Comparison of Intranasal Steroid Application Using Nasal Spray and Spray-Sol to Treat Allergic Rhinitis: A Preliminary Investigation
Abstract
1. Introduction
2. Materials and Methods
- Spray-sol group: nasal administration of BDP (CLENIL 0.8 mg/2 mL suspension for nebulization, one single-dose vial 2 mL for aerosol) plus 3 mL of isotonic saline solution, dividing the content equally between the two nostrils, twice a day (in the morning after breakfast and in the evening before bedtime) for four weeks. Patients used BDP with Spray-sol according to the following instructions:
- ○
- remove one single-dose vial and heat it between your hands before administering the solution;
- ○
- open the vial by turning the cap, connect it to the Luer-Lock syringe, and aspirate the solution to be sprayed;
- ○
- fix the Luer-Lock syringe by screwing it into the appropriate Spray-sol nozzle;
- ○
- divide the solution contained in the syringe equally between the two nostrils;
- ○
- before storing the device, rinse the syringe under running water and wash Spray-sol.
- Spray group: nasal administration of BDP solution using a common nasal spray (Rinoclenil 100 microgram nasal spray, suspension), one spray per nostril twice daily (in the morning after breakfast and in the evening before going to bed) for four weeks.
- The two groups recorded no statistically significant differences regarding age, BMI, nasal endoscopy, and TNSS.
2.1. Spray-Sol Characteristics
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ciprandi, G.; Marseglia, G.L.; Castagnoli, R.; Valsecchi, C.; Tagliacarne, C.; Caimmi, S.; Licari, A. From IgE to clinical trials of allergic rhinitis. Expert Rev. Clin. Immunol. 2015, 11, 1321–1333. [Google Scholar] [CrossRef] [PubMed]
- Brożek, J.L.; Bousquet, J.; Agache, I.; Agarwal, A.; Bachert, C.; Bosnic-Anticevich, S.; Brignardello-Petersen, R.; Canonica, G.W.; Casale, T.; Chavannes, N.H.; et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines–2016 revision. J. Allergy Clin. Immunol. 2017, 140, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Bousquet, J.; Schünemann, H.J.; Togias, A.; Bachert, C.; Erhola, M.; Hellings, P.W.; Klimek, L.; Pfaar, O.; Wallace, D.; Ansotegui, I.; et al. Next-generation Allergic Rhinitis and Its Impact on Asthma (ARIA) guidelines for allergic rhinitis based on Grading of Recommendations Assessment, Development and Evaluation (GRADE) and real-world evidence. J. Allergy Clin. Immunol. 2020, 145, 70–80.e3. [Google Scholar] [CrossRef] [PubMed]
- Samoliński, B.; Nowicka, A.; Wojas, O.; Lipiec, A.; Krzych-Fałta, E.; Tomaszewska, A. Intranasal glucocorticosteroids–not only in allergic rhinitis In the 40th anniversary of intranasal glucocorticosteroids’ introduction. Otolaryngol. Pol. = Polish Otolaryngol. 2014, 68, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Casale, M.; Pace, A.; Moffa, A.; Vella, P.; Sabatino, L.; Lopez, M.A.; Salvinelli, F. Post-operative nebulized sodium hyaluronate versus spray after functional endoscopic sinus surgery for chronic rhinosinusitis. J. Biol. Regul. Homeost. Agents 2017, 31 (Suppl. S2), 81–89. Available online: https://pubmed.ncbi.nlm.nih.gov/29202566/ (accessed on 17 February 2022). [PubMed]
- Moffa, A.; Casale, M.; Fiore, V.; Rinaldi, V.; Giancaspro, R.; Lopez, M.; Baptista, P.; Gelardi, M.; Cassano, M. Impact of intranasal nebulized ectoine on morbidity and short-term quality of life after pediatric adenoidectomy. J. Biol. Regul. Homeost. Agents 2020, 34, 1213–1218. [Google Scholar] [CrossRef] [PubMed]
- Moffa, A.; Carbone, S.; Costantino, A.; Fiore, V.; Rinaldi, V.; Baptista, P.; Cassano, M.; Casale, M. Potential role of topical ectoine for prevention of pediatric upper respiratory tract infection: A preliminary observational study. J. Biol. Regul. Homeost. Agents 2019, 33, 1935–1940. [Google Scholar] [CrossRef] [PubMed]
- Ciprandi, G.; Gelardi, M. Open and clean: The healthy nose. Acta Bio Medica Atenei Parm. 2019, 90 (Suppl. 2), 4. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research (CDER). FDA Guidance for Industry, Nasal Spray and Inhalation Solution, Suspension, and Spray Drug Products—Chemistry, Manufacturing, and Controls Documentation. 2002; pp. 10–15. Available online: https://www.fda.gov/media/70857/download (accessed on 19 December 2022).
- Varricchio, A.; Brunese, F.P.; La Mantia, I.; Ascione, E.; Ciprandi, G. Choosing nasal devices: A dilemma in clinical practice. Acta Biomed. 2023, 94, e2023034. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Lane, A.P. Topical Drug Delivery for Chronic Rhinosinusitis. Curr. Otorhinolaryngol. Rep. 2013, 1, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Laube, B.L. Devices for aerosol delivery to treat sinusitis. J. Aerosol Med. Depos. Clear. Eff. Lung 2007, 20 (Suppl. 1), S5–S17; discussion S17–S8. [Google Scholar] [CrossRef] [PubMed]
- Casale, M.; Vella, P.; Moffa, A.; Oliveto, G.; Sabatino, L.; Grimaldi, V.; Pietro, F.; Salvinelli, F. Hyaluronic acid and upper airway inflammation in pediatric population: A systematic review. Int. J. Pediatr. Otorhinolaryngol. 2016, 85, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Adappa, N.D.; Wei, C.C.; Palmer, J.N. Nasal irrigation with or without drugs: The evidence. Curr. Opin. Otolaryngol. Head Neck Surg. 2012, 20, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Moffa, A.; Costantino, A.; Rinaldi, V.; Sabatino, L.; Trecca, E.M.C.; Baptista, P.; Campisi, P.; Cassano, M.; Casale, M. Nasal Delivery Devices: A Comparative Study on Cadaver Model. Biomed Res. Int. 2019, 2019, 4602651. [Google Scholar] [CrossRef] [PubMed]
- Albu, S. Novel drug-delivery systems for patients with chronic rhinosinusitis. Drug Des. Devel. Ther. 2012, 6, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.S.; Wight, R.G.; Stevens, J.C.; Beckingham, E. The nasal valve: A physiological and clinical study. J. Laryngol. Otol. 1988, 102, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Sastre, J.; Mosges, R. Local and systemic safety of intranasal corticosteroids. J. Investig. Allergol. Clin. Immunol. 2012, 22, 1–12. [Google Scholar] [PubMed]
- Casale, M.; Rinaldi, V.; Sabatino, L.; Moffa, A.; Ciccozzi, M. Could nasal irrigation and oral rinse reduce the risk for COVID-19 infection? Int. J. Immunopathol. Pharmacol. 2020, 34, 2058738420941757. [Google Scholar] [CrossRef] [PubMed]
- Casale, M.; Sabatino, L.; Frari, V.; Mazzola, F.; Dell’Aquila, R.; Baptista, P.; Mladina, R.; Salvinelli, F. The potential role of hyaluronan in minimizing symptoms and preventing exacerbations of chronic rhinosinusitis. Am. J. Rhinol. Allergy 2014, 28, 345–348. [Google Scholar] [CrossRef] [PubMed]
| Nasal Endoscopy Evaluation | None | Mild | Moderate | Severe |
|---|---|---|---|---|
| Edema | 0 | 1 | 2 | 3 |
| Irritation | 0 | 1 | 2 | 3 |
| Secretion | 0 | 1 | 2 | 3 |
| Crustiness | 0 | 1 | 2 | 3 |
| TNSS | Likert SCALE: 0—No Symptoms; 1—Mild Symptoms; 2—Moderate Symptoms; 3—Severe Symptoms | |||
|---|---|---|---|---|
| Nasal Congestion | 0 | 1 | 2 | 3 |
| Sneezing | 0 | 1 | 2 | 3 |
| Rhinorrhea | 0 | 1 | 2 | 3 |
| Nasal Itching | 0 | 1 | 2 | 3 |
| Spray-Sol Group (12 Patients) | Spray Group (14 Patients) | |
|---|---|---|
| Mean age (yr) | 45.52 | 44.80 |
| Weight (kg) | 82.30 | 83.00 |
| Height (cm) | 176.00 | 175.70 |
| Nasal endoscopy evaluation | ||
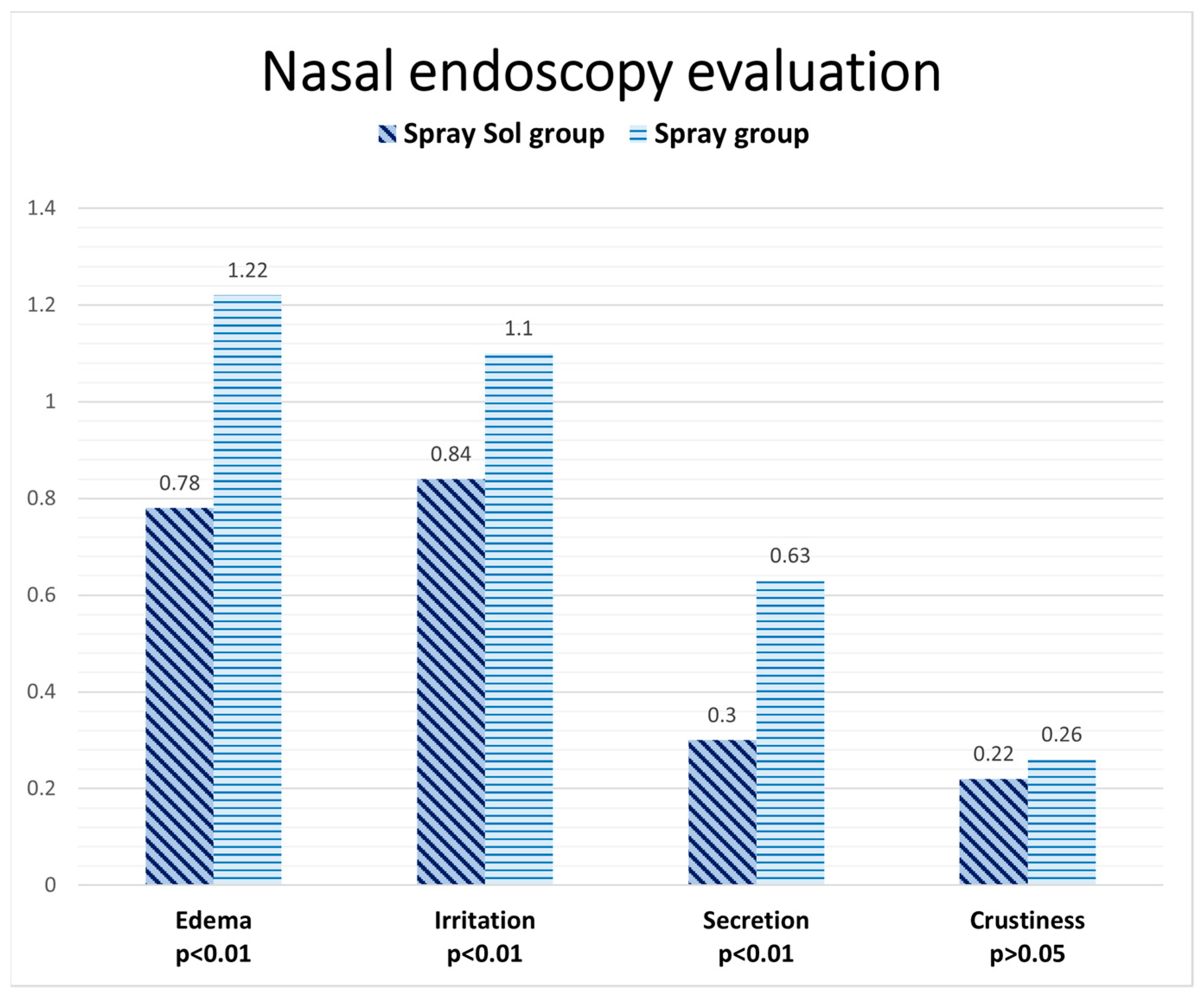
| Edema | 2.2 ± 0.41 | 2.1 ± 0.32 |
| Irritation | 2.2 ± 0.41 | 2.1 ± 0.32 |
| Secretion | 1.33 ± 1.05 | 0.6 ± 0.52 |
| Crustiness | 0.26 ± 0.46 | 0.5 ± 0.53 |
| TNSS | ||
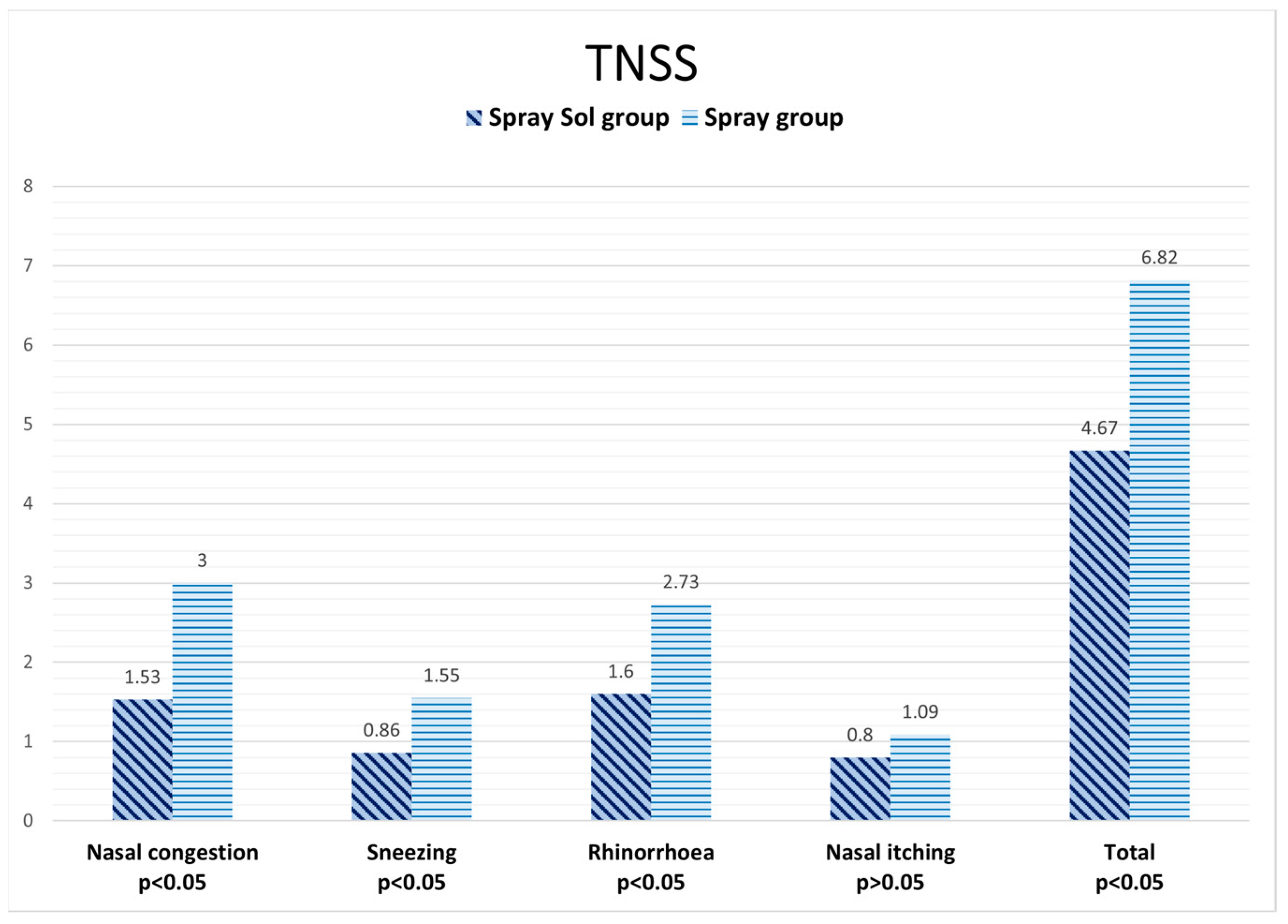
| Nasal congestion | 1.57 ± 0.98 | 2.25 ± 1.16 |
| Sneezing | 1 ± 1 | 1.125 ± 0.83 |
| Rhinorrhea | 1.14 ± 1.06 | 1.125± 0.64 |
| Nasal itching | 0.71 ± 0.76 | 0.75 ± 0.71 |
| Total score | 2.58 ± 2.94 | 3.82 ± 3.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moffa, A.; Giorgi, L.; Carnuccio, L.; Lugo, R.; Baptista, P.; Casale, M. Comparison of Intranasal Steroid Application Using Nasal Spray and Spray-Sol to Treat Allergic Rhinitis: A Preliminary Investigation. J. Clin. Med. 2023, 12, 3492. https://doi.org/10.3390/jcm12103492
Moffa A, Giorgi L, Carnuccio L, Lugo R, Baptista P, Casale M. Comparison of Intranasal Steroid Application Using Nasal Spray and Spray-Sol to Treat Allergic Rhinitis: A Preliminary Investigation. Journal of Clinical Medicine. 2023; 12(10):3492. https://doi.org/10.3390/jcm12103492
Chicago/Turabian StyleMoffa, Antonio, Lucrezia Giorgi, Luca Carnuccio, Rodolfo Lugo, Peter Baptista, and Manuele Casale. 2023. "Comparison of Intranasal Steroid Application Using Nasal Spray and Spray-Sol to Treat Allergic Rhinitis: A Preliminary Investigation" Journal of Clinical Medicine 12, no. 10: 3492. https://doi.org/10.3390/jcm12103492
APA StyleMoffa, A., Giorgi, L., Carnuccio, L., Lugo, R., Baptista, P., & Casale, M. (2023). Comparison of Intranasal Steroid Application Using Nasal Spray and Spray-Sol to Treat Allergic Rhinitis: A Preliminary Investigation. Journal of Clinical Medicine, 12(10), 3492. https://doi.org/10.3390/jcm12103492









