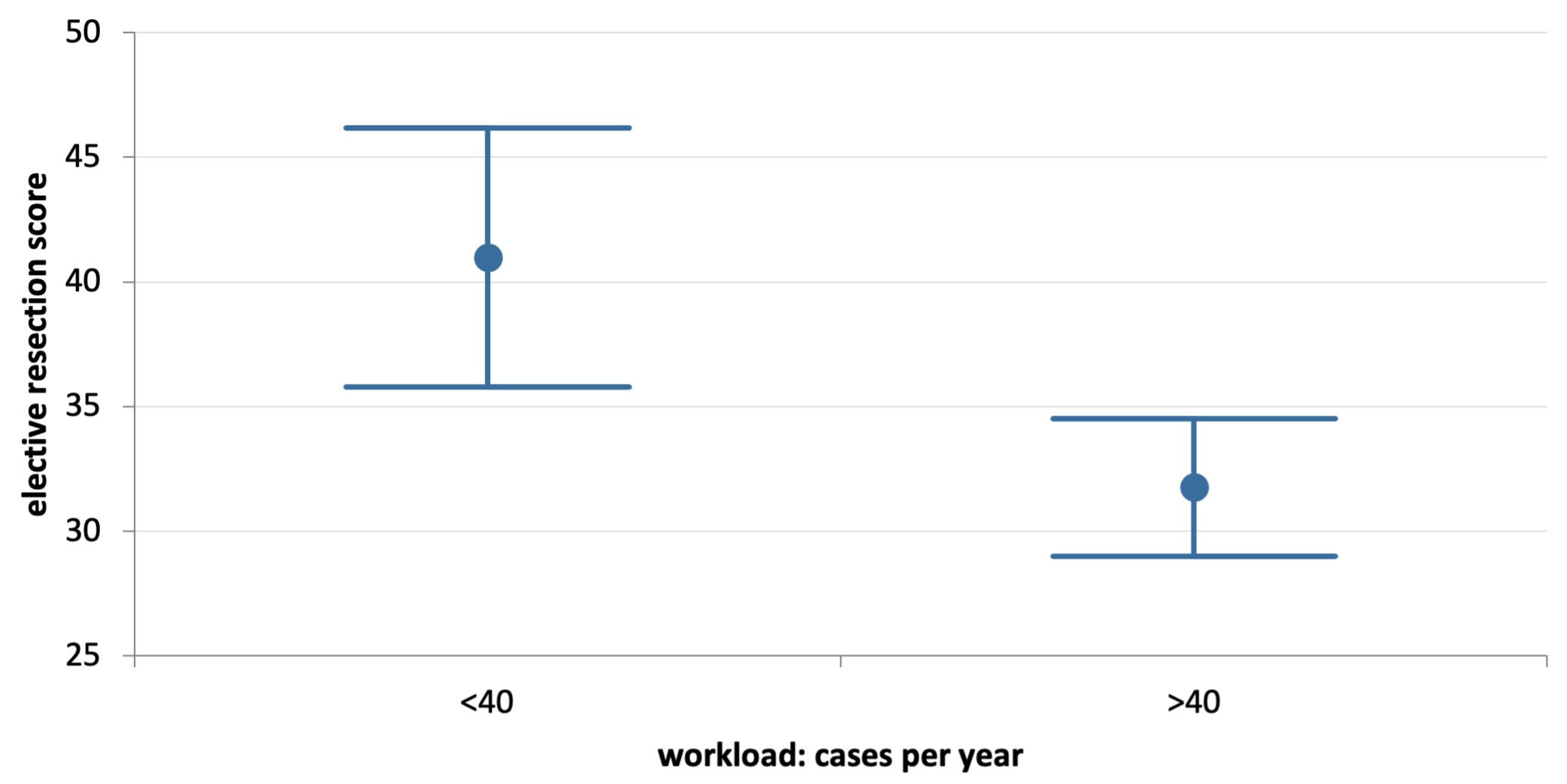
| Workload | <40 CRC/year | 146 | 41.0 ± 31.7 | 46.3 ± 18.3 | 0.081 |
| >40 CRC/year | 456 | 31.8 ± 30.0 | 45.7 ± 16.7 | 0.000 | |
| p | 0.001 | 0.710 | |||
| Affiliation | Academic | 303 | 33.4 ± 30.9 | 46.8 ± 17.1 | 0.000 |
| Non-academic | 258 | 33.9 ± 30.7 | 45.0 ± 17.2 | 0.000 | |
| Private/Other | 41 | 39.4 ± 28.5 | 44.3 ± 16.8 | 0.345 | |
| p | 0.500 | 0.382 | |||
| Seniority | Consultant | 368 | 32.0 ± 31.0 | 45.9 ± 17.0 | 0.000 |
| Non-consultant | 234 | 35.7 ± 30.0 | 45.7 ± 17.3 | 0.000 | |
| p | 0.275 | 0.857 | |||
| Colorectal cancer MDT | Yes | 542 | 33.9 ± 30.9 | 45.9 ± 16.9 | 0.000 |
| No | 60 | 35.2 ± 27.8 | 45.2 ± 19.0 | 0.023 | |
| p | 0.743 | 0.780 |
| Significant Variables | Coefficient | p |
|---|---|---|
| Age (>50 yo vs. <50 yo) | 9.0 | 0.001 |
| Workload (>40/y vs. <40/y) | −8.2 | 0.004 |
| Intercept | 37.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tebala, G.D.; Di Cintio, A.; Ricci, F.; Avenia, S.; Cirocchi, R.; Desiderio, J.; Di Nardo, D.; Di Saverio, S.; Gemini, A.; Ranucci, M.C.; et al. Primary Tumour Treatment in Stage 4 Colorectal Cancer with Unresectable Liver and Lung Metastases and No Peritoneal Carcinomatosis—Current Trends and Attitudes in the Absence of Clear Guidelines. J. Clin. Med. 2023, 12, 3499. https://doi.org/10.3390/jcm12103499
Tebala GD, Di Cintio A, Ricci F, Avenia S, Cirocchi R, Desiderio J, Di Nardo D, Di Saverio S, Gemini A, Ranucci MC, et al. Primary Tumour Treatment in Stage 4 Colorectal Cancer with Unresectable Liver and Lung Metastases and No Peritoneal Carcinomatosis—Current Trends and Attitudes in the Absence of Clear Guidelines. Journal of Clinical Medicine. 2023; 12(10):3499. https://doi.org/10.3390/jcm12103499
Chicago/Turabian StyleTebala, Giovanni Domenico, Antonio Di Cintio, Francesco Ricci, Stefano Avenia, Roberto Cirocchi, Jacopo Desiderio, Domenico Di Nardo, Salomone Di Saverio, Alessandro Gemini, Maria Chiara Ranucci, and et al. 2023. "Primary Tumour Treatment in Stage 4 Colorectal Cancer with Unresectable Liver and Lung Metastases and No Peritoneal Carcinomatosis—Current Trends and Attitudes in the Absence of Clear Guidelines" Journal of Clinical Medicine 12, no. 10: 3499. https://doi.org/10.3390/jcm12103499
APA StyleTebala, G. D., Di Cintio, A., Ricci, F., Avenia, S., Cirocchi, R., Desiderio, J., Di Nardo, D., Di Saverio, S., Gemini, A., Ranucci, M. C., Trastulli, S., Cianchi, F., Scatizzi, M., Catena, F., & the MeCC-4 International Collaborative. (2023). Primary Tumour Treatment in Stage 4 Colorectal Cancer with Unresectable Liver and Lung Metastases and No Peritoneal Carcinomatosis—Current Trends and Attitudes in the Absence of Clear Guidelines. Journal of Clinical Medicine, 12(10), 3499. https://doi.org/10.3390/jcm12103499








